Abstract
Soil cadmium (Cd) pollution resulting from anthropogenic activities has become a major concern for microbial and biochemical functions that are critical for soil quality and ecosystem sustainability. Organic amendments can reduce Cd toxicity to the microbial community and enzymatic activity in Cd-polluted soils and thus would increase the ecological dose (ED) values. However, there has been less focus on the effect of organic amendments on microbial and biochemical responses to Cd toxicity in non-calcareous soils using the concept ED. The aim of this study was to assess the impact of compost application on microbial activity, microbial biomass, turnover rates of carbon and nitrogen, and enzymatic activities as the key ecological functions in a non-calcareous soil spiked with different Cd concentrations (0–200 mg kg−1). Results showed that soil amendment with compost decreased Cd availability by 48–76%, depending on the total soil Cd content. The application of compost reduced the negative influence of Cd eco-toxicity on most soil microbial and biochemical functions by 20–122%, depending on the Cd level and the assay itself. The ED values, derived from the sigmoidal dose-response and kinetic models, were 1.10- to 2.24-fold higher in the compost-amended soils than the unamended control soils at all Cd levels. In conclusion, the potential risks associated with high levels of Cd pollution can be alleviated for microbial and biochemical indicators of soil quality/health with application of 2500 kg ha−1 compost as a cost-effective source of organic matter to non-calcareous soils. The findings would have some useful implications for organic matter-limited non-calcareous soils polluted with Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil microbial communities are the main driving forces responsible for the long-term sustainability of ecosystems because they regulate the formation and decomposition of soil organic matter (SOM) and plant residues, the cycling and availability of essential nutrients, carbon (C) cycling and sequestration, biodegradation of many harmful organic pollutants as well as biotransformation of toxic metals in the soil (Nannipieri et al. 2003; Soares and Rousk 2019; Xu et al. 2019). Microbial communities are a potential source of enzyme release and production in the soil environment, which are the major catalysts of biogeochemical reactions (Dick 1997; Nannipieri et al. 2012, 2002). These catalysts play an important role in microbial life cycle and metabolism, and the critical biogeochemical processes that are essential for the maintenance of soil quality and ecosystem functioning (Caldwell 2005; Dick 1997; Nannipieri et al. 2018, 2002). As soil microorganisms and enzymes reflect environmental conditions, they can be used as early indications of ecological changes in soil quality/health due to perturbation and pollution by toxic metals (Dick 1997; Karaca et al. 2010, 2002; Nannipieri et al. 2003).
A large number of microbial and biochemical properties manifesting the activity, biomass and diversity as well as enzyme production of soil microbial communities are used as sensitive indicators for soil quality/health evaluation and for ecological risk assessments in polluted environments (Karaca et al. 2010; Kızılkaya et al. 2004; Lorenz et al. 2006; Tejada 2009; Vig et al. 2003).
Soil pollution with cadmium (Cd) is an important environmental concern due to its high mobility and toxicity as well as different source contributions in the soil-plant system (Hamid et al. 2019; Khan et al. 2017; Smolders and Mertens 2013). Numerous studies have demonstrated a negative influence of Cd on soil microbial and biochemical properties, often with reduced microbial activity, microbial biomass and enzyme activity in Cd-polluted environments (Epelde et al. 2016; He et al. 2019; Karaca et al. 2002; Li et al. 2020; Lorenz et al. 2006; Moreno et al. 2006, 2002; Tan et al. 2017; Tejada 2009). Nevertheless, the response of soil microbial community and biogeochemical processes to Cd pollution primarily depends upon soil properties, which play key roles in the bioavailability and toxicity potential of Cd (Khan et al. 2017; Vig et al. 2003). Amongst others, soil pH and SOM are important determinants of Cd mobility and availability (Hamid et al. 2019; Khan et al. 2017; Smolders and Mertens 2013) because of their impacts on Cd adsorption, precipitation, or complexation, its speciation in the soil solution and fractionation within the soil solid phases (Mohamed et al. 2010; Vaca-Paulín et al. 2006).
Considering the critical role of SOM and organic amendments on soil response to Cd pollution, several studies have paid considerable attention to the addition of soil amendments to lower/alleviate Cd toxicity to the microbial community (He et al. 2019; Li et al. 2020; Moreno et al. 2003, 2002; Pardo et al. 2014) and growing plants (Hamid et al. 2019; Hanc et al. 2009; Li et al. 2020; Pardo et al. 2014). The availability and toxicity of Cd can decrease with the addition of cost-effective organic amendments due to its strong adsorption or complexation with organic ligands (Hamid et al. 2019; Karaca 2004; Khan et al. 2017; Mohamed et al. 2010; Tejada 2009). Application of organic amendments to the soil has been used as immobilizing agents practically suitable for Cd remediation at polluted sites (Hamid et al. 2019; Khan et al. 2017; Li et al. 2020; Mohamed et al. 2010; Pardo et al. 2014). While organic amendments can reduce Cd toxicity to the microbial community in calcareous soils with high pH (Li et al. 2020; Moreno et al. 2003; Tejada 2009), there has been less focus on the effect of organic amendments on microbial responses to Cd toxicity in non-calcareous soils under Iranian conditions. The presence of high calcium carbonates and alkalinity in calcareous soils can lead to co-precipitation of Cd ions with major hydroxides or calcium-carbonates that can immobilize/stabilize Cd and reduce its concentration in the soil solution (Moreno et al. 2001; Renella et al. 2004; Smolders and Mertens 2013). Therefore, it is difficult to separate these effects from the effect of SOM per se on Cd mobility and toxicity in highly calcareous soils, because they commonly occur concurrently. On the other hand, low nutrient availability and biological activity are the major challenges in Cd-polluted non-calcareous soils with low SOM content (Dušek 1995; Moral et al. 2005).
Several studies (Moreno et al. 2003, 2002; Tejada et al. 2008) have considered the effect of organic amendments on various soil microbial and biochemical functions in metal-polluted soils by determining the 50% ecological dose (ED50) value, the concentration of a toxic metal at which microbial and biochemical functions are inhibited by 50% relative to the uninhibited (maximum) value in an unpolluted soil (Babich et al. 1983; Speir et al. 1999). They demonstrated higher ED50 values with the application of sewage sludge in Cd- or nickel (Ni)-polluted soils (Moreno et al. 2003, 2002) and with addition of organic amendments in Ni-polluted soils (Tejada et al. 2008) under controlled laboratory conditions. The ED50, first established by Babich et al. (1983), is a suitable indicator of the sensitivity of a soil function to metal toxicity under different soil and exposure conditions (Hinojosa et al. 2008; Moreno et al. 2006; Renella et al. 2003). Plausibly, the ED50 value is robust and can also be used to identify the factors affecting the speciation and mobility of heavy metals in soils and controlling their availability (Moreno et al. 2006, 2001; Tejada et al. 2008); the time of exposure to a particular metal (Moreno et al. 2003, 2002; Tejada et al. 2008) as well as differences in soil or environmental conditions such as texture, organic matter content and pH (Doelman and Haanstra 1986; Moreno et al. 2006, 2001; Tan et al. 2014; Wang et al. 2018) or even soil use and management (Moreno et al. 2006). Although too high for the continuing soil functioning (Moreno et al. 2002; Speir et al. 1999; Tejada et al. 2008), this toxicity cue can be applied for assessing the ecological risk of toxic metals and other chemicals to the quality and health of the soil ecosystem (Epelde et al. 2016).
