Abstract
The slightly polluted source water of Yellow River was pretreated in a horizontal subsurface flow constructed wetland (HSFCW) and a lateral subsurface flow constructed wetland (LSFCW) in the Ji’nan city Reservoir, Shandong, China. During almost one years run, the results showed that at the hydraulic loading rate of 1 m/day, the removal efficiencies of chemical oxygen demand (COD), total nitrogen (TN), ammonium nitrogen (NH4 +-N) and total phosphorus (TP) in the HSFCW were 48.9, 51.4, 48.7 and 48.9 %, respectively, and the corresponding removal efficiencies in the LSFCW were 50.51, 53.12, 50.44 and 50.83 %, respectively. The HSFCW and LSFCW had a similar high potential for nutrients removal and LSFCW was slightly better. According to the China standard for surface water resources (GB3838-2002), mean effluent COD can reach the Class I (≤15 mg/L), and NH4 +-N and TP and TN can reach nearly the Class I (≤0.015 mg/L), the Class III (≤0.05 mg/L) and the Class IV (≤1.5 mg/L), respectively. It can be concluded that the slightly polluted source water from Reservoir was pretreated well by the constructed wetland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, more and more domestic sewage and industrial wastewater were discharged into rivers due to the rapid industrial development and intensive agriculture in China,which caused the water quality deterioration. The statistics of China Environmental State Bulletin in 2011 showed that water qualities of the seven main water systems, namely the Yangtze River, the Yellow River, the Pearl River, the Songhua River, the Huai River, the Hai River and the Liao River was all slightly polluted (MEPPRC 2012). However, most of the cities and towns in the drainage area of these rivers take the water from the seven river systems as the mainly sources of their drinking water. Because water from the seven main water systems is contaminated, and current treatment is inadequate, it is difficult to meet the Standards of Daily Drinking Water (GB5749-2006) (2006). In order to improve drinking water quality and reduce the treatment requirements of the water treatment plants, it may be necessary to implement simple and cost-effective techniques for pretreatment of such slightly polluted source water by constructed wetlands (CWs).
Wetlands are cost-effective, simple, low energy cost and natural technology for water treatment, which have received remarkable attention in recent years. They have been applied for various water treatment processes (Faulwetter et al. 2009), such as domestic sewage (Juang and Chen 2007; Valipour et al. 2009; Rawson et al. 2010; Mufarrege et al. 2011; Ye et al. 2012), industrial wastewater (Hallinger et al. 2011; Mufarrege et al. 2011), lake pollution (Li et al. 2008; Cui et al. 2011; Zhang et al. 2011; Hallinger et al. 2011; Zhang et al. 2012; Wu et al. 2013), heavy oil-production water (Ji et al. 2007; Baker et al. 2012), agricultural runoff (Yang et al. 2008; Milenkovski et al. 2010; Moustafa et al. 2011; Díaz et al. 2012; Murdock et al. 2013), rainstorm runoff in cities (Greenway 1997; Lai and Lam 2009). However, few studies have investigated on polluted source water for potential drinking use. Thus, the aim of this paper was to evaluate the possibility of using the subsurface flow constructed wetlands to pretreat slightly polluted source water prior to intensive treatment for drinking.
Materials and methods
Pilot-scale systems description
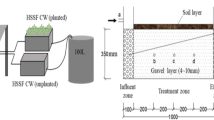
The site of this study was located in Ji’nan city Reservoir of Shandong Province (latitude 36°40′N and longitude 117°E). Ji’nan city Reservoir serves as the main water source for drinking plant and the water comes from the Yellow River, which is slightly polluted. A subsurface constructed wetland consisting of two parallel units of equal area (9 m2) was constructed at the Ji’nan city Reservoir (Fig. 1). Each unit was 6 m long and 1.5 m wide with a slope of 1 %. One unit was a horizontal subsurface flow constructed wetland (HSFCW) while the other was a lateral subsurface flow constructed wetland (LSFCW). The units were filled with two layers of gravel and a layer of soil. The bottom layer consisted of gravel with an average diameter of 4.8 cm, a depth of 30 cm, and a porosity of 50 %. The middle layer contains gravel with an averaged diameter of 2.0 cm, a depth of 30 cm, and 50 % porosity. The top layer was 20 cm of soil available locally. The bottom of the wetland was made of concrete and brick, and the effluent water level was made adjustable. Several types of PVC pipes were used to deliver the source water flow uniformly into the CWs. Polyethylene draining pipes were used to collect the treated water after passed through the CWs.
