Abstract
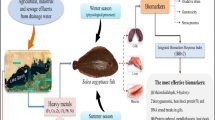
Subcellular biochemical biomarkers are valuable early warning indicators of environmental contaminant effects. Thus, the present study evaluated several biomarkers and the relationships among them in wild freshwater mussels (Lasmigona costata) from a gradient of metal exposure and differential levels of other urban-related influences in the Grand River (ON, Canada). The biomarkers examined are related to metal exposure [gill ion and metal concentrations (Na, K, Ca, Mg, Cd, Cu, Ni, Pb and Zn)], oxidative status [reactive oxygen species (ROS), catalase (CAT), superoxide dismutase (SOD), antioxidant capacity (ACAP)], sulfhydryl (SH) metabolism [glutathione (GSH), protein sulfhydryl groups (SH protein), glutathione S-transferase (GST), glutathione reductase (GR)], and lipid peroxidation. Gill metal concentration increased proportionally to waterborne metal concentration and disturbances in osmotic and divalent cations (Ca and Mg) concentrations were observed. This suggests that the observed effects are associated with metal exposure, although simultaneous relationships with other contaminants are also possible. Oxidative status biomarkers (ROS, SOD, CAT and ACAP) were more sensitive to urban-influences than gill metal concentration. In contrast, biomarkers involving SH metabolism (GSH, SH protein, total SH, GR and GST) were more correlated with gill metal concentration. Oxidative damage occurred when both metal and urban-related influences were high. Mechanistically, the way of dealing with oxidative stress changed when mussels were exposed to high levels of contaminants. The reduction in ROS content, SOD and CAT activity, and ACAP accompanying the stimulation of detoxification metabolism via SH (GSH and SH protein contents, GST and GR activities) and their association with gill metal concentration are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers are commonly impacted by chemical contaminants because they often act as the receiving environment for the byproducts of various human activities, including agricultural and road runoff, municipal wastewater, and industrial effluents. Many of the anthropic-derived compounds including metals, salts, hydrocarbons, pharmaceuticals and pesticides found in runoff and effluents are known to compromise ecosystem health and cause biological dysfunction (Nemerow 1991; Depledge et al. 1995; Box et al. 2007; Viarengo et al. 2007). The Grand River (Ontario, Canada) is an example of an ecosystem disturbed by human activities. Different classes of waterborne contaminants including polychlorinated biphenyls (Frank and Logan 1988), atrazine (Frank and Logan 1988; Cooke 2006; Wang et al. 2011), bisphenol A (Wang et al. 2011), pharmaceuticals and personal care products (Metcalfe et al. 2003; Gillis et al. 2014a), and metals [Ontario Provincial Water Quality Monitoring Network (Ontario PWQMN 2012)] have been found in the urbanized region of the Grand River. The influence of cities located around the Grand River on the quality of its waters has been well documented through both focused studies on specific groups of compounds (Frank and Logan 1988; Metcalfe et al. 2003, 2010, Wang et al. 2011) and a long-term water quality monitoring program (Ontario Ministry of the Environment, PWQMN). The occurrence of metals has been particularly well documented, and many of them (Cd, Cu, Pb and Zn, for example) have been shown to increase moving downstream (Ontario PWQMN 2012). In fact, the local conservation authority (Grand River Conservation Authority) has referred to the water quality in urban areas of the Grand River as ‘impaired’ (Cooke 2006). Recently, negative impacts on wild biota from the Grand River have been also reported (Tetreault et al. 2011; Gillis 2012; Gillis et al. 2014b), demonstrating that at least some of the waterborne contaminants present in this ecosystem are bioavailable and toxic.
Freshwater mussels are often considered as sentinel indicators of aquatic ecosystem health. Population surveys have found that the diversity of mussels in the Grand River watershed has declined by at least 30 %, losses which have been attributed, at least in part, to water pollution, impoundments and the destruction of riparian buffer zones adjacent to watercourses (Metcalfe-Smith et al. 1998; Metcalfe-Smith et al. 2000). Mackie (1996) attributed temporal and spatial losses in the mussel community to a range of anthropogenic impacts, including agricultural runoff, roadway crossings, cattle crossings, and industrial discharges. While these are effects at the population level, it has been proposed that impacts observed at the community level in contaminant-exposed animals begin as alterations at the sub-cellular (even biomolecular) level (Monserrat et al. 2007). In fact, bioavailable waterborne contaminants have been shown to influence organismal physiology at the sub-cellular level, causing injuries such as those resulting from oxidative stress (Lushchak 2011) or ionoregulatory disturbance (Jorge et al. 2013). According to the concepts of (Depledge et al. 1995) reviewed by Monserrat et al. (2007), the term biomarker refers to biological responses at the sub-organismal level that can be measured to indicate the presence of contaminants. In fact, these authors consider that biomarkers can provide an early warning indicator of exposure to toxic compounds, allowing changes in biological systems to be identified before effects are evident at the organismal or community level. This contaminant/biomarker interaction approach has been employed in the Grand River using the freshwater mussel Lasmigona costata as a biomonitor (Gillis 2012; Gillis et al. 2014a, b). In addition to impacts at the organismal level (e.g. reduced condition factor; Gillis 2012), these studies have reported evidence of oxidative stress (lipid peroxidation) both in mussels deployed in the plume of a municipal wastewater treatment plant (Gillis et al. 2014a) and in wild mussels living downstream of a large urban area (Gillis et al. 2014b).
The role of metal exposure in inducing oxidative stress is well described for bivalves and several other aquatic organisms (Stohs and Bagchi 1995; Jorge et al. 2013; Machado et al. 2013). Indeed, the hypothesis that metallic cations can stimulate lipid peroxidation has been proposed for the marine bivalves, Crassostrea gigas (oyster) and Mytilus edulis (mussel) (Géret et al. 2002). While some relationships between specific contaminants and various biomarker responses have been established (Géret et al. 2003; Box et al. 2007; Martín-Díaz et al. 2009; Gonzalez-Rey and Bebianno 2011), there has been limited analysis of oxidative biomarkers with respect to the physiology involved in these responses in wild organisms. Furthermore, other environmental contaminants (in addition to metals) can also induce oxidative stress (Lushchak 2011). In fact, oxidative stress and detoxification biomarkers have been found to respond to several other urban-related contaminants, such as caffeine, pharmaceuticals and personal care products (Martín-Díaz et al. 2009). Correspondingly, the functioning of oxidative stress-related parameters in wild organisms exposed to complex contaminant mixtures remains largely unknown.