While the effect of organic amendments on Cd mobility and toxicity is widely recognized, very few studies have been conducted to examine the impact of organic amendments, particularly compost, on Cd toxicity to the soil microbial community and enzyme activity in non-calcareous soils of Iran with a high risk of Cd pollution using the concept ED. Such information is important for identifying and adopting management practices which are most effective in immobilizing Cd and hence contributing to the mitigation of the toxic effect of Cd on the soil microbial community. In this context, the main aim of the study reported here was to investigate the effect of compost application on Cd toxicity to the soil microbial community and enzyme activity by calculating ED50 values. Our main research question is to what extent compost addition to the soil influences Cd availability and subsequently the basic soil ecological functions, by determining the ED50 values. Our key hypothesis was that application of compost would lower Cd toxicity, which could be reflected by an increase in soil ecological functions and the ED50 values as an integrated index of soil ecological sensitivity.
Materials and methods
Soil sampling and treatments
A non-calcareous (calcium carbonates <5%, pH ≈ 7) clay soil sample from the surface layer (~20 cm depth) was collected from a cropland field in DoAb-Samsami region (mean annual rainfall of 875 mm and mean annual temperature of 9.8 °C) without pollution exposure (Table 1). The study soil was classified as Pachic Haploxerolls (The United States Department of Agriculture Soil Taxonomy) or Haplic Phaeozems (The Food and Agriculture Organization). This soil was selected to minimize the possible impact of carbonates (He et al. 2019; Moreno et al. 2006; Pardo et al. 2014; Renella et al. 2004), which are common constituents of many soils in arid and semi-arid areas, on Cd availability and toxicity. Soil pH and SOM content are generally the primary factors associated with Cd solubility in the soil solution (Smolders and Mertens 2013). The soil (20 kg) was air-dried and passed through a 2-mm mesh sieve prior to analysis. The soil texture (the hydrometer method), pH (in 1:2.5 soil–water), electrical conductivity (in 1:2.5 soil–water), CaCO3 (the titration method), organic carbon (the Walkley and Black method), total nitrogen (the Kjeldahl method), available phosphorus concentration (the Olsen’s method), available potassium concentration (extraction by 1 M ammonium-acetate) and cation exchange capacity (ammonium-acetate, pH 7.0) were determined. DTPA-TEA (diethylene triamine penta acetic acid-triethanol amine)-extractable Cd concentration at pH 7.3 (Lindsay and Norvell 1978) and total Cd pool were determined using atomic absorption spectrophotometry (AAS Model GBC 932 Plus). Total soil Cd was determined using digestion of soil (2 g) in 12.5 mL of concentrated (4 M) HNO3 at 80 °C overnight (Sposito et al. 1982).
The compost was prepared from a mixture of agricultural plant residues, grasses and cow manure; and left to rest and decay for 60 days. At the end of the composting process, the compost subsamples were air-dried and ground to 1 mm for chemical analysis including electrical conductivity (EC), pH, C, nitrogen (N), phosphorus (P), potassium (K) and Cd contents as outlined for soil analysis (Table 1). In Iran, compost production using organic municipal wastes or plant residues has increased dramatically leading to the establishment of large-scale composting facilities/plants making compost widely available to be used as a valuable soil conditioner. We used a completely randomized design with 5 × 2 full-factorial treatment combinations in three replicates with the following factors: (1) five Cd levels (0–200 mg kg−1) and (2) two rates of compost application (0 and 10 g kg−1 soil, equivalent to 1% w/w or about 2500 kg ha−1). Soil samples were spiked with Cd at five concentrations of 0, 50, 100, 150 and 200 mg kg−1 to represent soil Cd pollution in the vicinity of mining and industrial areas in Iran (Chehregani et al. 2009; Rafiei et al. 2010). The selected Cd concentrations are much higher than the maximum concentration of total Cd permitted in soils (2.3 mg Cd kg−1) in the context of European Union legislation (Smolders and Mertens 2013). We used this limit as a reference because no such values have been established for soil protection in Iran. The soils were artificially contaminated with cadmium sulfate (CdSO4·8/3H2O) on a dry weight basis in plastic jars. The control soils (0 mg Cd kg−1) were watered with distilled water and Cd-treated soils with appropriate Cd solutions to achieve the above Cd concentrations. Soils were mixed completely using plastic spoons for 3 min to ensure homogenization and for a uniform distribution of the added Cd ions in the soil matrix. Distilled water was used to adjust a soil moisture content of about 60–70% of the soil water holding capacity (WHC). The soils were incubated at room temperature (about 20 ± 5 °C) for 30 days to achieve an equilibrium condition and for Cd aging process (Renella et al. 2005). Metal aging is a factor that affects metal availability and is needed for the experiments with freshly spiked soils (Oorts et al. 2007). After this period, the soils in 15 jars were amended with compost (<1 mm) at a rate of 10 g kg−1 soil, and the other 15 jars were left unamended. The treated soils were then homogenized thoroughly for a uniform distribution of the added compost in the soil background. The amended and unamended Cd-polluted soils were pre-incubated at 60–70% WHC and in the dark at constant room temperature for 10 days to lessen the effects of soil preparation and disturbance, and to reactivate the soil microbial populations and communities. Ultimately, the jars were incubated for 10 weeks at 25 ± 1 °C and 65 ± 3% WHC.
