Abstract
This study focused on the occurrence of several EDCs including bisphenol A, estrone (E1), the 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) in fourteen rivers of Portugal. Samples analysis revealed a widespread contamination of BPA especially in Ave, Cávado, Douro, Ferro, Sousa and Vizela Rivers. Achieving 98.4 ng/L for the highest concentration. The estrogens achieved above the method quantification limit (MQL) were E1 in Águeda River and E2 in Ave, Lima and Tâmega Rivers. The maximum concentration detected for E1 was 26.9 ng/L. EE2 was detected only below MQL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endocrine disrupting chemicals (EDCs), a group of compounds able to interfere with hormone-controlled physiological processes, are increasingly widespread in the environment. Steroid estrogens like the natural hormones estradiol and estrone, as well as the synthetic hormone ethinylestradiol as well as plasticizers are supposed to contribute mainly to estrogenic activity in surface water from adjacent, domestic and industrial effluents and even from sewage treatment plants (WWTP) (Quednow and Püttmann 2008).
The natural estrogens such as estrone (E1) and 17β-estradiol (E2) are mainly derived from excreta of humans and livestock. The 17α-etinylestradiol (EE2) is a synthetic steroid and is the most common used as contraceptive and in some hormonal therapies and is also eliminated by urine to the environment. Bisphenol-A (BPA) is an industrial chemical used to make a hard clear plastic known as polycarbonate. BPA is known as an EDC and is almost ubiquitous in the environment (Chang et al. 2011).
Usually, analysis using gas chromatography-mass spectrometry (GC–MS) (Kolpin et al. 2002; Kim et al. 2007) or liquid chromatography-tandem mass spectrometry (LC–MS/MS) (Kolpin et al. 2002; Labadie and Hill 2007) were applied to determine EDCs in rivers at ng/L to μg/L levels. In order to employ high-resolution GC for the analysis of these compounds, derivatization is required to increase analyte volatility and thermal stability and thus improve chromatographic separation.
Although the occurrence of BPA and steroid hormones in the environment has received a great deal of attention worldwide, little is known about their fate in Portuguese aquatic systems (Ribeiro et al. 2009).
Therefore, the aim of this study was to assess the potential impact of levels of BPA, estrone, 17β-estradiol and 17α-etinylestradiol in 14 rivers from the North of Portugal (21 sampling points).
Materials and Methods
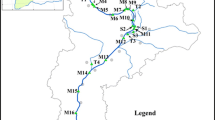
Fourteen rivers located in the northern and central areas of Portugal were studied between May and July 2010. Samples were collected near the estuary, both on the left and the right margins. In the tributary rivers only one set point of harvest was performed, from one of the river margin (Fig. 1). From Douro river another sample was collected 20 km from the mouth (Point 13).
Samples were collected in amber glass bottles (1L), previously rinsed with the river water, acidified with glacial acetic acid (1 %, v/v) after collection and stored at 4°C. Samples were processed in 24 h.
Altogether 21 river samples were taken. During the sampling, a global position system (GPS) was used to locate the sampling sites (Table 1). Geographical positions of sampling points and average temperature are shown in Fig. 1.
The internal standard (IS) was deuterated bisphenol A (BPA-d16, 98 atom % D), was from Sigma-Aldrich (Steinheim, Germany). The steroids E1, E2 and EE2 were supplied by Sigma (purity > 98 %) (St. Louis, MO, USA). The BPA with purity > 99 % and the derivatizing agent, MSTFA were obtained from Aldrich (purity > 98 %). Methanol and ethyl acetate were organic trace analysis grade SupraSolv and were supplied by Merck (Darmstadt, Germany). Acetic acid (glacial) 100 % was from Carlo Erba (Rodano, Italy). Ultrapure water was highly purified by a Milli-Q gradient system (18.2 mΩ cm) from Millipore (Milford, MA, USA).
