Abstract
The physiological responses and Cu accumulation of Paulownia fortunei (Seem) Hemsl. were studied under 15.7–157 μmol L−1 Cu treatments in liquid culture for 14 days; the impacts of Cu concentration in the seedlings were evaluated under Cu mine tailing culture with acetic acid and EDTA treatment for 60 days. Results showed that the concentrations of Chl-a, Chl-b and Carotenoids significantly increased (p < 0.05) at 15.7–78.7 μmol L−1Cu treatment and significantly decreased at 157 μmol L−1 treatment after 14 days of Cu exposure. The activities of superoxide dismutase (SOD) and catalase (CAT) significantly increased as Cu levels were enhanced and the activities of both SOD and CAT under 157 μmol L−1 Cu stress were 2.9 and 1.9 times higher than that of control, respectively. The concentrations of proline and soluble sugars in the leaves of P. fortunei significantly increased as the Cu concentrations were elevated. Cu concentrations in roots, stems and leaves of P. fortunei increased significantly as Cu levels increased and reached 1911, 101 and 93 μg g−1 dry weights (DW) at 157 μmol L−1 Cu treatment, respectively. The seedlings of P. fortunei cultivated in Cu tailing experienced unsuccessful growth and loss of leaves in all treatments due to poor nutrition of the Cu tailing. The dry weight of P. fortunei increased under all the treatments of acetic acid after 60 days exposure. However, dry weight significantly decreased under both levels of EDTA. The Cu concentrations increased significantly in roots and decreased in leaves when each was treated with both concentrations of acetic acid. The Cu concentrations in the roots, stems and leaves increased significantly, and the concentrations of Cu in the stems and leaves under the treatment of 2 μmol L−1 EDTA reached 189.5 and 763.1 μg g−1 DW, respectively. The result indicated that SOD, CAT, proline and soluble sugars played an important role in coping with the oxidative stress of copper. Acetic acid could promote growth and EDTA at the experimental levels, which could also enhance Cu absorption and translocation into the stems and leaves of P. fortune. Furthermore, acetic acid and EDTA could be rationally utilized in Cu-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution has become an urgent problem throughout the world over the past several decades. Many industrial processes are major contributors to extensive environmental pollution (Cieśliński et al. 1998; Fernández-Caliani et al. 2009; Han et al. 2008). Heavy metals are the main pollutants to the soil and water in different forms. Several heavy metals have been introduced into the environment due to human activities like mining, including garbage and sewage sludge disposal in field sites (Lyubenova et al. 2009). Metal mine tailing and acid mine drainage are sources of major environmental pollution to soil around tailing sites. Furthermore, leachate from such sites (rich in sulfate, iron, and soluble heavy metals) has the potential to contaminate the ground water as well as the local watercourses (Hakkou et al. 2008).
Copper (Cu) is an essential nutrient for organisms. However, over-absorption of Cu may be harmful to plants. Cu is known as an environmentally important metal because of its toxicity and its widespread pollution (Kováčik et al. 2010a). While plant growth and physiology are severely disturbed under Cu stress, one of the main and most common mechanisms behind heavy metal toxicity has been attributed to oxidative stress (Lu et al. 2010). Decreases of chlorophyll content in leaves of plants after exposure to copper is frequently reported in the literature; as a result, plant growth is restrained (Basak et al. 2001; Wójcik et al. 2009).
An active oxygen-detoxifying system could respond to many environmental stresses including heavy metals (De Vos et al. 1992; Foyer et al. 1994). Exposure to heavy metals is known to activate a universal mechanism of defense called “the stress response” in all living organisms, which includes the production of stress proteins to best adapt to adverse conditions (Borkan and Gullans 2002). Failure to quench oxygen free radicals or subsequent propagation of chain reactions may lead to toxic effects and cause oxidative stress (Mazhoudi et al. 1997). In plant cells, the defense systems of plants coping with reactive oxygen species includes enzymes such as superoxide dismutase (SOD), peroxidase (POD), glutathione reductase (GR), catalase (CAT) (Jouili and El Ferjani 2004; Huang et al. 2011), and low molecular weight antioxidants, including proline and soluble sugars (Jouili and El Ferjani 2003).