Soil microbial and enzyme analysis
Soil C and N mineralization, as an index of C and N turnover, and microbial activity, were monitored during the incubation period (70 days). For the soil C mineralization (i.e., CO2 production), a vial containing 10 ml of 1 M NaOH as alkali was placed inside the jars for CO2 absorption. The amount of CO2 evolved from the soil was determined by back-titrating the alkali with 0.5 N HCl after precipitating the carbonates with 10% BaCl2 solution (Alef and Nannipieri 1995). The C mineralization was expressed as the cumulative (total) CO2 production or total C mineralized (TCM) over 70 days and reported as mg C kg−1 soil. The soil net N mineralization was determined according to the method described by Raiesi (2012) at weekly intervals for 70 days as the net increases in ammonium-N plus nitrate-N. Soil subsamples were extracted for 30 min with 50 mL of 1 M KCl before (t = 0) and at 7-day intervals, and the inorganic N (NH4 and NO3) concentrations were determined in the soil extracts colorimetrically at 410 (NO3) or 660 (NH4) nm (Alef and Nannipieri 1995). As with C mineralization, the net N mineralization was expressed as the total concentration of net N mineralized or total N mineralized (TNM) throughout the incubation. Microbial ammonification and nitrification are the major sources of plant-available N in the soils (Azeez and Van Averbeke 2010; Li et al. 2018; Raiesi et al. 2018). The potential net ammonification rate (PAR) was then calculated as the cumulative net ammonification (the difference in ammonium-N before and after the incubation) divided by 70-days of the incubation period. Likewise, the potential net nitrification rate (PNR) was estimated as the difference in nitrate-N before and after the incubation and the increase in nitrate-N during the course (70 days) of incubation. Results for C and N turnover were calculated on a 105 °C oven-dry weight basis.
At the end of the soil incubation, microbial basal respiration (BR) as the overall microbial activity, substrate (glucose)-induced respiration (SIR), microbial biomass N (MBN) content and enzyme activities were determined. The BR was quantified and considered as the amount of CO2 produced over 15 days and expressed as mg C kg−1 soil day−1 as outlined above for the C mineralization experiment. Substrate-induced respiration (SIR) was measured by adding 2 ml of 1% (w/w) glucose solution to a soil subsample (1 g) and the evolved CO2 over 5–6 h was determined (Lin and Brookes 1999) as described above. The SIR value was used as an indirect estimate of soil microbial biomass and the potentially active microbial community (Blagodatskaya and Kuzyakov 2013). The fumigation-incubation method was used to measure soil MBN pool (Joergensen 1995). Subsamples were fumigated with chloroform for 24 h in a desiccator lined up with vacuum pump. Fumigated and non-fumigated control samples were incubated (65 ± 3% WHC, 25 ± 1 °C) for 10 days and the quantity of inorganic N (NH4 and NO3) was determined as described above. The difference between the inorganic N accumulation in fumigated and non-fumigated samples was considered as MBN using a factor of 0.57 (Joergensen 1995). Soil MBN was expressed as mg kg−1 soil.
The microbial enzyme activities were assayed based on the colorimetric determination of the product released by the enzyme, when soil subsamples were incubated with an adequate substrate under standard conditions, following the methods described in Alef and Nannipieri (1995). Urease activity (URE) was assayed using 200 mM urea as the substrate under standard conditions (24 h at 37 °C). Alkaline (ALP) and acid (ACP) phosphatase activities were determined using 15 mM p-nitrophenyl phosphate disodium (PNP) as the substrate in a modified universal buffer (MUB) at pH 11 and 6; respectively, incubated for 1 h at 37 °C. Absolute enzyme activities were expressed as micrograms of product produced per gram of oven-dry weight (105 °C) per specified time. Soil pH, EC and DTPA-extractable Cd concentration were also determined at the end of the incubation period. The DTPA-extractable Cd fraction in the soil solution was considered as a measure of the potentially available Cd to the soil microorganisms (Moreno et al. 2006; Tejada 2009).
Mathematical models for the ED50 determination
We used the sigmoidal dose-response logistic model (Haanstra et al. 1985) and the kinetic model (Speir et al. 1999) to assess the inhibition of the tested soil properties by Cd. The first model (Model 1) describes a logistic curve, which represents the relationship between the measured parameter and the natural logarithm of soil Cd concentration. The mathematical equation for the sigmoidal dose-response model is:
where y is the response variable (the test soil property), x is the natural logarithm of the total Cd added to the soil, a is the uninhibited value of y, b is a slope factor showing the inhibition rate, and c is the natural logarithm of ED50. The ED50 values were finally calculated using the following expression:
The algebraic expression of the kinetic model (Model 2) was:
This model describes the full inhibition of the measured parameter (y) by the concentration of (Cd) inhibitor (x). The constants a and b are positive. The constant a represents the uninhibited value of the measured parameter, and the constant b depends on the curve slope. The ED50 values for Model 2 were calculated using the following expression:
Statistical analysis
A two-way analysis of variance (ANOVA) procedure was conducted to examine the influence of Cd levels, compost application and their interaction as sources of variation on the measured soil biological variables. First, the residuals were tested for equal variance and normal distribution before statistical analysis to meet the assumptions underlying parametric statistical tests. Differences among mean values were separated based on the least significant difference (LSD) test, and were considered to be statistically significant at 5% level of significance (P < 0.05). All statistical analyses were performed using the statistical software Minitab (Minitab 18.1). The sigmoidal dose-response and kinetic functions (Models 1 and 2) were fitted to soil data for the calculation of the parameters in the models separately for each compost treatment using SigmaPlot for Windows version 12.0, which uses the robust Marquardt–Levenberg algorithm to minimize the sum of squares of the residuals. Goodness of fit of the model was estimated from both the coefficient of determination (R2) and the standard error of the estimate (SEE) of the non-linear regression determined at P < 0.05. As an ED50 value is too extreme for environmental risk assessment, lower doses of ED10 and ED5 (the concentration of Cd causing 10 or 5 % inhibition), which would be more meaningful (Haanstra et al. 1985; Moreno et al. 2003; Speir et al. 1999; Tejada et al. 2008), were also calculated using the algebraic expressions presented in Moreno et al. (2002). The EDx values (mg Cd kg−1 soil) were calculated using the total Cd concentrations initially added to the soil because the two mathematical models were poorly fitted using the DTPA-extractable Cd concentration (x = % inhibition expressed as the added Cd concentration).
Results and discussion
Compost effect on Cd availability
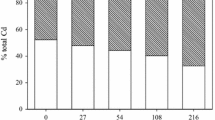
Results showed that the available soil Cd concentration extracted with DTPA increased proportionally with increasing level of spiked Cd, but the increases were much lower in compost-amended soils (Table 2, Fig. 1A). This is reflected in the slope from the linear regression for each compost treatment, which was much lower for the treated (0.049) than untreated (0.158) soils (Fig. 1A). Compost application lowered the available Cd concentrations by 48–76% at all Cd levels as compared with the corresponding unamended soils, indicating a remarkable reduction in Cd mobility and availability in the presence of compost. This is further confirmed by the percentages of DTPA-extractable Cd calculated with respect to the total concentration of added Cd, which were also lower (67–76%) in the compost-amended than the unamended soils (Fig. 1B). Our result agrees with previous observations (Karaca 2004; Li et al. 2020; Pukalchik et al. 2017; Raiesi and Sadeghi 2019; Tejada 2009), where the addition of various organic amendments including compost successfully decreased Cd availability and toxicity in the soil. Several short-term laboratory experiments indicated that the solubility and availability of soil Cd declined with the addition of organic resources such as sewage sludge (Moreno et al. 2003), compost (Hanc et al. 2009; Karaca 2004; Pardo et al. 2014; Vaca-Paulín et al. 2006), organic wastes (Tejada 2009), humic substances (Pukalchik et al. 2017) and even uncomposted plant residues (Raiesi and Sadeghi 2019; Safari Sinegani and Jafari Monsef 2016).