The main goal of the present study was to investigate how exposure to a complex mixture of contaminants under natural conditions affects various biochemical indicators of oxidative stress. The Grand River was considered as the site model and the freshwater mussel L. costata as the biological model. The Grand River, a major drainage of Lake Erie (Laurentian Great Lakes), has historically supported one of the most diverse assemblages of freshwater mussels in Canada (Metcalfe-Smith et al. 1998). The mussel L. costata is found throughout this urban-impacted watershed and considered to be relatively tolerant to contaminants (Metcalfe-Smith et al. 2000; Gillis 2012) and thus a suitable model to examine the biochemical and cellular effects of contaminant exposure (Nogueira et al. 2013; Gillis et al. 2014b). It is probable that the negative impacts of poor water quality observed in this species would also be experienced by other more sensitive, and possibly at risk, mussel species that share this habitat. Unfortunately, it is not ecologically reasonable, nor possible to collect large numbers of the rarer (i.e. Species at Risk) mussels for investigation. In the present study, we focus on biochemical responses in the gill tissue of L. costata exposed to the complex mixture of contaminants present in the Grand River (ON). Endpoints analyzed include the concentrations of metals and major ions, as well as the activity of several enzymes involved in the oxidative stress detoxification pathways. Results reported here contribute to our understanding of the defense mechanisms that freshwater mussels employ to protect themselves from the biological effects that result from multi-contaminant exposure. Also, they support the use of some of the studied parameters as biomarkers of environmental health.
Materials and methods
Study area and species
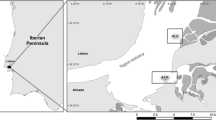
The Grand River watershed is situated in southern Ontario, one of the most populated areas in Canada. Thirty municipal wastewater treatment plants discharge into the Grand River watershed, and in addition the river receives anthropogenic inputs from agriculture and urban runoff. The study area is located upstream, within, and downstream of a large (population approximately 1 million) urban center (Kitchener–Waterloo–Cambridge) (Grand River Conservation Authority 2013).
Four locations were chosen, covering both rural and urban areas and following an increasing gradient of metal contamination and distinct urban-related influences (Fig. 1). The furthest upstream site, West Montrose (WM), is upstream of urban centers, and therefore receives very low urban-related influences. It is therefore considered the reference site in the present study. Moving downstream, the second site, Kiwanis (KW), located within the city of Waterloo, receives intermediate urban-related influences. The concentrations of waterborne metals at KW are elevated compared to WM (Gillis 2012). The third site, Doon (DN), is located downstream of WM and KW, within the city of Kitchener, has higher waterborne metal levels, and is considered as the site receiving the highest urban-related influences. The furthest downstream site, Glenn Morris (GM), is located downstream of Kitchener–Waterloo, Guelph and Cambridge and has the highest waterborne metal levels. Although GM receives inputs from multiple cities, it is located much further downstream (~8.5 km downstream of the closest city of Cambridge and ~24 km downstream of DN) and is thus considered to be a site with intermediate urban-related influences. Upstream of GM (but downstream of DN), the Grand River also receives additional input from the Speed River, which crosses mostly agricultural areas and receives significant ground water input (Loomer and Cooke 2011). Some biological aspects (e.g. immunological responses, condition factors, metal levels) of wild mussels from these same study sites have been previously examined (Gillis 2012; Gillis et al. 2014b).
Twenty-five adult L. costata (Rafinesque 1820) (98–112 mm length) were collected using viewing boxes (wooden boxes with a bottom glass viewing panel) at each sampling site in July 2011. After collection, mussels were transferred to the laboratory at the Canada Centre for Inland Waters (Burlington, ON, Canada) where their gills were promptly dissected. A portion of the gill tissue was used immediately for reactive oxygen species (ROS) content measurement, while the remaining tissue was immediately frozen (−80 °C) for further analyses. Because the gender of L. costata is not apparent from external shell morphology, mixed sexes were collected. Upon dissection, it was noted that some mussels were gravid (i.e. brooding glochidia (larvae) in their gills). However, only non-gravid mussels (i.e. glochidia-free gills) were used in the present study. Therefore the sample sizes for biomarker analyses were 20, 13, 18 and 15 mussels for WM, KW, DN and GM sites, respectively.
Water chemistry
Water chemistry parameters [pH, ions (Ca, K, Mg, Na, Cl, Fl, total SO4) and dissolved organic carbon (DOC) concentrations, and hardness] were measured at the time of mussel collection. Ca, K, Mg and Na concentrations were analyzed through atomic absorption spectrophotometry, while Cl, Fl and total SO4 concentrations were measured by ion chromatography. Hardness was calculated from Ca and Mg concentrations. DOC concentration was measured using a total organic carbon (TOC) analyzer (Phoenix 8000 UV-Persulfate TOC analyzer). All analyses were conducted by the National Laboratory for Environmental Testing (Environment Canada, Burlington ON, Canada).
Biomarkers
In order to assess a large suite of potential biomarkers associated with oxidative status, including those related to the physiological mechanisms involved in metal exposure response, four groups of biomarkers were analyzed.
The first group considered the biomarkers specifically induced by metal exposure. Metals are well known to accumulate in bivalve tissues (Viarengo and Nott 1993; Serafim et al. 2011; Gillis 2012) and cause osmotic and ionic imbalances (Mazon et al. 2002; Giacomin et al. 2013; Jorge et al. 2013). Based on these facts, water, major ions (Na, K, Ca, and Mg) and metals (Cd, Cu, Ni, Pb and Zn) concentrations were measured in mussel gills. Water content was estimated based on the wet and dry (48 h at ~60 °C) weight of a gill tissue subsample. Dried gill tissue was then completely digested in 1 mL of Ultra Pure nitric acid (Suprapur®, Merck, Haar, Germany) and diluted in Nanopure water. Zn, Na, K, Ca, and Mg concentrations in the digested sample were measured by flame atomic absorption spectrophotometry (Varian AA 220FS, Mulgrave, Australia). Cd, Cu, Ni, and Pb concentrations in the digested sample were determined by graphite furnace atomic absorption spectrophotometry (GF AAS; Varian Spectra AA-220 with a Spectra AA GTA-11 furnace, Mulgrave, Australia). National Water Research Institute’s certified reference materials (TM24 and TM25) were used for metals QA/QC (average recovery 100.97 ± 6.98 %).