Prior to environmental regulations, ore processed from mine tailings was usually left exposed and uncontained or dumped into nearby streams and rivers (Richmond 2000). These fluvial deposits of tailings can be extremely acidic and contain large amounts of heavy metals that hinder plant growth (Richmond 2000), presenting a challenging environment for plant establishment and growth (Boyter et al. 2009). Though soil with heavy metal contamination may also degrade plant communities and their productivity, certain green plants can be used to remove pollutants from the environment or to render them harmless, which are defined as phytoremediation (Raskin et al. 1997). Regardless of the plants used, the abundance of heavy metals absorbed by plant roots is considered the key factor limiting the efficiency of phytoextraction.
During the processes of phytoextraction, organic acids are important plant root exudates and microbial metabolites in terms of their ability to increase the dissolution of metals from highly insoluble mineral phases in soil, thereby increasing metal mobility in the vicinity of roots and enhancing their availability to plants (López-Bucio et al. 2000; Clemens et al. 2002). Thus, a methodology using organic acids to assist phytoextraction of heavy metals has been developed to help in the remediation of heavy metals contaminated soil (Evangelou et al. 2006; Wang et al. 2009). EDTA could be the most efficient of a tested chelator in increasing shoot heavy metals concentration in corn (Zea mays), white bean (Picea vulgaris) (Luo et al. 2005), wheat (Triticum aestivum) (Wang et al. 2009) and Indian mustard (Brassica juncea) (Epstein et al. 1999; Wu et al. 2004). However, the growth and physiology of plants are effected under the treatment of higher EDTA concentration (Epstein et al. 1999). Furthermore, it has been understood that EDTA has a low biodegradability and is very persistent in the environment (Wasay et al. 1998). Finally, acetic acid is very cheap and easily decomposed in the nature; the function of acetic acid in the heavy metal (Pb) accumulation by plant has been reported by hydroponics research (Wang et al. 2007).
Wang et al. (2009) reported that acetic acid could promote Cu accumulation in wheat in contaminated soil. However, the use of acetic acid in mine tailings has not been found. Wenzel et al. (2003) indicated that chelate-assisted phytoextraction is limited by the risk of groundwater pollution due to metal mobilization. Future efforts should thus focus on natural, continuous technologies without the application of chelates using high biomass perennial plants such as willow or poplars (Evangelou et al. 2007). P. fortunei (Seem) Hemsl. is a fast-growing and Cu-tolerant plant; we made an on-the-spot investigation in the copper mine in Dexing Copper Mine, Jiangxi, China, which has not been reported. The purposes of this study was to investigate: (1) the effects of Cu on the physiological responses, such as the concentration of photosynthetic pigments, solute sugar and proline, and the activities of some oxidative enzymes (SOD, POD and CAT) under Cu stress; (2) the effects of different Cu levels on Cu accumulations of P. fortunei growing in the liquid culture; and (3) the growth and Cu absorption of P. fortunei growing in Cu mine tailings treated with different concentration of EDTA and acetic acid. As a result, the goal of these experiments was to find the phytoremediation techniques in increasing litterfall and organic matter inputs in mine tailings or in decreasing Cu content in mine tailings by clean up the litterfall and metal dispersion through natural agencies.
Materials and methods
Plants materials and growth conditions
Seedling cultures of P. fortunei were prepared with the methods according to Han et al. (2007). Seeds were collected from the trees grown in the Campus of Jiangxi University of Finance and Economics, China. The surface of seeds were sterilized by immersing them in 5% (v/v) sodium hypochlorite for 20 min and then washing them carefully with tap water. The seeds were then planted in containers with clean sand and watered with a solution of water and 1/2 Hoagland nutrient solution. When the seedlings reached 10 cm in height, they were removed from the containers to liquid cultures of different Cu stress and copper mine tailing cultures of different Cu concentrations to be observed for responses to different physiological parameters. The experiment was conducted at ambient temperature (15–25°C) under natural light in experiment plots at the Experimental Teaching Center of Ecological Environment of Jiangxi Province, Jiangxi University of Finance and Economics, Nanchang, China.