The absolute (A) and relative (B) concentrations of DTPA-extractable Cd in spiked soils unamended or amended with compost. Values are mean and bars represent the standard error (SE) of the mean (n = 3). Different letters indicate significant differences (P < 0.05) among mean values by LSD test. Linear regression equation in the form of y = bx + a, line of best fit and coefficient of determination (R2) are provided
Decreased Cd availability in soils treated with organic amendments is mainly attributed to the chemical processes of Cd adsorption, ion exchange, and the formation of insoluble organo-Cd complexes, or to changes in soil properties following utilization of organic resources (Hamid et al. 2019; Hanc et al. 2009; He et al. 2019; Khan et al. 2017). Adding organic matter in the form of compost should lead to higher specific surface area, cation exchange capacity and organic carbon pool, providing more binding sites for Cd and other metals and consequently less mobile Cd in available forms (Hamid et al. 2019; Hanc et al. 2009; Shuman et al. 2002). Soil amendment with organic matter may increase the negatively charged functional groups (including phenolic hydroxyl, carboxyl and carbonyl groups) with the potential to enhance Cd retention and consequently would result in decreased Cd solubility and mobility successfully (Karaca 2004; Khan et al. 2017; Tejada 2009).
Increased soil pH and organic matter content following the application of organic amendments are two of the most important soil factors that control Cd availability and toxicity in soil (Hamid et al. 2019; Khan et al. 2017; Smolders and Mertens 2013). In this study, compost addition had no effect on soil pH (Table 2); therefore, an increase in organic matter pool is the only factor regulating the effect of compost on the availability and mobility of Cd in the soil solution. In other words, the large reductions in Cd solubility observed in the amended soils, compared with unamended soils, could be related mostly to the formation of humic substances and humified complexes. Humic substances formed during the decomposition of organic amendments are known to decrease the availability of Cd by the formation of highly stable inner-sphere organo-Cd complexes (Pukalchik et al. 2017; Tejada 2009). These reactive substances have the ability to bind metal ions through their high content of oxygen-containing functional groups, including carboxyl and phenolic hydroxyl (Mohamed et al. 2010; Stevenson 1994). The observed decline in Cd solubility in the compost-treated soils may also be due to the high concentrations of phosphate (1430 mg kg−1) in the compost used in this experiment (Table 1). Phosphate-rich compounds applied to metal-contaminated soils can effectively limit Cd availability due to the formation of low-solubility Cd phosphate minerals (Brown et al. 2004; Karaca et al. 2002).
Compost effect on microbial and biochemical functions
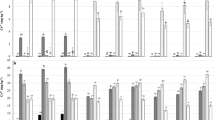
The ANOVA results indicated the soil microbial properties and processes (except PAR and ACP) were significantly influenced by the interactions between Cd and compost treatments (Table 2). The measured microbial endpoints indicate that Cd addition and stress inhibited the microbial and biochemical functions (processes) in this non-calcareous soil either amended or unamended with compost, while the effect of compost addition was always beneficial at all Cd levels (Table 2, Figs 2–4), with exception of the MBN content in unpolluted soils (Fig. 3D) and the ALP activity at moderate levels of Cd (50–100 mg Cd kg−1) addition (Fig. 4B). However, the extent to which soil properties and functions are inhibited by Cd addition or stimulated by compost application depends on the Cd level and on the soil attributes and functions involved. These findings show that Cd had a detrimental influence on the microbial and biochemical indicators of soil health/quality, and that a proportion of the soil microflora was probably inactivated or killed, especially in the soils unamended with compost. Similarly, earlier studies (Moreno et al. 2006; Raiesi et al. 2018; Renella et al. 2005; Tejada 2009) also reported reduced microbial properties and activities for microbial enzymes that are fundamental to the cycling of N and P by high concentrations of Cd in the soil under laboratory conditions. Furthermore, consistent with previous observations (Moreno et al. 2006, 2003; Tejada et al. 2006), there was a strong dose-dependent decline in the measured soil microbial properties and processes (Supplementary Fig. S1).
The total carbon mineralization, TCM (A), basal respiration, BR (B) and substrate-induced respiration, SIR (C) in Cd-spiked soils unamended or amended with compost. Values are mean and bars represent the standard error (SE) of the mean (n = 3). Different letters indicate significant differences (P < 0.05) among mean values by LSD test
The potential net ammonification rate, PAR (A), potential net nitrification rate, PNR (B), total nitrogen mineralization, TNM (C) and microbial biomass nitrogen, MBN (D) in Cd-spiked soils unamended or amended with compost. Values are mean and bars represent the standard error (SE) of the mean (n = 3). Different letters indicate significant differences (P < 0.05) among mean values by LSD test
The activity of acid phosphatase, ACP (A), alkaline phosphatase, ALP (B) and urease, URE (C) in Cd-spiked soils unamended or amended with compost. Values are mean and bars represent the standard error (SE) of the mean (n = 3). Different letters indicate significant differences (P < 0.05) among mean values by LSD test
Our data demonstrated that the negative effects of Cd toxicity were often lower in the compost-amended than the corresponding unamended soils, confirming that the observed reduction of DTPA-extractable Cd contents in compost-treated soils was reflected in the increased microbial metabolic processes and microbial growth. This shows that the decreased Cd toxicity in compost-treated soils was accompanied by increased microbial activity, microbial biomass, turnover rates of C and N as well as enzyme activity. In other words, the finding suggests that the potential risks associated with high levels of Cd can be reduced for a range of different soil microbial and biochemical properties with compost application. As explained previously, a possible hypothesis is that with addition of compost to improve soil organic carbon content, Cd can be immobilized and become less mobile, biologically available and less toxic to the soil microbial community. The improved organic matter content following addition of organic materials has a high capacity to immobilize and reduce the solubility of Cd (Li et al. 2020; Tejada 2009). Our finding was in accordance with previous observations (Li et al. 2020; Moreno et al. 2002; Raiesi et al. 2018; Raiesi and Sadeghi 2019; Renella et al. 2005; Tejada 2009; Tejada et al. 2011), where the use of organic amendments in the soils polluted with Cd improved the soil microbial properties and processes compared with non-treated soils mostly under controlled laboratory conditions. Likewise, addition of cotton gin compost, municipal solid waste, sewage sludge and poultry manure to Ni-polluted (Moreno et al. 2003; Tejada et al. 2008) and Cr-polluted (Tejada et al. 2011) soils increased soil enzymatic activities and microbial properties and reduced the inhibition of soil properties. In a multi-polluted mine soil, application of organic amendments, especially compost, increased soil enzyme activities and improved soil health due to the decreased soil eco-toxicity (Pardo et al. 2014). Increases in microbial activity, microbial biomass and microbial enzyme activities in response to compost addition may themselves actively influence SOM content and thus Cd availability and toxicity. Apparently, compost amendment increased the microbial population resistant to Cd or decreased its availability.