Data on gill metal (Cd, Cu, Ni, Pb and Zn) concentration are expressed as microgram of metal per gram of dry tissue because of the variations in water content observed among study sites (Fig. 2). As Ca, K, Na, and Mg have important physiological roles as ionic and osmotically active particles, concentrations are expressed as micromole per mg of dry tissue. For all biochemical biomarkers, data are expressed per milligram of protein in the gill tissue sample. Protein concentration was measured employing the Bradford reagent using a commercial reagent kit (Sigma-Aldrich, St. Louis, MO, USA).
Biomarkers of metal exposure in terms of gill water content and transition metal concentrations in freshwater mussels (Lasmigona costata) collected along a contaminant gradient in the Grand River [West Montrose (WM), Kiwanis (KW), Doon (DN), and Glen Morris (GM), ON, Canada]. Contents of water (A), cadmium (B), nickel (C), copper (D), lead (E), and zinc (F). Bars and error bars represent mean and standard error of mean values, respectively. Bars not sharing the same letter are significantly different (a < 0.05) among parameters
The second group of biomarkers considered the biochemical parameters related to the oxidative status mostly in terms of the free radical metabolism. This group included an index of ROS production (relative ROS content), the activities of enzymes involved in ROS detoxification [superoxide dismutase (SOD) and catalase (CAT) activity], and an indicator of total ROS scavenging capacity [antioxidant capacity against peroxyl radicals (ACAP)]. ROS content was assessed by fluorescence (Gemini™ XPS Fluorescence Microplate Reader, Molecular Devices, Sunnyvale, CA, USA) using 2′,7′-dichlorofluorescein diacetate (H2DCFDA) following the procedures described by Amado et al. (2009). SOD activity was analyzed according to McCord and Fridovich (1969), using a method based on the reduction of cytochrome c (Biovision, Mountain View, CA, USA). Although attempts were made to measure SOD activity using the method based on the oxidation of epinephrine (Misra and Fridovich 1972), they were not successful. CAT activity was measured according to the method described by Beutler (1975), which is based on the degradation of hydrogen peroxide. ACAP was quantified as the relative area of the curve generated by the H2DCFDA fluorescence comparing its fluorescence in presence and absence of 2,2′-azobis (2 methylpropionamidine) dihydrochloride (ABAP), as described by Amado et al. (2009).
The third group of biomarkers was composed of indicators of detoxification processes through sulfhydryl (SH) metabolism. Although these parameters are known to play a role in detoxifying the products of oxidative stress (Monserrat et al. 2007; Lushchak 2011), they are actually important in several other metabolic pathways, such as in the degradation and excretion of organic and inorganic compounds and NADPH-NADP cycling (Sedlak and Lindsay 1968; Carlberg and Mannervik 1975; Keen et al. 1976). This group included parameters such as non-protein SH, total SH protein, total SH groups (measured as the sum of GSH and SH protein), and glutathione reductase (GR) and glutathione S-transferase (GST) activities. Total SH, non-protein SH and protein SH concentrations were measured according to Sedlak and Lindsay (1968). As most non-protein SH is due to reduced glutathione (Sedlak and Lindsay 1968), this parameter will be referred as glutathione (GSH) content. GST activity was measured using a method based on the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB), as described by Keen et al. (1976). GR activity was measured using a method based on the degradation of reduced nicotinamide adenine dinucleotide (NADPH), as described by Carlberg and Mannervik (1975).
Finally, the fourth “group” of biomarkers consisted of a single indicator often used as an integrative parameter of oxidative damage in environmental studies, the lipid peroxidation (LPO) level. Despite the fact that LPO is also related to oxidative stress, it is treated differently because it is the only actual ‘effect’ biomarker (Lushchak 2011; Machado et al. 2013) considered in the present study because it is directly related to biological damage. This biomarker was assessed with the thiobarbituric acid reactive substances method (TBARS), using a commercial reagent kit (Cayman Chemical Company, Ann Arbor, MI, USA).
Statistical analysis
Data have been expressed as means ± 1 SEM (standard error of mean). Data normality was tested using the Shapiro–Wilk test. Differences in biomarker responses among study sites were detected by analysis of variance (ANOVA) followed by the Tukey test or the Kruskal–Wallis ANOVA followed by the Dunn test for data with non-normal distribution. In Figs. 2, 3, 4, 5, bars not sharing the same letter are significantly different (a < 0.05). The highest mean value in a set of biomarker responses is identified with the letter “a”, while statistically significant lower means are identified by subsequent letters in alphabetical order. Canonic correlation was applied to identify relationships among water chemistry data, gill metal concentrations, and gill biomarker responses (a < 0.05). These analyses were performed using the BioEstat software (Instituto Mamirauá, Manaus, Brazil). Linear correlation (a < 0.05) for each pair of parameters was assessed with the function ‘lm’ available at R software (R Core Team 2013), according to Chamber (1992). Correspondence analysis was performed to assess the relative association and similarities of study sites and biomarkers. The correspondence analysis was carried out with the function ‘ca’ also available on library ‘ca’ for the R software (R Core Team 2013; Nenadic and Greenacre 2007), and for this analysis all variables were expressed as percentage of control site.