Individual stock standard solutions of the studied compounds were prepared in methanol at a by exact weighing and accurate dilution. Mixture was then prepared in ethyl acetate, containing 1.5 mg/L of each compound. Stock standard solutions were stored in glass-stoppered flasks at 4°C, in the dark.
Water matrix calibration solutions were 0.075, 0.150, 0.375, 0.450, 0.525 and 0.750 μg/L in each EDCs. Matrix-standard calibration solutions (residue-free matrix spiked with standards) were prepared by spiking 1.0 L of ultrapure water with different volumes of the 1.5 mg/L. It was added 0.5 % (v/v) of methanol to the water under analysis and pH ~ 4 was adjusted with glacial acetic acid.
BPA-d16 at 0.750 μg/L was chosen as quality control internal standard for assessing the effectiveness of the extraction technique.
Prior to extraction, the IS was added. Water samples were previously filtered with Whatman GF/B glass microfiber filters. Each filter was washed several times with small amounts of methanol (approximately 0.5 % (v/v) of the sample) that were then added to the filtered.
SPE was conducted in a SPE vacuum manifold system from Phenomenex (USA). The Strata™ SDB-L cartridges were previously conditioned with it was used methanol and ultrapure water (7:7 mL). Cartridges were then dried under high vacuum and analytes eluted with 2 × 2.5 mL methanol, followed by 2 × 2.5 mL of ethyl acetate, at a flow rate of 1 mL/min. The eluate was evaporated to dryness in a rotative evaporator (Rotavapor R -200) equipped with a heating bath B—490 (at ~40°C) and a vacuum pump (Vac V -500). Therefore, the dried residues were resuspended in ethyl acetate to a final volume of 500 μL and submitted to the derivatization.
SPE recoveries were accessed using ultrapure water spiked at 200 μg/L with the compounds under study. Calibration matrix standards and river water samples extracts were derivatized in the tested optimal conditions. 75 μL of the extracts in ethyl acetate were mixed with 150 μL of MSTFA. Closed vials were placed at 85°C for 100 min (Quintana et al. 2004). After that, they were cooled to room temperature and injected in the chromatographic system.
The identification of the peaks was made by means of a chromatogram of a standard solution. The compounds BPA, E1, E2 and EE2 were identified by retention time and mass spectra.
As it was possible to select more than one ion, the identities of the peaks were confirmed through ratios of their respective ions abundances. Comparison with comprehensive mass-spectral libraries allowed an unequivocal identification of target compounds.
The ions selected for quantification and identification purposes were mass-to-charge ratio (m/z) are described in Table 2.
Analyses of BPA, E1, E2 using GC–MS were carried out in a Shimadzu GCMS-QP2010 Gas Chromatograph Mass Spectrometer equipped with a fused-silica capillary column coated with 5 % diphenylmethylsiloxane, (30 m × 0.25 mm ID, 0.25 μm film thickness) from Teknokroma.
Helium (99.9999 %) at a constant flow rate of 1.5 mL/min was used as the carrier gas. Injections (1μL) were made in the splitless mode with a 1.0 min purge-off time and injector temperature set at 275°C. Samples were analyzed using the following oven program temperature: initial temperature 50°C (held for 1 min), increased by 20°C/min to 220°C (held for 17 min), increased again by 20°C/min to 250°C and held at this temperature for 10 min. In this device the temperature of GC–MS interface was maintained at 250°C and ionization by 70 eV electron impact. The transfer line was set at 275°C and source at 200°C.
Positive fragment ions (m/z—ions mass/charge ratio) were analyzed over 45–500 m/z mass range in full scan mode and in selected-ion monitoring (SIM) mode. Selected ions used for quantification are in Table 2. Instrument control and mass spectrometry data were managed by a personal computer running the LabSolutions GCMS software (2.50 SU3 version).