It should be noted that the increase in potential nitrification does not necessarily mean that the application of compost to Cd-polluted soils would stimulate the chemoautotrophic process of nitrification. Alternatively, the addition of compost (with high N content and low C/N ratio, Table 1) may stimulate either the heterotrophic process of ammonification, which can provide ammonium as the essential substrate for the nitrification process, or the heterotrophic nitrification, which generally has a low yield of product and proceeds only slowly (Li et al. 2018; Zhang et al. 2019). The heterotrophic nitrification activity is performed by a wide range of soil microorganisms such as heterotrophic bacteria, fungi and actinobacteria (Li et al. 2018; Zhang et al. 2019). Certainly, the compost input released ammonium that supported chemoautotrophic nitrification activity.
In this experiment, ALP activity behaved differently in response to compost addition, being significantly higher in compost-amended soils than unamended control soils in treatments without Cd addition (Fig. 4B). In contrast, the soils amended with compost showed significantly lower ALP activity than the unamended soils in treatments with the moderate levels of Cd (50–100 mg Cd kg−1). We can give no explicit explanation for the decline of ALP activity in the compost-treated soils, and further research is needed to determine the reason for this observation. It is likely that the decreased ALP activity as a consequence of compost addition could occur directly through the possible co-location of ALP molecules (or enzyme substrates) and Cd ions on the surfaces of organic compounds and humic substances produced during compost decomposition, resulting in inhibition of the catalytic activity. Furthermore, the possibility that the adsorbed Cd inhibits the activity of extracellular ALP stabilized by humic substances cannot be excluded. Nonetheless, the validity of this hypothesis should be independently verified.
Pearson coefficients indicated significant correlations among pairs of soil attributes (Table 3). The concentrations of both total Cd and DTPA-extractable Cd had a significant negative correlation with all the measured soil properties, suggesting that soil microbial functions would decrease with increasing concentrations of total or available Cd. For most soil properties, correlation coefficients were greater with total Cd contents than DTPA-extractable Cd concentrations (Table 4). This suggests that total Cd concentration does provide adequate evidence concerning its availability and toxicity in the soil, and consequently its potential impact on the soil microbial community and enzymatic activity. Although the available Cd fraction correlates well with the toxicity parameters and determines Cd impact on the microorganisms (Lindsay and Norvell 1978; Moreno et al. 2006; Tejada 2009; Vig et al. 2003), this study shows that total Cd concentration equally represents Cd toxicity, and therefore would be co-equally representative of soil Cd toxic effects on the soil microorganisms in freshly Cd-spiked soils.
The addition of labile C forms and nutrients in compost could also be an important driver for the increased microbial activity and biomass as a microbial substrate, and enzyme activity as an enzyme substrate over the incubation period. The compost used in this study was rich in organic matter and an important source of nutrients for the soil microorganism and it did not contain any potentially harmful metals such as Cd (Table 1). Soil amendment with compost and other organic resources increases the availability of substrate C, which stimulates microbial growth and biomass or the stimulation of microbial activity and hydrolase activities through additions of labile nutrients (Li et al. 2020; Pardo et al. 2014; Renella et al. 2005; Tejada 2009). This could counterbalance any inhibitory effect of the toxic metals (Tejada 2009). Incorporation of organic amendments, such as compost and plant residues, into these soils repeatedly promotes microbiological and enzyme activity for maintenance of soil quality (Bastida et al. 2009; Raiesi and Sadeghi 2019; Ros et al. 2003; Vafa et al. 2016). Compost can also indirectly affect soil enzymatic activities by changing the microbial community which is responsible for the production of enzymes (Epelde et al. 2016; Tejada et al. 2009). This is supported by the strong correlations between enzyme activities and microbial biomass or microbial activity (Table 3). Considering Cd-compost interactions and microorganisms-compost interactions, the findings showed the potential significance of environmental and nutritional co-benefits of compost application for the microbial community and quality of Cd-polluted soils. Compost as an organic substrate itself may have a selective effect, or it may modify the responses to Cd through changes in the physiological state and activity or growth of the soil microorganisms (Tejada 2009). However, as the inhibition of soil microbial and biochemical properties in the freshly Cd-spiked soils is higher than in soils where Cd is in equilibrium conditions for a much longer period (Vig et al. 2003), field experiments using long-term Cd-contaminated soils and compost applications are required to confirm these findings. Mostly, the bioavailability of Cd decreases with the duration of its contact with solid phases in the short-term due to the changes in its chemical forms and aging process (Renella et al. 2004; Vig et al. 2003), however, its toxicity in the soil is persistent and its bioavailability does not decline in the long-term (Smolders and Mertens 2013).
Compost effect on the ED values as an index of soil ecological sensitivity
Two simple mathematical models described previously were used to establish a relationship between soil ecological functions and total Cd concentrations, and to estimate ecological dose (ED) values for the inhibition of each function. The determination coefficient values (R2) from the nonlinear regression analysis for the measured soil properties are listed in Table 4. A selection of the dose-response and kinetic curves is also presented in Supplementary Fig. S1 and S2 (Supplementary materials), demonstrating dose-dependent effects of Cd addition on the soil microbial conditions. With few exceptions, both models demonstrated a good fitness of the data with the R2 values ranging from 0.710 to 0.991 (Table 4). The R2 values were low for the ALP activity in unamended soils with the kinetic model (0.774); and the BR (0.879–0.887) and MBN (0.710–0.888) values in compost-amended soils with both mathematical models. In addition, Model 1 had higher R2 values for soil properties associated with microbial respiration, C turnover and enzyme activities, suggesting a higher degree of fitting. However, for soil parameters related to N dynamics, Model 2 showed a better performance.