Biomarkers of metal exposure in terms of major ion concentrations (alkali and alkaline metals) in gills of freshwater mussels (Lasmigona costata) collected along a contaminant gradient in the Grand River [West Montrose (WM), Kiwanis (KW), Doon (DN), and Glen Morris (GM)] ON, Canada. Concentrations of sodium (A), potassium (B), calcium (C), and magnesium (D). Bars and error bars represent mean and standard error of mean values, respectively. Bars not sharing the same letter are significantly different (a < 0.05) among parameters
Biomarkers related to oxidative stress (free radical metabolism) in gills of freshwater mussels (Lasmigona costata) collected along a contaminant gradient in the Grand River [West Montrose (WM), Kiwanis (KW), Doon (DN), and Glen Morris (GM)] ON, Canada. Reactive oxygen species content (A), superoxide dismutase activity (B), catalase activity (C), and antioxidant capacity against peroxyl radicals (D). Bars and error bars represent mean and standard error of mean values. Bars not sharing the same letter are significantly different (a < 0.05) among parameters
Biomarkers related to oxidative stress and detoxification through SH metabolism and lipid peroxidation in gills of freshwater mussels (Lasmigona costata) collected along a contaminant gradient in the Grand River [West Montrose (WM), Kiwanis (KW), Doon (DN), and Glen Morris (GM)] ON, Canada. Concentrations of GSH (non-protein SH groups) (A), SH protein (B), and total SH groups (C), glutathione S-transferase activity (D), glutathione reductase activity (E), and lipid peroxidation (F). Bars and error bars represent mean and standard error of mean values. Bars not sharing the same letter are significantly different (a < 0.05) among parameters
Results
Water chemistry parameters
A summary of the concentrations of major ions and metals, as well as the other water quality parameters measured in the study sites is shown in Table 1. In general, pH and concentrations of Fl, Cl, SO4, Mg, Na and K) were higher at KW, lower at DN, and intermediate at GM and WM. Hardness levels (216–252 mg CaCO3 L−1) indicate that waters at study sites were moderately hard to very hard. Chloride concentration ranged from 26 to 99 mg L−1, i.e., below the EC50(24h) for glochidia (mussel larvae) (Gillis 2011). According to data from the last 10 years of monitoring (Ontario Provincial Water Quality Monitoring Network 2012), none of these water chemistry parameters measured in the present study were out of the natural range reported for the Grand River waters.
Biomarkers
As mentioned earlier, all comparisons were made considering the WM site as reference. Overall, the concentrations of trace metals in the gills of wild mussels tended to increase moving downstream (Fig. 2). The increase in gill metal levels was significant for Cu, Ni, Pb and Zn, with the concentrations of Pb and Zn increasing by about five fold through the study area (Fig. 2). The matrix of correlation for all gill parameters is presented in the supplementary material (Fig. S1). Gill Zn concentration was positively correlated to Cu concentration (r = 0.62, N = 64) and Pb concentration (r = 0.69, N = 64). Additionally, gill tissue water content was 10 % lower at GM, suggesting that mussels from this area have more concentrated body fluids. The gill concentrations of the monovalent cations Na and K were not significantly different across the study sites, while concentrations of the divalent cations Ca and Mg were significantly higher (about 20 %) in the gills of downstream mussels (Fig. 3). Ca and Mg levels were positively and linearly correlated (r = 0.74, N = 64), as well as with the gill concentrations of some trace metals, such as Ni (r = 0.54, N = 64) and Pb (r = 0.52, N = 64). On the other hand, gill Ca concentration was negatively and linearly correlated with gill K concentration (r = −0.68, N = 64), while gill Mg concentration was positively and linearly correlated with gill Pb (r = 0.54, N = 64) and Zn (r = 0.48, N = 64) concentrations.
With respect to biomarkers related to the ability to cope with ROS, mussels from DN showed a significantly lower ROS content when compared to those from the three other study sites (Fig. 4). SOD and CAT activities also showed a similar trend, with a significant positive and linear correlation between them (r = 0.62, N = 64). Both SOD and CAT activity decreased moving downstream, with DN mussels exhibiting 60 % of the level measured in WM mussels, though enzyme activity was nearly restored to reference (i.e. WM) levels in GM mussels (Fig. 4). Similarly, ACAP was reduced by about 60 % in DN mussels.
Concerning biomarkers of the detoxification processes involving the SH metabolism, GSH content was significantly lower in DN mussels (Fig. 5) than in mussels from all other study sites. Although the SH protein concentration in DN mussels was not significantly different from those of mussels from the other study sites, DN mussels had most of their SH groups in a protein-bound form. On the other hand, total SH concentration was significantly lower (about 25 %) in GM mussels (Fig. 5). The matrix of correlation for all gill biochemical parameters is presented in the supplementary material (Table S2). There was a significant and positive correlation between total SH and GSH concentrations (r = 0.93, N = 52), between total SH and protein SH concentrations (r = 0.83, N = 52), and consequently also between GSH and protein SH concentrations (r = 0.56, N = 52). GSH conjugation and recycling, represented by the activities of GST and GR, respectively, were significantly elevated (about 100 and 30 %, respectively) at the downstream study sites (DN, GM). LPO level (quantified as gill malondialdehyde concentration) was significantly elevated in DN mussels, the lowest mean value being observed in KW mussels (Fig. 5). LPO level was negatively and linearly correlated to SOD activity (r = −0.50, N = 63).
Interdependence of responses
Canonical correlation analysis that included data from water chemistry, gill ion and metal concentrations and biochemical biomarkers showed significant associations among the parameters analyzed (Table 2), though for most of the observed relationships, interactions appear to be non-linear because no strong linear correlations among biomarkers were found (see supplementary material Figs. S1 and S2). In the correspondence analysis, similarities among variables can be noted by the distance between their positions on the plot, e.g. closer variables presented higher association (Fig. 6). The dimension 1 accounted for 59.36 % of variance, and it presented clear association to gill metal concentration. The dimension 2 represented 27.67 % of variance and it was associated to urban-related influences. The dimension 3 (not shown in Fig. 6) accounted for 12.97 % of the variances, and must be associated to other environmental stressors. Oxidative stress-related biomarkers were the most sensitive biomarkers to urban-related influences. Concentration of GSH was sensitive to gill metal concentration, linked to upstream sites. In turn, gill metal concentration was more linked to downstream sites. Compared to other parameters, lipid peroxidation was associated with GR and GST, which jointly with Pb gill concentration, were more linked to high metal and urban-related influences.