In April 2008, reeds obtained from a local area were transplanted into each unit at a density of 18 plants/m2. After planting, water levels were kept constant at 20 cm below the soil surface. In June 2008, data collection started after the plants had been established.
Operation and monitoring
A grit chamber was used to remove large suspended solid material (>10 mm) from Yellow River source water and then was pumped into the distribution system into the CWs. Large suspended solids were filtered out to prevent rapid clogging of the CWs. The constructed wetland system was operated continuously during the experimental period and both units were maintained at a hydraulic loading of 1 m/day. Monitoring of the CWs was conducted between June 2008 and November 2009.
Sampling frequency and analytical methods
From June 18, 2008 to November 29, 2009, the influent and effluent of the pilot-scale CWs were sampled approximately 8–10 times per month. Water samples were analyzed for chemical oxygen demand (COD), total nitrogen (TN), ammonium nitrogen (NH4 +-N), nitrate nitrogen (NO3 −-N), total phosphorus (TP), dissolved oxygen (DO), conductivity and pH. DO, conductivity and pH were measured with PB-608 dissolved oxygen analyzer, DDS-307A conductivity meter and PHS-25 pH-meter (Shanghai Precision & Scientific Instrument Co., LTD, China). Other parameters mentioned above were analyzed according to standard methods as described in the Standard Methods for the Examination of Water and Wastewater (NEPAC 2002). Chemical oxygen demand (COD) was determined by the potassium dichromate method, total nitrogen (TN) was determined by the peroxide potassium sulfate-ultraviolet spectrophotometry, NH4 +-N by the nessler’s reagents spectrophotometer, NO3 −-N by the phenol disulfonic acid spectrophotometry, TP by the molybdenum-antimony anti-spectrophotometric method.
In order to determine nutrient storage in plant and estimate the nutrient mass balance, plant biomass was sampled in May 2009 and September 2009. Plant samples were harvested from two 0.5 m2 sample plots of each wetland unit, and then separated into above-ground and underground parts. Plant samples were washed first with tap water then distilled water to remove any adhering sediments. Each sample was cut into small pieces, well-mixed, dried for two days to reduce the moisture content and later oven-dried to constant weight at 105 °C for dry weight determination. The concentration of nitrogen in plant tissues was determined by using the Kjeldahl method while phosphorus was by the molybdenum-antimony anti-spectrophotometric method (Bao 2005).
All statistical analyses in this study were performed by using the SPSS software package, including analysis of variance (ANOVA), Bartlett’s and Levine’s test for homogeneity of variance and normality, and Duncan’s multiple range test for differences between means.
Results
Water quality and performance efficiency
According to the Environmental Quality Standard for Surface Water SEPA (2002), mean effluent COD could reach the Class I (≤15 mg/L), and NH4 +-N and TP and TN can nearly the reach Class I (≤0.015 mg/L), the reach Class III (≤0.05 mg/L) and the reach Class IV (≤1.5/mg L), respectively. Concentrations of COD, TN, NH4 +-N, NO3 −-N and TP were significantly lower in the effluent than in the influent (p < 0.01), while there were no significantly differences between HSFCW and LSFCW (p > 0.05) (Table 1). The removal efficiencies of COD, TN, NH4 +-N, TP and NO3 −-N in the HSFCW were 48.9, 51.4, 48.7, 48.9 and 53.9 %, respectively, and 50.5, 53.1, 50.4, 50.8 and 55.6 %, respectively in the LSFCW.
Removal efficiency of CWs under various seasons
The nutrient removal efficiencies fluctuated in each wetland during the 14 months of investigation (Figs. 2, 3, 4, 5, 6). Both HSFCW and LSFCW showed high removal efficiencies for COD, TN and TP in summer than in other seasons (p < 0.1).