Table 4 shows the results for ecological dose (EDx) values (ED50, ED10 and ED5) of Cd for the inhibition of different soil characteristics calculated from the best fit models. The EDx values of soil properties predicted by both models were much smaller than those reported in other studies in different soils and incubation times (Doelman and Haanstra 1986; Moreno et al. 2003, 2001). However, our ED50 values obtained for ALP (60–90 mg kg−1) in the control soil fall within the range (4.5–699 mg kg−1) previously reported (Renella et al. 2003; Tan et al. 2014). Likewise, the ED50 values recorded for ACP (71–95 mg kg−1) were well within the range (14–2936 mg kg−1) reported by (Renella et al. 2003) in different soils at various incubation times. Furthermore, most of the EDx values obtained for Cd in the control soils (Table 4) were much greater than the maximum level of total soil Cd permissible in soils (2.3 mg Cd kg−1 soil) set by the legislation of the European Union for the protection of soil microorganisms (Smolders and Mertens 2013), with a few exceptions where only the ED5 values of MBN and URE with the kinetic model (2 mg Cd kg−1) in the control soils were below 3 mg Cd kg−1 soil (Table 4).
The EDx values derived from both models were highly variable depending on the different soil properties, the mathematical model used and the compost treatment (Table 4). The EDx values depend on the specific soil property or process concerned, probably due to the fact that soil microbial and biochemical functions are frequently highly context-dependent.
The EDx values calculated from the two models were almost similar for TCM and PAR in the unamended soils and for PAR, PNR and URE in the compost-amended soils (Table 4). The magnitude of the change in the EDx values and the response of a specific soil bio-indicator to Cd pollution depend largely, among other factors, on climate, soil type, pollution history and exposure time as well as the quality and quantity of the organic amendment used for soil remediation (Hinojosa et al. 2008; Moreno et al. 2006, 2003, 2001; Speir et al. 1995; Tejada et al. 2008).
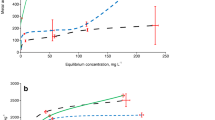
Depending on the assay, soil amendment with compost increased the calculated ED values, except for a few parameters (Table 4). Based on the ED50 values derived from Model 1, the added Cd was 1.10–2.24 times more toxic to microbial functions in unamended soils than in soils amended with compost (Fig. 5). This finding shows that Cd toxicity thresholds (total concentrations) increased with the addition of compost. In other words, the finding provides strong evidence that Cd is less toxic in compost-amended than unamended soils. Apparently, most of the Cd ions were adsorbed on compost surfaces or removed from the soil solution as organically-complexed Cd, and rendered non-toxic or unavailable to the soil micro-organisms.
The sequences of ED50 sensitivity of different soil attributes (excluding ALP) to Cd ecotoxicity in unamended (A) and compost-amended (B) soils. Higher DE50 values indicate that the soil microbial attributes are less sensitive to Cd toxicity, since 50% inhibition of an ecological soil characteristic occurs at a higher Cd level (PNR potential nitrification rate, TNM total nitrogen mineralization, PAR potential ammonification rate, TCM total carbon mineralization, BR basal respiration, MBN microbial biomass nitrogen, SIR substrate-induced respiration, ACP acid phosphatase, URE urease)
The decreased DTPA-extractable Cd contents of compost-treated soils (Fig. 1) were accompanied by increased ED values for microbial activity, biomass, turnover rates of C and N as well as enzyme activity (Table 4). This suggests that the risks associated with high level of Cd can be reduced for a range of different soil microbial and biochemical properties. The results of this study corroborate earlier findings (Moreno et al. 2003, 2002); where application of sewage sludge as an organic amendment increased the ED values for the inhibition of different soil properties and processes (respiration, biomass and enzymatic activities) in Cd-polluted soils with high pH (8.6) under laboratory conditions. (Tejada et al. 2008) have also reported a consistent increase in the ED50 values for the activities of different enzymes (urease, alkaline phosphatase and arylsulfatase) with addition of crushed cotton gin compost and poultry manure to a Typic Xerofluvent soil experimentally polluted with nickel. Also, (Moreno et al. 2003) observed that the ED values for the activities of urease, phosphatase, β-glucosidase and protease-BAA increased after the addition of sewage sludge to a semi-arid soil contaminated with Ni in the laboratory. (Pardo et al. 2014) calculated the 50% ecological concentration (EC50) values (concentration at which a 50% of lethal or toxic effect can be observed) and observed that compost addition increased the EC50 values in a multi-contaminated mine soil. The ED values are useful as an integrated parameter for assessing metal eco-toxicity since they are very sensitive to metal pollution, time of exposure to metal and differences in soil characteristics (Hinojosa et al. 2008; Moreno et al. 20062001; Speir et al. 1995; Tejada et al. 2008). These values can be particularly used to establish which microbiological and biochemical properties of soil are most sensitive to heavy metal contamination (Moreno et al. 2001; Speir et al. 1995). The ED10 and ED5 values were used to establish a threshold boundary of Cd (Moreno et al. 2003, 2002) and Ni (Moreno et al. 2003) levels in order to avoid irreversible effects on soil functionality in experimentally polluted soils. Critical risk limits can also be established using the ED50 value of a specific microbial property for a specific soil type (Epelde et al. 2016).
Based on the estimated ED50 values, the sensitivity of the measured soil properties to Cd pollution depended on the soil attribute considered and compost treatment (Table 4, Fig. 5). We found that microbial biomass (MBN and SIR) and URE activity were more sensitive than microbial activity (TCM, BR and TNM) and ACP activity to soil pollution by Cd, regardless of whether the compost was used or not (Fig. 5). Indeed, the lower the DE50 value, the more sensitive the soil attributes. Thus, the size of microbial biomass may be considered as one of the soil properties most sensitive for evaluating Cd effects on microbial functions. Interestingly, Cd was 1.26 and 1.19 times more toxic to PAR than PNR in unamended and compost-amended soils, respectively (Fig. 5), confirming that ammonification activity was more sensitive Cd pollution than nitrification activity. In unamended soils, the greatest inhibition and hence the lowest ED50 values were observed in the following order for MBN, URE, SIR, ACP and TCM (Fig. 5A), which is indicative of greater sensitivity of these biological properties to the adverse effects of Cd in the absence of compost. However, the lowest observed ED50 values were in the following order for URE, ACP, SIR, MBN and BR, excluding ALP in compost-amended soils (Fig. 5B). Similarly, the sensitivity of the soil microbial properties to Cd and Ni pollution varied with addition of other organic amendments (Moreno et al. 2003, 2002; Tejada et al. 2008).
Conclusions
We studied the effect of compost as a cost-effective organic amendment on the microbial community activity, biomass and functions in a non-calcareous soil polluted with Cd. The findings showed the significance of SOM as the most important factor in determining the availability and eco-toxicity of Cd following addition of compost to Cd-polluted soils with a neutral pH. Consistent with our hypothesis, compost amendment demonstrated a great potential to decrease Cd toxicity to the soil ecological functions and biological quality through increases in the ED values. The findings show Cd toxicity generally causes lower inhibition of microbial and biochemical properties in the presence of compost as an organic amendment. This has environmental implications and co-benefits for organic matter-limited soils polluted with Cd. The increased ED values with compost application can be used to perform and establish the remediation guidelines and strategies of Cd-contaminated soils and, thus, to maintain soil health and quality. Remediation of Cd-contaminated soils can be achieved by adding compost at 2500 kg ha−1, which in addition to the soil remediation can also alleviate Cd toxicity and provide the labile C substrates and nutrients for microbial assimilation. However, field experiments using long-term Cd-polluted soils and compost application are required to confirm these short-term experimental results obtained under laboratory conditions.