Correspondence analysis for the biomarker responses in gills of freshwater mussel (Lasmigona costata) collected along a contaminant gradient in the Grand River, ON, Canada. The study sites West Montrose (WM), Kiwanis (KW), Doon (DN), and Glen Morris (GM) are plotted as solid blue circles. Gill ion (K, Ca, and Mg) and metal (Cd, Cu, Ni, Pb, and Zn) concentrations and biochemical biomarkers [reactive oxygen species (ROS), superoxide dismutase (SOD), catalase (CAT), antioxidant capacity against peroxyl radicals (ACAP), reduced glutathione (GSH), sulfhydryl-protein groups (SH protein), total sulfhydryl groups (SH total), glutathione S-transferase (GST), glutathione reductase (GR), and lipid peroxidation (LPO)] are shown as solid red triangles. The relative association among biomarkers and study sites can be assessed by comparing the distances between them. The arrows point out that dimension 1 represents association to the metal contamination, while dimension 2 represents association to urban-related influences. The gray-filled boxes identify the quadrants in terms of association to low or high waterborne metal and urban-related influences
Discussion
The use of biomarkers to assess anthropogenic impacts is gaining importance in environmental science. Bivalves are particularly useful biomonitors because they are sessile benthic filter-feeders, thus are exposed to contaminants found in both water and sediment. Freshwater mussels in particular are an ecologically relevant group known to be significantly impacted by human influences. In fact 70 % of North American species are either endangered, threatened, or in decline, and poor water quality is considered as a contributing factor (Neves et al. 1997; Lydeard et al. 2004; Strayer et al. 2004). In both marine and freshwater mussels, oxidative stress-related biomarkers have been employed in environmental health assessments (Géret et al. 2002; Gagné et al. 2011; Gonzalez-Rey and Bebianno 2011). However, most studies are focused on the absolute or relative responses of some of these biomarkers rather than investigating the physiological processes behind the response.
While the quantification of lipid peroxidation, as an end product of oxidative stress, demonstrates that contaminant exposure does indeed have a negative impact on these organisms, the activation of the specific biochemical responses leading to the response of this biomarker remained unknown. Thus, the response and relationships of several biological parameters were evaluated in gills of wild freshwater mussels from an urban-impacted ecosystem. Mussels were collected from a site with low urban-related influences and low metal contamination (WM), a site with intermediate urban-related influences and intermediate metal contamination (KW), another site exhibiting high urban-related and high metal contamination (DN), and finally a site with high metal contamination but intermediate urban-related influences (GM). Acknowledging that environmental selection for contaminant-tolerant animals or and/or adaptation to the poor water quality may have already occurred in the wild mussel population studied, the responses observed in the wild mussels provide important information on linkages among biomarkers, specifically those related to metal exposure, oxidative stress, detoxification pathways, and oxidative damage after long-term (>10 years) exposure. Moreover, as discussed below, the trends observed here align well with another study in which the same species of mussel was caged (i.e. field deployed) in this study area (Gillis et al. 2014a).
Biomarkers responses
The elevated metal concentrations in gills of wild mussels living within and downstream of an urban area provide clear evidence that the biota is being exposed to bioavailable metals in the study area (Fig. 2). This is in agreement with previous reports that suggested that metal bioaccumulation can serve as a useful indicator of exposure in urban-impacted ecosystems (Gillis 2012; Gillis et al. 2014b). Additionally, mussels from the downstream site of GM, which had the highest gill metal concentrations, also had significantly lower gill water content. This finding suggests that mussels downstream of the urban area show a disturbance in cell volume and/or osmoregulation. While gill Na and K concentrations did not change among study sites, gill Ca and Mg concentrations were higher in mussels collected downstream, following the same trend observed for trace metals. Indeed this was reinforced by the several significant correlations observed among gill Ca, Mg and metal concentrations (Fig. 3). These divalent cations are also osmotically active particles. Therefore, disturbances in the levels of these ions paralleled by changes in gill water content suggest that mussels are facing ionic and osmotic disturbances. In fact, osmotic responses are widely reported as being characteristic of metal-related distress (Grosell et al. 2002; Box et al. 2007; Alsop and Wood 2011). Nevertheless, the concentrations of the two monovalent ions analyzed (Na, K), whose regulation has been shown to be disrupted by metal exposure in the microgram per liter range (Oliveira and Souza 2007; Giacomin et al. 2013; Jorge et al. 2013; Nogueira et al. 2013), did not change according to the gradient in gill metal levels in the wild mussels examined. Thus, the possible storage of Ca for use in building Ca-rich metal granules for the purpose of metal detoxification and excretion, as previously reported for marine organisms (Viarengo and Nott 1993), cannot be disregarded. If this is the case for L. costata, this would corroborate the idea of the occurrence of metal-induced physiological adaptations in these contaminant-exposed wild mussels.
The responses of oxidative stress-related biomarkers (ROS, SOD, CAT and ACAP) exhibited similar patterns (Fig. 4), i.e., all these parameters showed their lowest mean value in DN mussels and tended to be higher in mussels from the less urban-impacted sites. In fact, mussels from DN had the lowest ROS content, which could be explained by a depressed aerobic metabolic rate, likely due to the exposure to urban-derived contaminants and poor water quality. Changes in ROS levels may explain the differential SOD and CAT responses. These incrementally decreased downstream, reaching the lowest level in mussels from the DN site, and then being partially recovered in mussels from the GM site. SOD is one of the first detoxification enzymes to be activated by ROS production (Lushchak 2011). This enzyme degrades the superoxide anion, thereby producing hydrogen peroxide, which is the substrate for CAT (McCord and Fridovich 1969). As previously mentioned, the activities of both enzymes were significantly inhibited in mussels from the DN site, which is consistent with the low ROS content observed in these mussels. Also, there was a significant correlation (r = 0.62, N = 64, p < 0.05) between the activity of these two enzymes, suggesting that they are coupled and functional for ROS detoxification in the mussels analyzed. ACAP, the most integrative parameter in this group of biomarkers, showed the highest levels in mussels from the upstream control site (WM). As ACAP integrates SOD, CAT and several other enzymatic and non-enzymatic antioxidants (Amado et al. 2009), the response observed for this biomarker indicates that the sum of important components of the total oxidant scavenging capacity behaved the same way as SOD and CAT did alone.