Inffluent COD concentration (closed diamonds), effluent COD concentration (triangles) and removal efficiencies (open diamonds) in the constructed wetland (a horizontal subsurface flow constructed wetland; b lateral subsurface flow constructed wetland) of the Ji’nan city Reservoir (2008,6-2008,12 and 2009,5-2009,11)
Inffluent TN concentration (closed diamonds), effluent TN concentration (triangles) and removal efficiencies (open diamonds) in the constructed wetland (a horizontal subsurface flow constructed wetland; b lateral subsurface flow constructed wetland) of the Ji’nan city Reservoir (2008,6-2008,12 and 2009,5-2009,11)
Inffluent TP concentration (closed diamonds), effluent TP concentration (triangles) and removal efficiencies (open diamonds) in the constructed wetland (a horizontal subsurface flow constructed wetland; b lateral subsurface flow constructed wetland) of the Ji’nan city Reservoir (2008,6-2008,12 and 2009,5-2009,11)
Inffluent NH4 +-N concentration (closed diamonds), effluent NH4 +-N concentration (triangles) and removal efficiencies (open diamonds) in constructed wetland (a horizontal subsurface flow constructed wetland; b lateral subsurface flow constructed wetland) of the Ji’nan city Reservoir (2008,6-2008,12 and 2009,5-2009,11)
Inffluent NO3 −-N concentration (closed diamonds), effluent NO3 −-N concentration (triangles) and removal efficiencies (open diamonds) in constructed wetland (a horizontal subsurface flow constructed wetland; b lateral subsurface flow constructed wetland) of the Ji’nan city Reservoir (2008,6-2008,12 and 2009,5-2009,11)
PH, DO and Conductivity content
Mean pH, DO, and conductivity were significantly lower in the effluent than in the influent (Table 1). No insignificant difference was detected between HSFCW and LSFCW (p > 0.05).
Plant biomass production
In May 2009, the mean height (±SD) of reeds were 50 (±11) cm for both of the wetlands, and in September 2009 the mean plant height was 175 (±25) and 178 (±23) cm for HSFCW and LSFCW, respectively (Table 2).
Discussion
The objective of this study was to construct the subsurface constructed wetland system that would pretreat the Yellow River water and improve the efficiency of water purification plants subsequently. A horizontal subsurface flow constructed wetland (HSFCW) and a lateral subsurface constructed flow wetland (LSFCW) were constructed, the LSFCW had a more complex flow pattern and a slightly longer flow path. Water flow had a better contact with matrix surface and plant root in the LSFCW. Therefore, the performance efficiency of the LSFCW was slightly better than the HSFCW system.
Purification within the CWs was achieved by a wide variety of physical, chemical, and (micro) biological processes, including sedimentation, filtration, precipitation, sorption, plant uptake, microbial decomposition, and nitrogen transformations. In the current study, the concentration-based removal efficiency for COD (32–65 %) was lower than the 61–94 % efficiency reported by Rousseau et al. (2004). Because the COD concentration of slightly polluted source water from the Yellow River was low, the lower organic load resulted in the lower COD removal efficiency compared with removal efficiencies reported in the literature for sewage water, which has a greater organic load (Rousseau et al. 2004). Korkusuz et al. (2005) stated that it was difficult to reduce COD concentrations below 50 mg/L. In this study, influent concentration of COD remained constantly below 45 mg/L (range 10.9–44.5 mg/L) throughout study period (Fig. 2) with a COD concentration of about 2.5–21.5.mg/L in effluent. Similar results were reported by Li et al. (2008), who studied the performance of three parallel CWs to improve surface water quality. In their cases, the influent concentration of COD ranged from 5.4 to 10.3 mg/L (12-month running), with an average COD removal efficiency of 17–40 %.
The removal of COD was accomplished by a combination of physical and microbial mechanisms. The removal of the organic matter was mostly a result of the microbial activity of aerobic and anaerobic bacteria, which function even at the temperature as low as 5 °C (Greenway and Woolley 1999; Steer et al. 2002; Vymazal 2002; Hiley 2003). Porous media and plant roots could keep the water temperature in the winter higher than the air temperature by 2–3 °C, thus allowing higher microbial activity to continue (Hiley 2003). This may explain why the removal efficiency of COD was kept above 30 % at such a low temperature in December (Fig. 2).