References
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, London
Azeez JO, Van Averbeke W (2010) Nitrogen mineralization potential of three animal manures applied on a sandy clay loam soil. Bioresour Technol 101:5645–5651
Babich H, Bewley RJF, Stotzky G (1983) Application of the “ecological dose” concept to the impact of heavy metals on some microbe-mediated ecologic processes in soil. Arch Environ Contam Toxicol 12:421–426
Bastida F, Pérez-de-Mora A, Babic K, Hai B, Hernández T, García C, Schloter M (2009) Role of amendments on N cycling in Mediterranean abandoned semiarid soils. Appl Soil Ecol 41:195–205
Blagodatskaya E, Kuzyakov Y (2013) Active microorganisms in soil: critical review of estimation criteria and approaches. Soil Biol Biochem 67:192–211
Brown S, Chaney R, Hallfrisch J, Ryan JA, Berti WR (2004) In situ soil treatments to reduce the phyto- and bioavailability of lead, zinc, and cadmium. J Environ Qual 33:522–531
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Chehregani A, Noori M, Yazdi HL (2009) Phytoremediation of heavy-metal-polluted soils: screening for new accumulator plants in Angouran mine (Iran) and evaluation of removal ability. Ecotoxicol Environ Saf 72:1349–1353
Dick RP (1997) Soil enzyme activities as integrative indicators of soil health. In: Pankhurst CE, Doube BM, Gupta VVSR (Eds.) Biological indicators of soil health. CAB International, Wallingford, CT, pp 121–156
Doelman P, Haanstra L (1986) Short-and long-term effects of heavy metals on urease activity in soils. Biol Fertil Soils 2:213–218
Dušek L (1995) The effect of cadmium on the activity of nitrifying populations in two different grassland soils. Plant Soil 177:43–53
Epelde L, Muñiz O, Garbisu C (2016) Microbial properties for the derivation of critical risk limits in cadmium contaminated soil. Appl Soil Ecol 99:19–28
Haanstra L, Doelman P, Voshaar JHO (1985) The use of sigmoidal dose response curves in soil ecotoxicological research. Plant Soil 84:293–297
Hamid Y, Tang L, Sohail MI, Cao X, Hussain B, Aziz MZ, Usman M, He ZL, Yang X (2019) An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci Total Environ 660:80–96
Hanc A, Tlustos P, Szakova J, Habart J (2009) Changes in cadmium mobility during composting and after soil application. Waste Manage 29:2282–2288
He D, Cui J, Gao M, Wang W, Zhou J, Yang J, Wang J, Li Y, Jiang C, Peng Y (2019) Effects of soil amendments applied on cadmium availability, soil enzyme activity, and plant uptake in contaminated purple soil. Sci Total Environ 654:1364–1371
Hinojosa MB, Carreira JA, Rodríguez-Maroto JM, García-Ruíz R (2008) Effects of pyrite sludge pollution on soil enzyme activities: ecological dose-response model. Sci Total Environ 396:89–99
Joergensen RG (1995) Microbial biomass. In: Alef K, Nannipieri P (Eds.) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp 375–417
Karaca A (2004) Effect of organic wastes on the extractability of cadmium, copper, nickel, and zinc in soil. Geoderma 122:297–303
Karaca A, Cetin SC, Turgay OC, Kizilkaya R (2010) Soil enzymes as indication of soil suality. In: Shukla G, Varma A (Ed.), Soil enzymology. Springer-Verlag, Berlin, Heidelberg, pp 119–148
Karaca A, Naseby DC, Lynch JM (2002) Effect of cadmium contamination with sewage sludge and phosphate fertiliser amendments on soil enzyme activities, microbial structure and available cadmium. Biol Fertil Soils 35:428–434
Khan MA, Khan S, Khan A, Alam M (2017) Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ 601–602:1591–1605
Kızılkaya R, Aşkın T, Bayraklı B, Sağlam M (2004) Microbiological characteristics of soils contaminated with heavy metals. Eur J Soil Biol 40:95–102
Li S, Sun X, Liu Y, Li S, Zhou W, Ma Q, Zhang J (2020) Remediation of Cd-contaminated soils by GWC application, evaluated in terms of Cd immobilization, enzyme activities, and pakchoi cabbage uptake. Environ Sci Pollut Res 27:9979–9986
Li Y, Chapman SJ, Nicol GW, Yao H (2018) Nitrification and nitrifiers in acidic soils. Soil Biol Biochem 116:290–301
Lin Q, Brookes PC (1999) An evaluation of the substrate-induced respiration method. Soil Biol Biochem 31:1969–1983
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sc Soc Am J 42:421–428
Lorenz N, Hintemann T, Kramarewa T, Katayama A, Yasuta T, Marschner P, Kandeler E (2006) Response of microbial activity and microbial community composition in soils to long-term arsenic and cadmium exposure. Soil Biol Biochem 38:1430–1437
Mohamed I, Ahamadou B, Li M, Gong C, Cai P, Liang W, Huang Q (2010) Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J Soils Sediments 10:973–982
Moral R, Gilkes RJ, Jordán MM (2005) Distribution of heavy metals in calcareous and non-calcareous soils in Spain. Water Air Soil Poll 162:127–142
Moreno JL, García C, Hernández T (2003) Toxic effect of cadmium and nickel on soil enzymes and the influence of adding sewage sludge. Eur J Soil Sci 54:377–386
Moreno JL, García C, Landi L, Falchini L, Pietramellara G, Nannipieri P (2001) The ecological dose value (ED50) for assessing Cd toxicity on ATP content and dehydrogenase and urease activities of soil. Soil Biol Biochem 33:483–489
Moreno JL, Hernández T, Pérez A, García C (2002) Toxicity of cadmium to soil microbial activity: effect of sewage sludge addition to soil on the ecological dose. Appl Soil Ecol 21:149–158
Moreno JL, Sanchez-Marín A, Hernández T, García C (2006) Effect of cadmium on microbial activity and a ryegrass crop in two semiarid soils. Environ Manage 37:626–633
Nannipieri P, Ascher J, Ceccherini MT, Lan DlL, Pietramellar G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari S (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemcial processes in soil. In: Burns RG, Dick RP (Ed.), Enzymes in the environment: activity, ecology and applications. Marcel Dekker, Inc., New York, NY, pp 1–34
Nannipieri P, Trasar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19
Oorts K, Ghesquiere U, Smolders E (2007) Leaching and aging decrease nickel toxicity to soil microbial processes in soils freshly spiked with nickel chloride. Environ Toxicol Chem 26:1130–1138