Analyses of the target compounds using GC–MS/MS were carried out in a Trace GC Ultra device coupled to MS from Thermo Polaris Q was used with a Zebron ZB-XLB (30 m × 0.25 mm id, 0.25 μm film) column from Phenomenex. The carrier gas used was high purity helium (99.9999 %), with a constant flow of 1.3 mL/min. The injections (1 μL) were made in splitless mode with purge time of 0.5 min and the injector temperature set at 270°C. The program of the oven temperatures used was similar between the GC equipments. The mass spectrometer with ion trap from Thermo Polaris Q and interface were maintained at 250°C with an electron impact ionisation of 70 eV. The positive ion fragments (m/z) were analyzed over the mass range m/z 50–650 full scan mode and SIM mode. The instrumental control and data from mass spectrometry were managed from a computer with software GCMS Xcalibur (version 1.3). In the analysis by GC–MS/MS of all target compounds, ions from the first electron impact ionisation (EI) of the target compounds were selected and fragmented a second time with collision-induced dissociation (CID) of helium gas in the ion trap, using a voltage collision excitation of 1.00 V. The spectra for these fragments were scanned from the resulting ions with m/z belonging to the selected mass range. The selection of ions was organized according to the different segments. The parameters used for qualitative GC–MS/MS analysis are presented in Table 2.
In order to develop and optimize the SPE procedure followed by GC–MS for an effective and reproducible detection of low EDCs concentration, parameters such as: specificity and selectivity, linearity and linear range, limits of detection and quantification, precision, accuracy, trueness (recovery), stability and robustness were accessed.
Results and Discussion
Specificity and selectivity were evaluated by comparing the chromatograms of matrix-blank samples (different samples of ultrapure water, glass bottled mineral water and lab tap water) and an aqueous solution of the analytes at concentrations near the limit of quantification.
No significant interference has been detected at the retention time of the compounds for estrogens. Regarding BPA it was observed a small peak even in the glass bottled mineral water that was used as our blank sample and was always subtracted from samples BPA concentration. This fact may be due to the equipment used in sample preparation namely the SPE plastic cartridges.
Selectivity was also assessed by the comparison of the analytes mass spectra with spectra from libraries with a similarity ≥90 % which gave the evidence that the proposed method has a selectivity/specificity in accordance with the standards set forth by the validation authorities.
Calibration graphs showed good linear responses for the concentration range of all compounds with correlation coefficients (r w ) higher than 0.997. Calibration in the SIM mode was therefore performed using external standardization.
Good sensitivity with method detection limit (MDL), obtained for BPA, E1, E2 and EE2 were 7.8, 4.9, 2.5 and 3.15 ng/L. Method quantification limits (MQL) were also in the ng/L range with values below 25.88 ng/L.
Comparing with other limits (Ribeiro et al. 2009), it appears that they are similar, with an order of magnitude and similar units (ng/L), considering it is thus suitable for the detection and quantification of the target compounds.
Much is necessary to be learned pertaining to the effects on humans, plants, and animals exposed to low-level of EDCs. Furthermore, little is known about the potential interactive effects (synergistic or antagonistic toxicity) that may occur from complex mixtures of these compounds in the environment.
Our study showed that the water contained varying amounts of EDCs in accordance with other studies, at times reached deleterious amounts (Voutsa et al. 2006).
Data are displayed in Table 3. It is shown that BPA was detected in all river samples but only in nine samples above MQL, with levels between 29.8 and 98.4 ng/L. The estrogens were detected in 81 % of samples but only Águeda River (26.9 ng/L) for E1, Ave south (8.9 ng/L), Lima south (11.5 ng/L) and Tâmega (9.5 ng/L) for E2, get values above MQL.
The obtained levels were similar in both margins sampling points except for Ave, and Cávado Rivers where concentrations were higher in south margins. These results can be related with Coriolis force (Balla 2009). The sample of Douro River collected 20 km upstream was less polluted with these compounds.
The detected EDCs were confirmed by GC–MS/MS, as demonstrated by the retention time and the characteristic fragmentation patterns.