Because of relatively low NH4 +-N loads in the current study, the remedial effects were lower than the mean value reported by Vymazal (2007). Even though, the mean NH4 +-N concentrations in the effluent of HSFCW and LSFCW were better. According to China standard for surface water resources, it nearly reached Class I (≤0.015 mg/L) water quality.
The conversion of ammonium to nitrate can be pronounced in the systems. When the mean NH4 +-N removal efficiency in LSFCW was higher, the mean NO3 −-N concentration should be higher in the LSFCW than that in the HSFCW system. However, it was found that NO3 −-N concentration was 1.44 mg/L in the HSFCW than that of 1.39 mg/L in the LSFCW, which was removed by denitrification. And this result revealed that denitrification in LSFCW was more effective than in HSFCW. Moreover, six strains of nitrobacteria and seven strains of denitrifying bacteria were isolated from the HSFCW; while, eight strains of nitrobacteria and eight strains of denitrifying bacteria were isolated from the LSFCW. Thus, denitrification was more effective in LSFCW had been proved.
Denitrification is the most commonly defined as the process in which nitrate is converted into dinitrogen via intermediates nitrite, nitric oxide and nitrous oxide (Hauck 1984; Paul and Clark 1996; Jetten et al. 1997). In the constructed wetland systems, denitrification is dependent upon the presence of nitrate, nitrogen, and organic carbon. Environmental factors, such as pH, temperature, surface area for microbial attachment, and concentration of dissolved oxygen also effected denitrification (Kozub and Liehr 1999). Plant litter and plant/root exudates continually added carbon sources into the system (Hunt and Poach 2001). Therefore, there are abundant carbon sources for the microbial denitrification in the constructed wetlands. The wetland plant root network and the matrix provided extensive surface area for microbial attachment (Oostrom and Russell 1994). Paul and Clark (1996) reported that the optimum pH range for denitrification is between pH 6 and 8. In the current study, the mean pH of influent water (7.96) was within the optimum range for denitrification. In addition, the pH in the effluent was always lower than that of the influent. This difference may result from microbial degradation producing organic acids, ion exchange of medium, and/or physiological activities of plants which enhanced acidity.
In CWs, oxygen can be transported by plant photosynthesis to the rhizosphere and by the surface reaeration of the bulk water. Denitrification rates were found to be very sensitive to the presence of oxygen, but anaerobic conditions for denitrification could be found in CWs even with out-flowing oxygen concentrations of 4 mg/L (Schulz et al. 2003). Therefore, the mean influent DO (4.66) was within the range for denitrification in this study. According to the above analyses, denitrification conditions were suitable in this experiment. Therefore, CWs exhibited efficient denitrification.
Figure 3 illustrates the TN concentrations variations in influent and effluent of the constructed wetlands. The mean TN concentration in the effluent was 1.73 mg/L in the HSFCW higher than that in the LSFCW systems (1.67 mg/L), which may be attributed to better biology contact in LSFCW.
Throughout November, TN concentration in the effluent was kept at a lower level, which can be explained that plants uptake played a significant role on nitrogen removal (Jing et al. 2001), because the microorganisms could play important role for nitrogen removal function optimally at temperatures above 15 °C. In the current study, average TN removal efficiency over 50 % in HSFCW and LSFCW was higher than 44.6 % reported by Vymazal (2002), which might probably due to the relatively lower pollution loading in this experiment.
Removal of phosphate in wetlands was known to be mediated by precipitation, adsorption, plant uptake, and microbial assimilation. Chemical precipitation with Fe3+, Al3+ and Ca2+ in wetland sediment, adsorption onto gravel/soil particles and anion exchange of hydrous oxides were well documented as main mechanisms for phosphate removal in wetlands (Bubba et al. 2003). However, the ability of adsorption and plant uptake can be saturated, indicating that they have a finite capacity and therefore, cannot contribute to long-term sustainable phosphate removal. In this study, it needed a longer time to reach the adsorption saturation, because the TP concentration of slightly polluted source water was very low (0.111 ± 0.156 mg/L). Moreover, the mean effluent TP concentrations (0.059 and 0.057 mg/L in HSFCW and LSFCW, respectively) could nearly reach Class III (≤0.05 mg/L) water quality, according to standards for surface water resources SEPA (2002). In addition, phosphorus removal (Fig. 4) also showed a seasonal change. Lower phosphorus removals in November could be explained by the fact that litter and microbial biomass were decomposed during winter, and phosphorus was released from the precipitates (Knight and Kadlec 1996), resulting in phosphorus solubilization in water. Phosphate removal was not particularly sensitive to temperature for it is mainly governed by adsorption.