For biomarkers involved in detoxification through SH metabolism, the pattern of responses was quite different (Fig. 5). The total levels of SH groups were significantly lower in mussels from the GM site, while protein SH tended to increase in mussels from the KW and DN sites. On the other hand, GSH concentration was reduced in mussels from the DN site, which may be explained by the higher GST and GR activity observed in mussels from this site. Other studies also indicate that freshwater mussels from sites downstream of urban-related influences including municipal wastewater effluents had increased GST activity or elevated LPO (Farcy et al. 2011, Gillis et al. 2014b). Elevation of GST was reported for Elliptio complanata caged downstream of a municipal wastewater treatment plant (Farcy et al. 2011), which was accepted as an indicator of the presence of bioavailable xenobiotics in the plume. Moreover, the lowest GR activity was observed in mussels from the KW site, which showed higher GSH concentration. This would be expected since the function of GR is to recycle GSH (Monserrat et al. 2007), so there would be no need to elevate GR activity when the GSH available is abundant. Finally, the activity of GST, the enzyme that conjugates GSH with xenobiotic compounds (Keen et al. 1976), exhibited a trend of increased activity in the gills of mussels collected downstream of the urban inputs. Taken altogether, there appears to be a clear trend of increasing SH usage in response to exposure to elevated levels of contaminants. Compared to the control condition (e.g., the WM site), mussels exposed to intermediate levels of metals and intermediate urban-related influences (e.g., the KW site) primarily responded to the exposure by raising the SH protein and non-protein (total SH) production in their gills. However, when exposed to high levels of metals and the highest urban-related influences (e.g. the DN site), mussels showed the highest SH protein content and GST and GR activity in order to neutralize toxicants, which culminated in a reduced GSH concentration. Finally, for mussels exposed to the site showing the highest level of metals and intermediate urban influences (GM site), the SH protein and total SH groups also appear to be depressed. Higher demands of metallothioneins for metal detoxification might play a role in this observed decrease of SH concentrations. Metallothioneins are low-weight SH rich proteins which are often induced by low chronic or intermediate-high acute metal exposure, however at high chronic or very high acute metal exposure the levels of metallothioneins are often depleted (Viarengo et al. 2007). Thus, the decline of concentrations of SH protein and total SH in mussels from GM may be respectively linked to the consumption of both metallothioneins and GSH for metal detoxification. Gillis et al. (2014b) recently observed similar results, in terms of SH metabolism, though only the content of metallothioneins was considered. In addition, the current study found intermediate levels of GST and GR activities in mussels from the GM site, indicating that they were upregulated relative to the enzyme activity levels observed in mussels from the reference site (WM), but not to the same extent as in mussels from the DN site, where there were high metal levels and urban influences.
The results of the present study suggest that detoxification of free radicals in downstream mussels with higher gill metal concentrations tended to stimulate the activity of enzymes involved in SH metabolism, such as GR and GST, rather than the activity of those directly involved in free radical detoxification, such as SOD and CAT. Dealing with oxidative stress in this way would probably be energetically more expensive because GR is a NADPH-dependent enzyme (Carlberg and Mannervik 1975) and GST needs a constant supply of GSH (Keen et al. 1976). On the other hand, despite the higher energy demand, the SH metabolism provides oxidative scavenging capacity as well as an effective way to detoxify metals and other toxicants (Monserrat et al. 2007). The alteration in the way that these exposed mussels deal with oxidative stress may be a trade-off between energetic costs and detoxification abilities. In terms of preventing oxidative damage, this shift in metabolism appears to work for mussels from the KW and GM sites. Mussels from these sites showed metabolic shifts and limited lipid damage (LPO), such that their LPO levels were similar to those found in mussels from the reference site (WM) in spite of the higher metal levels and urban-related influences observed in KW and GM. In fact, mussels from GM, the site with the highest metal exposure did not exhibit elevated LPO, which supports data from some studies performed in our laboratory showing that exposure to environmentally relevant levels of single metals, such as Cu, do not cause marked effects on oxidative stress-related parameters (Giacomin et al. 2013; Jorge et al. 2013). However, for mussels from the DN site, metabolic changes that reduced the level of ROS using the SH route were not enough to prevent significant oxidative damage to lipids (Fig. 5), suggesting that the detoxification capacity of L. costata was overwhelmed when mussels were exposed to high levels of metals associated with the presence of high urban-related influences.
In addition to providing explanations on the physiological pathways working under environmentally oxidant conditions, our findings confirm the occurrence of oxidative stress, measured as LPO, in L. costata from the Grand River (ON, Canada). Indeed, previous measurements of oxidative stress in mussels from this river have pointed to several anthropogenic stressors, including municipal wastewater treatment plant effluents (Gillis et al. 2014a), as potential inducers of oxidative stress. The present findings together with those previously reported for the digestive gland (Gillis et al. 2014a) and gills (Gillis et al. 2014b) clearly indicate the existence of oxidative stress in multiple tissues of the freshwater mussel L. costata when chronically exposed to complex environmental contaminants.
Interdependence of responses
The strong and significant linear correlations and canonical relationships found reinforce the idea that biomarkers related to gill ion concentrations, metal burden, oxidative stress, detoxification through SH, and oxidative damage are all interconnected either individually or as groups of biomarkers. However, several of these interactions are likely non-linear.
The relationships among the various biomarkers examined are shown in a correspondence analysis plot (Fig. 6). They are expressed in terms of relative distance between the positions of each biomarker and study site. Regarding the general responses of the biomarkers analyzed; data plots of mussels from each of WM, KW, DN and GM were arranged in relation to their association to metal contamination or urban-related influences. Responses of mussels from the DN and GM sites are considered similarly distant from GM and KW, respectively. This shaped arrangement is different from the line-shaped arrangement that would be expected if the metal exposure gradient alone would be influencing the responses, thereby illustrating the important impact of other urban-related influences on biomarker responses. In fact, for mussels from all study sites, the biomarkers that deal strictly with oxidative stress formed a group associated with low urban influences (Fig. 6), which further reinforces the sensitivity and significance of urban-related influences on the responses of the oxidative stress-related biomarkers. Metal bioaccumulation, expressed in terms of gill metal burden, formed a diverse group that is more related to mussels from the DN and GM sites (positive values of dimension 1). Biomarkers of detoxification through the SH metabolism were non-specific biomarkers associated with gill metal content. From low to high metal, there occurred changes from high GSH stocks, to depletion of GSH and higher GSH enzyme metabolism. In fact, GR and GST were the biomarkers whose responses were closest to some of the gill metal concentrations (Ca, Mg, Ni, Pb). In turn, LPO is an integrative parameter that is firstly coupled to GR and GST activities, secondly to SH protein content, and then to gill metals. In addition, the gill concentrations for some metals are equally distant to GR-GST and SOD-CAT-ACAP, which means that they are somehow linked to the responses of both of these groups of biomarkers. The observation that metals are influencing but not determining oxidative stress is physiologically consistent with the ability of metals to increase ROS production, leading to cellular and metabolic alterations and oxidative stress (Géret et al. 2003; Machado et al. 2013). However, the lack of strong association between gill metal levels and biomarkers suggests that other contaminants or stressors present in this ecosystem could be triggering some of the biomarker responses. In this context, it should be highlighted that in the present analysis, LPO appeared as the interactive effect of both high metal exposure and high other urban-related influences. This implies that non-metal contaminants, such as pharmaceuticals (Metcalfe et al. 2003, Wang et al. 2011), pesticides such as phenoxyherbicides and atrazine (Frank and Logan 1988; Cooke 2006; Wang et al. 2011) and others that are indeed present in the study area but were not assessed in the current study, determined the oxidative stress observed in chronically exposed mussels.