Taking account the average EDCs concentration from both Laboratories and both margins of each river, we can conclude that Ave, Cávado, Douro and Vizela Rivers are the most polluted concerning BPA, with concentrations above 39 ng/L (Table 3). In the vicinity of these rivers, several urban, industrial and agricultural activities take place. Domestic and industrial wastewaters, as well as surface runoff, end up in the neighborhood or directly in these rivers and could possibly be sources of BPA.
The Ave River, located in an agricultural region with poor sewage treatment plants and industrial pollution, especially textile industry, shown the presence of estrogens as well.
At the Douro River the estuary area included in a metropolitan area of high population density and near the industrial poles of the city of Porto and Gaia, BPA concentration was much higher than in Point 15 located at 20 km upstream.
Ferro is a tributary of Vizela River and is a stream of small size, which has important industrial plants located along its banks. BPA was quantified while E1 and E2 have been detected in both rivers.
In Sousa River was reported industrial and livestock pollution with sudden death of fish and EDCs have also been detected.
It has reported complains about Tâmega pollution about discharges of pig farming. E2 has been quantified in this river. A major source of estrogenic compounds in the aquatic environment is livestock excreta. Other studies showed that estrogens such as E1 and E2 released by dairy and swine can exceed those released by the municipal WWTP (Pal et al. 2010).
In Lima River a decrease of some fishes namely trout’s has been noticed without any particularly reason. Taking account this problem, the Ministério da Agricultura e Pescas trough the Despacho no 3732/2005 has forbidden the fishery to attempt to increase the number of fishes. E1 was detected but lower than MDL and E2 was quantified at 11.5 ng/L. These EDCs may contribute to alter the reproduction in this ecosystem and a decline in wild life however more studies are needed to understand the fish decrease.
Paiva was considered a few years ago the least polluted river in Europe, and is still the place of spawning trout. Some threats are the implementation of birds and fish farming and underground constructions. Although at low levels BPA, E1 and E2 have been detected.
Minho estuary is included in Natura 2000 network (EC, 2010), which aim to assure the long-term survival of Europe’s most valuable and threatened species and habitats. None of the EDCs target has been found.
In the centre of Portugal in Ria de Aveiro, Vouga and Águeda rivers BPA concentration was low, but a high concentration of E1 was detected at Águeda River.
These data are comparable with levels reported in USA Rivers, with BPA between 140 and 12,000 ng/L in 42 % of samples (Kim et al. 2007). The BPA levels in irrigation waters in China were between 45-265 ng/L (Labadie and Hill 2007). In Spain, BPA was detected in two rivers between 101 and 322 ng/L collected at the entrance of a domestic WWTP (Gallart-Ayala et al. 2010) and in Loudias River in Greece, 138 ng/L (Arditsoglou and Voutsa 2008).
Voutsa et al. (2006) reported occurrences of BPA in rivers from the entire world that are also consistent with our data, except the results from other work from Portugal that where excessively high (160–5,030 ng/L) (Quirós et al. 2005), when compared to our results or with concentrations of some very polluted rivers as the Spanish Llobrega.
The world screening assessment report states that BPA is entering or may be entering the environment in a quantity or concentration or under conditions that have or may have as immediate or long-term effect on the environment or its biological diversity. Additionally screening assessment reports states that BPA meets the criteria for persistence.
Regarding estrogens, in USA rivers, EE2 was detected between 831 and 73 ng/L, E2 between 0.2 and 0.16 μg/L and E1 between 112 and 27 ng/L (Kolpin et al. 2002).
Several studies showed that the concentrations of estriol, E1, E2 and EE2 in freshwaters, in most of the countries, exceed their respective Predicted No Effect Concentration (PNEC) values. EE2, even at trace levels of ng/L, in effluents from wastewater treatment plants is with potential to cause feminization of fish and other aquatic vertebrate species (Kidd et al. 2007).
Sample analysis revealed that concentrations of target compounds in 14 Rivers of North and Center of Portugal were generally similar to those that have been previously reported in other countries. The highest detected concentrations of BPA were found in Ave, Cávado, Douro and Vizela Rivers. E1 was quantified in Águeda River, E2 was quantified in Ave, Lima and Tâmega and EE2 was detected but above MQL in several rivers.