In addition, conductivity was lower in the effluent than that in the influent (Table 1). It was observed that ionic decreased after CWs treatment, indicating ionic was wetland adsorbed by the substrate and plants.
Plants in the wetland play an important role in treating wastewater. Plant roots and rhizomes are important for the microbial transformation processes and subsequently to wastewater purification process (Münch et al. 2007; Stottmeister et al. 2003). Moreover, plant nutrient uptake is another way for nutrient removal in the wetland. Wetland plants generally removed part of nutrients from influent water (Hammer 1992). Depending on plant species used, type of wastewater treated, and nutrient loading rates, plant nutrient uptake has been shown to account for between 3 and 47 % of nitrogen removal and 3 and 60 % of phosphorus removal (Tanner et al. 1995; Greenway and Woolley 2000). In this study, the biomass of above ground varied considerably over the course of the growing season. Table 2 showed that the biomass increased more than 24 % for the above ground from May to September. It was assumed that N and P uptake by plants occurs only from the water column, 69.15 and 70.45 g N and 5.87 and 6.42 g P could be removed from HSFCW and LSFCW respectively by harvesting of the above ground biomass of reed plants in September, which account for 5.85 and 5.78 % of N and 30.70 and 32.51 % of P that removed by HSFCW and LSFCW from May to September, respectively. These results indicated that harvesting of plants could play a significant removal way for lightly loaded treatment wetlands.
Conclusions
It was clear from this research that the subsurface flow constructed wetlands could be effectively used in pretreating slightly polluted source water in the water reservoir. CWs could improve the water quality of the drinking water source and mitigate the load of drinking water treatment plants in China. Compared with the seriously polluted water, slightly polluted source water can prevent rapid clogging of the media due to the low concentration of contaminants. Therefore, the constructed wetland system used for slightly polluted water treatment can be operated for a longer period. In addition, the LSFCW had a more complex flow pattern and a slightly longer flow path. Water flow had a better contact with matrix surface and plant root in the LSFCW; therefore, performance efficiency of the LSFCW was slightly better than the HSFCW system. All in all, CWs were suitable for source water pretreatment. Due to the low feeding load, the removal function of the system couldn’t be fully exhausted in a short period of time. Therefore, future experiments should be conducted to enhance the feeding load so that the effects of the system can be adequately exhibited. Moreover, the microbial community characteristics should be investigated by molecular biological methods to better understand the microbial community structure and provide useful insights into the mechanisms for enhanced removal efficiency of pollutants.