Pardo T, Clemente R, Alvarenga P, Bernal MP (2014) Efficiency of soil organic and inorganic amendments on the remediation of a contaminated mine soil: II. Biological and ecotoxicological evaluation. Chemosphere 107:101–108
Pukalchik M, Mercl F, Panova M, Břendová K, Terekhova VA, Tlustoš P (2017) The improvement of multi-contaminated sandy loam soil chemical and biological properties by the biochar, wood ash, and humic substances amendments. Environ Pollut 229:516–524
Rafiei B, Khodaei AS, Khodabakhsh S, Hashemi M, Nejad MB (2010) Contamination assessment of lead, zinc, copper, cadmium, arsenic and antimony in ahangaran mine soils, Malayer, West of Iran. Soil Sediment Contam 19:573–586
Raiesi F (2012) Land abandonment effect on N mineralization and microbial biomass N in a semi-arid calcareous soil from Iran. J Arid Environ 76:80–87
Raiesi F, Razmkhah M, Kiani S (2018) Salinity stress accelerates the effect of cadmium toxicity on soil N dynamics and cycling: does joint effect of these stresses matter? Ecotoxicol Environ Saf 153:160–167
Raiesi F, Sadeghi E (2019) Interactive effect of salinity and cadmium toxicity on soil microbial properties and enzyme activities. Ecotoxicol Environ Saf 168:221–229
Renella G, Adamo P, Bianco MR, Landi L, Violante P, Nannipieri P (2004) Availability and speciation of cadmium added to a calcareous soil under various managements. Eur J Soil Sci 55:123–133
Renella G, Mench M, Landi L, Nannipieri P (2005) Microbial activity and hydrolase synthesis in long-term Cd-contaminated soils. Soil Biol Biochem 37:133–139
Renella G, Ortigoza ALR, Landi L, Nannipieri P (2003) Additive effects of copper and zinc on cadmium toxicity on phosphatase activities and ATP content of soil as estimated by the ecological dose (ED50). Soil Biol Biochem 35:1203–1210
Ros M, Hernandez MT, García C (2003) Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biol Biochem 35:463–469
Safari Sinegani AA, Jafari Monsef M (2016) Chemical speciation and bioavailability of cadmium in the temperate and semiarid soils treated with wheat residue. Environ Sci Poll Res 23:9750–9758
Shuman LM, Dudka S, Das K (2002) Cadmium forms and plant availability in compost-amended soil. Commun Soil Sci Plant Anal 33:737–748
Smolders E, Mertens J (2013) Cadmium. In: Alloway BJ (Ed.), Heavy metals in soils. Springer, Dordrecht, pp 283–311
Soares M, Rousk J (2019) Microbial growth and carbon use efficiency in soil: links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol Biochem 131:195–205
Speir TW, Kettles HA, Parshotam A, Searle PL, Vlaar L (1995) A simple kinetic approach to derive the ecological dose value, ED50, for the assessment of Cr(VI) toxicity to soil biological properties. Soil Biol Biochem 27:801–810
Speir TW, Kettles HA, Parshotam A, Searle PL, Vlaar LNC (1999) Simple kinetic approach to determine the toxicity of As[V] to soil biological properties. Soil Biol Biochem 31:705–713
Sposito G, Lund LJ, Chang AC (1982) Trace metal chemistry in arid-zone field soils amended with sewage sludge: I. Fractionation of Ni, Cu, Zn, Cd, and Pb in solid phases. Soil Sc Soc Am J 46:260–264
Stevenson FJ (1994) Humus Chemistry: Genesis, Composition, Reactions. John Wiley & Sons, New York
Tan X, Kong L, Yan H, Wang Z, He W, Wei G (2014) Influence of soil factors on the soil enzyme inhibition by Cd. Acta Agr Scand B-S Soil Plant Sci 64:666–674
Tan X, Liu Y, Yan K, Wang Z, Lu G, He Y, He W (2017) Differences in the response of soil dehydrogenase activity to Cd contamination are determined by the different substrates used for its determination. Chemosphere 169:324–332
Tejada M (2009) Application of different organic wastes in a soil polluted by cadmium: effects on soil biological properties. Geoderma 153:254–268
Tejada M, Hernandez MT, Garcia C (2009) Soil restoration using composted plant residues: Effects on soil properties. Soil Till Res 102:109–117
Tejada M, Hernandez MT, Garcia C (2006) Application of two organic amendments on soil restoration: effects on the soil biological properties. J Environ Qual 35:1010
Tejada M, Moreno JL, Hernández MT, García C (2008) Soil amendments with organic wastes reduce the toxicity of nickel to soil enzyme activities. Eur J Soil Biol 44:129–140
Tejada M, Parrado J, Hernández T, García C (2011) The biochemical response to different Cr and Cd concentrations in soils amended with organic wastes. J Hazard Mater 185:204–211
Vaca-Paulín R, Esteller-Alberich MV, Lugo-De La Fuente J, Zavaleta-Mancera HA (2006) Effect of sewage sludge or compost on the sorption and distribution of copper and cadmium in soil. Waste Manage 26:71–81
Vafa HJ, Raiesi F, Hosseinpur A (2016) Sewage sludge application strongly modifies earthworm impact on microbial and biochemical attributes in a semi-arid calcareous soil from Iran. Appl Soil Ecol 100:45–56
Vig K, Megharaj M, Sethunathan N, Naidu R (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8:121–135
Wang Z, Tan X, Lu G, Liu Y, Naidu R, He W (2018) Soil properties influence kinetics of soil acid phosphatase in response to arsenic toxicity. Ecotoxicol Environ Saf 147:266–274
Xu Y, Seshadri B, Bolan N, Sarkar B, Ok YS, Zhang W, Rumpel C, Sparks D, Farrell M, Hall T, Dong Z (2019) Microbial functional diversity and carbon use feedback in soils as affected by heavy metals. Environ Int 125:478–488
Zhang Y, Wang J, Dai S, Zhao J, Huang X, Sun Y, Chen J, Cai Z, Zhang J (2019) The effect of C:N ratio on heterotrophic nitrification in acidic soils. Soil Biol Biochem 137:107562
Acknowledgements
Shahrekord University provided the funding for this study (Grant/Award Number: 96GRN1M1932). The authors greatly appreciate the useful comments by two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Raiesi, F., Dayani, L. Compost application increases the ecological dose values in a non-calcareous agricultural soil contaminated with cadmium. Ecotoxicology 30, 17–30 (2021). https://doi.org/10.1007/s10646-020-02286-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02286-1