Conclusions
The present study evaluated several biomarkers in the gills of wild freshwater mussels exposed to a gradient of metals and urban-related influences. Gill metal concentrations increased as the waterborne metal concentrations increased and were associated with the tissue concentrations of major divalent cations (Ca and Mg) and osmotic disturbances. The findings also suggest that mussel physiology is altered as a function of the oxidative stress observed when the animal is chronically exposed to a complex mixture of waterborne contaminants. It appears that enzymes involved in ROS scavenging and detoxification may be spared when the mechanisms linked to detoxification via SH metabolism (GSH and SH protein) are activated in mussels that are simultaneously exposed to elevated metals and other urban-related influences. Finally, oxidative damage occurs when both metal exposure and urban-related influences are elevated. Although the present and previous studies reported the level of biomarker responses (Gillis 2012, Gillis et al. 2014b), further studies are needed to reveal the activation of more specific biomarkers involved in the SH metabolism, such as metallothioneins, the propagation of damage within the organism, and the relationships between changes in the level of the biomarker responses and impacts on mussel populations.
References
Alsop D, Wood CM (2011) Metal uptake and acute toxicity in zebrafish: common mechanisms across multiple metals. Aquat Toxicol 105:385–393
Amado LL, Garcia ML, Ramos PB, Freitas RF, Zafalon B, Ferreira JLR, Yunes JS, Monserrat JM (2009) A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: Application to evaluate microcystins toxicity. Sci Tot Environ 407:2115–2123
Beutler E (1975) Red cell metabolism: a manual of biochemical methods. Grune & Straton, New York
Box A, Sureda A, Galgani F, Pons A, Deudero S (2007) Assessment of environmental pollution at Balearic Islands applying oxidative stress biomarkers in the mussel Mytilus galloprovincialis. Comp Biochem Physiol C 146:531–539
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Cooke S (2006) Water quality in the Grand River: a summary of current conditions (2000–2004) and long term trends. Grand River Conservation Authority. http://www.grandriver.ca/water/2006_WaterQuality_complete.pdf Accessed Jan 2014
Depledge MH, Aagaard A, Györkös P (1995) Assessment of trace metal toxicity using molecular, physiological and behavioural biomarkers. Mar Poll Bull 31:9–27
Farcy E, Gagné F, Martel L, Fortier M, Trépanier S, Brousseau P, Fournier M (2011) Short-term physiological effects of a xenobiotic mixture on the freshwater mussel Elliptio complanata exposed to municipal effluents. Environ Res 111:1096–1106
Frank R, Logan L (1988) Pesticide and industrial chemical residues at the mouth of the Grand, Saugeen and Thames rivers, Ontario, Canada, 1981–85. Arch Environ Contam Toxicol 17:741–754
Gagné F, André C, Cejka P, Hausler R, Fournier M (2011) Evidence of neuroendocrine disruption in freshwater mussels exposed to municipal wastewaters. Sci Tot Environ 409:3711–3718
Giacomin M, Gillis PL, Bianchini A, Wood CM (2013) Interactive effects of copper and dissolved organic matter on sodium uptake, copper bioaccumulation, and oxidative stress in juvenile freshwater mussels (Lampsilis siliquoidea). Aquat Toxicol 144–145:105–115
Géret F, Jouan A, Turpin V, Bebianno MJ, Cosson RP (2002) Influence of metal exposure on metallothionein synthesis and lipid peroxidation in two bivalve mollusks: the oyster (Crassostreagigas) and the mussel (Mytilus edulis). Aquat Living Resour 15:61–66
Géret F, Serafim A, Bebianno MJ (2003) Antioxidant enzyme activities, metallothioneins and lipid peroxidation as biomarkers in Ruditapes decussatus? Ecotoxicology 12:417–426
Gillis PL (2011) Assessing the toxicity of sodium chloride to the glochidia of freshwater mussel: implications for salinization of surface waters. Environ Pollut 159:1702–1708
Gillis PL (2012) Cumulative impacts of urban runoff and municipal wastewater effluents on wild freshwater mussels (Lasmigona costata). Sci Total Environ 431:348–356
Gillis PL, Gagné F, McInnis R, Hooey TM, Choy ES, André C, Hoque ME, Metcalfe CD (2014a) The impact of municipal wastewater effluents on field-deployed freshwater mussels on the Grand River (ON). Environ Toxicol Chem 33:131–143
Gillis PL, Higgins SK, Jorge MB (2014b) Evidence of oxidative stress in wild freshwater mussels (Lasmigona costata) chronically exposed to urban-derived contaminants. Ecotoxicol Environ Safety 102:62–69
Gonzalez-Rey M, Bebianno MJ (2011) Non-steroidal anti-inflammatory drug (NSAID) ibuprofen distresses antioxidant defense system in mussel Mytilus galloprovincialis gills. Aquat Toxicol 105:264–269
Grand River Conservation Authority (2013) http://www.grandriver.ca/. Accessed 10 Sept 2013
Grosell M, Nielsen C, Bianchini A (2002) Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol C 133:287–303
Jorge MB, Loro VL, Bianchini A, Wood CM, Gillis PL (2013) Mortality, bioaccumulation and physiological responses in juvenile freshwater mussels (Lampsilis siliquoidea) chronically exposed to copper. Aquat Toxicol 123:137–147
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for several activities of the glutathione-S-transferase. J Biol Chem 20:6183–6188
Loomer HA, Cooke SE (2011) Water quality in the Grand River watershed: current conditions and trends (2003–2008). Grand River Conservation Authority/ http://www.grandriver.ca/water/2011_WaterQualityReport.pdf. Accessed Dec 2013
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Lydeard C, Cowie RH, Ponder WF, Bogan AE, Bouchet P, Clark SA, Cummings KW, Frest TJ, Gargominy O, Herbert DG, Hershler R, Perez KE, Roth B, Seddon M, Strong EE, Thompson FG (2004) The global decline of nonmarine mussels. BioScience 54:321–330