References
Baker LF, Ciborowski JJH, MacKinnon MD (2012) Petroleum coke and soft tailings sediment in constructed wetlands may contribute to the uptake of trace metals by algae and aquatic invertebrates. Sci Total Environ 414:177–186
Bao SD (2005) Analysis of soil agricultural chemistry, vol 3. China Agriculture Press, Beijing
Bubba MD, Arias CA, Brix H (2003) Phosphorus adsorption maximum of sands for use as media in subsurface flow constructed reed beds as measured by the Langmuir isotherm. Water Res 37:390–3400
China Environmental State Bulletin: 2011 (2012) Ministry of Environmental Protection of the People’s Republic of China, Beijing
Cui F, Yuan B, Wang Y (2011) Constructed wetland as an alternative solution to maintain urban landscape lake water quality: trial of xing-qing lake in Xi’an city. Procedia Environ Sci C 10:2525–2532
Díaz FJ, O′Geen AT, Dahlgren RA (2012) Agricultural pollutant removal by constructed wetlands: implications for water management and design. Agric Water Manag 104:171–183
Faulwetter JL, Gagnon V, Sundberg C, Chazarenc F, Burr MD, Brisson J, Camper AK, Stein OR (2009) Microbial processes influencing performance of treatment wetlands: a review. Ecol Eng 35:987–1004
Greenway M (1997) Nutrient content of wetland plants in constructed wetlands receiving municipal effluent in tropical Australia. Water Sci Technol 35:135–142
Greenway M, Woolley A (1999) Constructed wetlands in Queensland: performance efficiency and nutrient bioaccumulation. Ecol Eng 12:39–55
Greenway M, Woolley A (2000) Changes in plant biomass and nutrient removal over 3 years in a constructed free water surface flow wetland in Cairns, Australia. In: Proceedings of the seventh international conference. on wetland systems for water pollution control 2. Lake Buena Vista, FL USA
Hallinger KK, Cornell KL, Brasso RL, Cristol DA (2011) Mercury exposure and survival in free-living tree swallows (Tachycineta bicolor). Ecotoxicology 20:39–46
Hammer DA (1992) Designing constructed wetlands systems to treat agricultural nonpoint source pollution. Ecol Eng 1:49–82
Hauck RD (1984) Atmospheric nitrogen chemistry, nitrification, denitrification, and their relationships. In: Hutzinger O (ed) The handbook of environmental chemistry, vol 1., Part C, the natural environment and biogeochemical cycles.Springer, Berlin, pp 15–27
Hiley P (2003) Performance of wastewater treatment and nutrientremoval wetlands. In: Mander U, Jenssen P (eds) Constructed wetlands for wastewater treatment in cold climates, (reedbeds) in cold temperature climates. WIT, Southampton, pp 1–18
Hunt PG, Poach ME (2001) State of the art for animal wastewater treatment in constructed wetlands. Water Sci Technol 44:19–25
Jetten MS, Logemann S, Muyzer G, Robertson LA, Vries SD, Loosdrecht MC, Kuenen JG (1997) Novel principles in the microbial conversion of nitrogen compounds. Antonie van Leeuwonheok 71:75–93
Ji GD, Sun TH, Ni JR (2007) Surface flow constructed wetland for heavy oil-produced water treatment. Biores Technol 98:436–441
Jing SR, Lin YF, Lee DY, Wang TW (2001) Nutrient removal from polluted river water by using constructed wetlands. Biores Technol 76:131–135
Juang DF, Chen PC (2007) Treatment of polluted river water by a new constructed wetland. Int J Environ Sci Technol l4:481–496
Knight RL, Kadlec RH (1996) Treatment Wetlands. Lewis, Boca Raton
Korkusuz EA, Beklioğlu M, Demirer GN (2005) Comparison of the treatment performances of blast furnace slag-based and gravel-based vertical flow wetlands operated identically for domestic wastewater treatment in Turkey. Ecol Eng 24:185–198
Kozub DD, Liehr SK (1999) Assessing denitrification rate limiting factors in a constructed wetland receiving landfill leachate. Water Sci Technol 40:75–82
Lai DYF, Lam KC (2009) Phosphorus sorption by sediments in a subtropical constructed wetland receiving stormwater runoff. Ecol Eng 35:735–743
Li LF, Li YH, Biswas DK, Nian YG, Jiang GM (2008) Potential of constructed wetlands in treating the eutrophic water: evidence from Taihu lake of China. Biores Technol 99:1656–1663
MEPPRC (Ministry of Environmental Protection of the People’s Republic of China) (2012) China Environmental State Bulletin: 2011. http://jcs.mep.gov.cn/hjzl/zkgb. Accessed 24 Jan 2014
Milenkovski S, Baath E, Lindgren PE, Berglund O (2010) Toxicity of fungicides to natural bacterial communities in wetland water and sediment measured using leucine incorporation and potential denitrification. Ecotoxicology 19:285–294