This implies that such compounds survive in wastewater treatment and biodegradation. Future research will be needed to identify those factors that are most important in determining the occurrence and concentration of EDCs in water resources.
The increasing knowledge about the origin, processing and effect of this new generation of environmental contaminants is essential to allow the definition/presentation of a more accurately official list of organic pollutants, to propose new mechanisms for water treatment to assure quality and to allow new environmental remediation strategies and health promotion.
References
Arditsoglou A, Voutsa D (2008) Determination of phenolic and steroid endocrine disrupting compounds in environmental matrices. Environ Sci Pollut R 15(3):228–236
Balla Z (2009) The influence of the Coriolis force on the rivers and the Baer law. Historical review. Annual Report of the Geological Institute of Hungary, pp 53–62
Chang BV, Yuan SY, Chiou CC (2011) Biodegradation of bisphenol-A in river sediment. J Environ Sci Heal A 46(9):931–937
Gallart-Ayala H, Moyano E, Galceran MT (2010) On-line solid phase extraction fast liquid chromatography-tandem mass spectrometry for the analysis of bisphenol A and its chlorinated derivatives in water samples. J Chromatogr A 1217(21):3511–3518
Kidd KA, Blanchfield PJ, Kenneth HM, Vince PP, Robert EE, Lazorchak JM et al (2007) Collapse of a fish population after exposure to a synthetic estrogen. PNAS 104(21):8897–8901
Kim SD, Cho J, Kim IS, Vanderford BJ, Snyder SA (2007) Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res 41(5):1013–1021
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB et al (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36(6):1202–1211
Labadie P, Hill EM (2007) Analysis of estrogens in river sediments by liquid chromatography-electrospray ionisation mass spectrometry. Comparison of tandem mass spectrometry and time-of-flight mass spectrometry. J Chromatogr A 1141(2):174–181
Pal A, Gin KY-H, Lin AY-C, Reinhard M (2010) Impacts of emerging organic contaminants on freshwater resources: review of recent occurrences, sources, fate and effects. Sci Total Environ 408(24):6062–6069
Quednow K, Püttmann W (2008) Endocrine disruptors in freshwater streams of Hesse, Germany: changes in concentration levels in the time span from 2003 to 2005. Environ Pollut 152(2):476–483
Quintana JB, Carpinteiro J, Rodriguez I, Lorenzo RA, Carro AM, Cela R (2004) Determination of natural and synthetic estrogens in water by gas chromatography with mass spectrometric detection. J Chromatogr A 1024(1–2):177–185
Quirós L, Céspedes R, Lacorte S, Viana P, Raldúa D, Barcelò D et al (2005) Detection and evaluation of endocrine-disruption activity in water samples from Portuguese rivers. Environ Toxicol Chem 24(2):389–395
Ribeiro C, Tiritan M, Rocha E, Rocha M (2009) Seasonal and spatial distribution of several endocrine-disrupting compounds in the Douro River Estuary, Portugal. Arch Environ Contam Toxicol 56(1):1–11
Voutsa D, Hartmann P, Schaffner C, Ginger W (2006) Benzotriazoles, alkylphenols and bisphenol A in municipal wastewaters and in Glatt River, Switzerland. Environ Sci Pollut R 13(5):333–395
Acknowledgments
This work has been supported by Fundação para a Ciência e a Tecnologia (grant no. PEst-C/EQB/LA0006/2011 and BD/47200/2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sónia Rocha and Valentina Domingues equally contributed to these work.
Rights and permissions
About this article
Cite this article
Rocha, S., Domingues, V.F., Pinho, C. et al. Occurrence of Bisphenol A, Estrone, 17β-Estradiol and 17α-Ethinylestradiol in Portuguese Rivers. Bull Environ Contam Toxicol 90, 73–78 (2013). https://doi.org/10.1007/s00128-012-0887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0887-1