Machado AAS, Hoff MLM, Klein RD, Cardozo JG, Giacomin MM, Pinho GLL, Bianchini A (2013) Biomarkers of waterborne copper exposure in the guppy Poecilia vivipara acclimated to salt water. Aquat Toxicol 138–139:60–69
Mackie GL (1996) Diversity and status of Unionidae (Bivalvia) in the Grand River, a tributary of Lake Erie, and its drainage basin. Ontario Ministry of Natural Resources, Peterborough
Martín-Díaz ML, Gagné F, Blaise C (2009) The use of biochemical responses to assess ecotoxicological effects of pharmaceutical and personal care products (PPCPs) after injection in the mussel Elliptio complanata. Environ Toxicol Pharmacol 28:237–242
Mazon AF, Monteiro EAS, Pinheiro GHD, Fernandes MN (2002) Hematological and physiological changes induced by short-term exposure to copper in the freshwater fish Prochilodus scrofa. Braz J Biol 62:621–631
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6065
Metcalfe CM, Miao XS, Koenig BG, Struger J (2003) Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ Toxicol Chem 22:2881–2889
Metcalfe CD, Chu S, Judt C, Li H, Oakes KD, Servos MR, Andrews DM (2010) Antidepressants and their metabolites in municipal waste water, and downstream exposure in an urban watershed. Environ Toxicol Chem 29:79–89
Metcalfe-Smith JL, Staton SK, Mackie GL, Lang NM (1998) Changes in the biodiversity of freshwater mussels in the Canadian water of lower Great Lakes drainage basin over the past 140 years. J Great Lakes Res 24:845–858
Metcalfe-Smith JL, Mackie GL, Maio JD, Staton SK (2000) Changes over time in the diversity and distribution of freshwater mussels (Unionidae) in the Grand River, southwestern Ontario. J Great Lakes Res 26:445–459
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Monserrat JM, Martínez PB, Geracitano LA, Amado LL, Martins CMG, Pinho GLL, Chaves IS, Ferreira-Cravo M, Ventura-Lima J, Bianchini A (2007) Pollution biomarkers in estuarine animals: Critical review and new perspectives. Comp Biochem Physiol C 146:221–234
Nemerow NL (1991) Stream, lake, estuary, and ocean pollution, 2nd edn. Van Nostrand Reinhold, New York
Nenadic O, Greenacre M (2007) Correspondence analysis in R, with two- and three-dimensional graphics: the ca package. J Stat Softw 20(3). http://www.jstatsoft.org/v20/i03/.Accessed 19 Dec 2013
Neves RJ, Bogan AE, Williams JD, Ahlstedt SA, Hartfield PW (1997) Status of aquatic mollusks in the southeastern United States: a downward spiral of diversity. In: Benz GW, Collins DE (eds) Aquatic fauna in peril: the southeastern perspective special publication 1, Southeast Aquatic Research Institute, Lenz Design and Communications, Decatur, GA, pp 43–85
Nogueira LS, Wood CM, Gillis PL, Bianchini A (2013) Isolation and fractionation of gill cells from freshwater (Lasmigona costata) and seawater (Mesodesma mactroides) bivalves for use in toxicological studies with copper. Cytotechnology 65:773–783
Oliveira LF, Souza MM (2007) Aquatic invertebrates under waterborne lead exposure: Ionic and osmotic balance. Comp Biochem Physiol A 148:S66–S79
Ontario Provincial Water Quality Monitoring Network (2012) Website. http://www.ene.gov.on.ca/environment/en/monitoring_and_reporting/provincial_water_quality_monitoring_network/. Accessed 13 Jan 2012
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accessed 9 Nov 2013
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Serafim A, Lopes B, Company R, Cravo A, Gomes T, Sousa V, Bebianno MJ (2011) A multi-biomarker approach in cross-transplanted mussels Mytilus galloprovincialis. Ecotoxicology 20:1959–1974
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol Med 18:321–336
Strayer DL, Downing JA, Haag WR, King TL, Layzer JB, Newton TJ, Nichols SJ (2004) Changing perspectives on pearly mussels, North America’s most imperiled animals. Bioscience 54:429–439
Tetreault GR, Bennett CJ, Shires K, Knight B, Servos MR, McMaster ME (2011) Intersex and reproductive impairment of wild fish exposed to multiple municipal wastewater discharges. Aquat Toxicol 104:278–290
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol C 146:281–300
Viarengo A, Nott JA (1993) Mechanisms of heavy metal cation homeostasis in marine invertebrates. Comp Physiol Chem C 104:355–372
Wang S, Oakes KD, Bragg LM, Pawliszyn J, Dixon G, Servos MR (2011) Validation and use of in vivo solid phase micro-extraction (SPME) for the detection of emerging contaminants in fish. Chemosphere 85:1472–1480
Acknowledgments
The authors thank Sarah Higgins, Rodney McInnis and Tina Hooey (Environment Canada) for field and laboratory assistance. Financial support was provided by the International Development Research Centre (IDRC, Ottawa, Canada), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES—Programa Ciências do Mar, Brasília, DF, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—Instituto Nacional de Ciência e Tecnologia de Toxicologia Aquática, Brasília, DF, Brazil). A. Bianchini is a Research Fellow from the Brazilian CNPq (Proc. # 304430/2009-9) and supported by the International Canada Research Chair Program from IDRC. C.M. Wood is supported by the Canada Research Chair Program. P.L. Gillis is supported by Environment Canada.
Ethical standards
All the methods for obtaining the results presented here comply with the current laws of Canada.
Conflict of interest
Everyone meriting authorship in this work has been above named and the authors state that they have no conflict of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Machado, A.A.S., Wood, C.M., Bianchini, A. et al. Responses of biomarkers in wild freshwater mussels chronically exposed to complex contaminant mixtures. Ecotoxicology 23, 1345–1358 (2014). https://doi.org/10.1007/s10646-014-1277-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1277-8