Moustafa MZ, White JR, Coghlan CC, Reddy KR (2011) Influence of hydropattern and vegetation type on phosphorus dynamics in flow-through wetland treatment systems. Ecol Eng 37:1369–1378
Mufarrege MM, Luca DGA, Hadadand HR, Maine MA (2011) Adaptability of Typha domingensis to high pH and salinity. Ecotoxicology 20:457–465
Münch CH, Neu T, Kuschk P, Röske I (2007) The root surface as the definitive detail for microbial transformation processes in constructed wetlands—a biofilm characteristic. Water Sci Technol l56:271–276
Murdock JN, Shields FDJ, Lizotte REJ (2013) Periphyton responses to nutrient and atrazine mixtures introduced through agricultural runoff. Ecotoxicology 22:215–230
NEPAC (2002) Standard method for the examination of water and wastewater. Environmental Science Press, Beijing
Oostrom VAJ, Russell AJ (1994) Denitrification in constructed wastewater wetlands receiving high concentrations of nitrate. Water Sci Technol 29:7–14
Paul EA, Clark FE (1996) Soil microbiology and biochemistry. Academic Press, San Diego
Rawson CA, Lim RP, Tremblay LA, Warne MSJ, Ying GG, Laginestra E, Chapman JC (2010) Benthic macroinvertebrate assemblages in remediated wetlands around Sydney, Australia. Ecotoxicology 19:1589–1600
Rousseau DPL, Vanrolleghem PA, Pauw ND (2004) Constructed wetlands in Flanders: a performance analysis. Ecol Eng 23:151–163
Schulz C, Gelbrecht J, Rennert B (2003) Treatment of rainbow trout farm effluents in constructed wetland with emergent plants and subsurface horizontal water flow. Aquaculture 217:207–221
SEPA (2002) Environmental quality standard for surface water (GB3838-2002). State Environmental Protection Administration of China, Beijing
Standards for Drinking Water Quality (GB5749-2006) (2006) Ministry of Health of the People’s Republic of China, Beijing
Steer D, Fraser L, Boddy J, Seibert B (2002) Efficiency of small constructed wetlands for subsurface treatment of single-family domestic effluent. Ecol Eng 18:429–440
Stottmeister U, Wiener A, Kuschk P, Kappelmeyer U, Kstner M, Bederski O, Müller RA, Moormann H (2003) Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol Adv 22:93–117
Tanner CC, Clayton JS, Upsdell MP (1995) Effect of loading rate and planting on treatment of dairy farm waste waters in constructed wetlands-II. Removal of nitrogen and phosphorous. Water Res 29:27–34
Valipour A, Raman VK, Ghole VS (2009) A new approach in wetland systems for domestic wastewater treatment using phragmites sp. Ecol Eng 35:1797–1803
Vymazal J (2002) The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecol Eng 18:633–646
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65
Wu JT, Wang L, Ma F, Yang JX, Li SY, Li Z (2013) Effects of vegetative-periodic-induced rhizosphere variation on the uptake and translocation of metals in Phragmites australis (Cav.) Trin ex. Steudel growing in the Sun Island Wetland. Ecotoxicology 22:608–618
Yang ZF, Zheng SK, Chen JJ, Sun M (2008) Purification of nitrate-rich agricultural runoff by a hydroponic system. Biores Technol 99:8049–8053
Ye JF, Wang L, Li D, Han W, Ye C (2012) Vertical oxygen distribution trend and oxygen source analysis for vertical-flow constructed wetlands treating domestic wastewater. Ecol Eng 41:8–12
Zhang YM, Zhang YC, Gao YX, Zhang HH, Cao JY, Cail JB, Kong XG (2011) Water pollution control technology and strategy for river-lake systems: a case study in Gehu Lake and Taige Cana. Ecotoxicology 20:1154–1159
Zhang FQ, Wang YS, Sun CC, Lou ZP, Dong JD (2012) A novel metallothionein gene from a mangrove plant Kandelia candel. Ecotoxicology 21:1633–1641
Acknowledgments
The authors would like to thank Ms. Chen Hong of Ji’nan city Reservoir, for their helps with field sampling and investigation. This work was financially supported by the National Natural Science Foundation of China (Nos. 41371072 and 41071332), Heilongjiang National Science Fund for Young Scholars (No. QC2009C70) and the Program for Young Academic Backbone of Harbin Normal University (No. 10KXQ-08).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Zhang, X., Wang, J. et al. Performance of the subsurface flow constructed wetlands for pretreatment of slightly polluted source water. Ecotoxicology 23, 699–706 (2014). https://doi.org/10.1007/s10646-014-1197-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1197-7