Physiological parameters and Cu accumulations in liquid cultures
The seedlings were transferred into 300 mL pots (5 seedlings/pot), and a 280 mL nutrient medium of ½ Hoagland nutrient solution was added. Each of five seedlings was fixed in one of five holes on a piece of foam board, and the seedling roots were covered in the pot. After 2 weeks, the plants were treated with nutrient solutions free of Cu (CK), with 15.7, 78.7 and 157 μmol L−1 Cu supplied as CuSO4·5H2O. The medium was replaced every 3-days, and a constant volume of the nutrient medium was maintained by adding distilled water every other day. Each experiment had triplicates. The leaves in the same position of the seedlings were taken for each physiological parameter examination in order to keep a consistent condition of the experiment after 14 days (d) of exposure.
After extraction with Dimethyl Formamide (DMF), chlorophyll a (Chl-a), chlorophyll b (Chl-b) and total carotenoids were measured spectrophotometrically as described by Mocquot et al. (1996). Total soluble sugars were estimated by anthrone-sulphuric acid method by using 0.2% anthrone in concentrated H2SO4 as reagent (McCready et al. 1950). Absorbance was recorded at 630 nm. The standard curve was plotted with 0-100 μg of Sucrose. Proline concentration and the activity of CAT were determined according to the methods of Li et al. (2000). The enzyme activity of CAT was expressed in μg H2O2 destroyed min−1 g−1 fresh weight (FW). The activity of SOD was assayed according to Beauchamp and Fridovich (1971). One unit of SOD was defined as the enzyme amount causing 50% inhibition reduction of NBT, and the enzyme activity was expressed in units per mg of protein. POD activity was determined using the guaiacol oxidation method (Fang and Kao 2000) and enzyme activity of POD was expressed in ΔOD470 min−1 g−1 FW.
In addition, all the samples were ground in a porcelain mortar and weighed (0.1–0.2 g per sample) before chemical analysis. The powders of samples were dissolved in a mixture of concentrated HNO3–HClO4 (87:13, v/v) and heated at 160°C for 5 h. After cooling, the extract was diluted, filtered and made up to 25 mL with 5% HNO3. The Cu concentration of the extract was determined using flame atomic absorption spectrometer (TAS990).
Growth and Cu concentration under Cu mine tailing cultures with organic acid treatment
Cu mine tailings were collected from tailing sites of Dexing Copper Mine, Dexing, Jiangxi Province, China. Eight samples were selected at random across the Cu mine tailing sites, and samples in each site were taken from the top layer of the deposit to the depth of 80 cm in order to examine the stability of chemical composition in Cu mine tailings which were long time discharged. Samples were then manually mixed, and their physicochemical properties are examined and showed in Table 1. After 1 week of initial adaptation of seedlings in Cu tailings, the plants (5 seedlings/pot) were treated with an equal quantity (100 mL) of tap water (CK) or containing 0.5 μmol L−1acetic acid (Ac0.5), 2 μmol L−1 acetic acid (Ac2), 0.5 μmol L−1 EDTA (EDTA0.5) and 2 μmol L−1 EDTA (EDTA2), respectively. Then an equal quantity of tap water was supplemented into each pot when the tailings were relatively dry until the seedlings were harvested. In this experiment, each treatment was thrice replicated.
After the seedlings were treated for 60 days, they were harvested and washed thoroughly with running tap water. Each seedling was then divided into three parts: leaves, stems and roots. The dry weights (DW) were measured after the leaves, stems and roots were dried at 80°C until a constant dry weight for biomass was reached. The Cu concentrations in the roots, stems and leaves were determined with the same method as described to determine liquid cultures in Cu.
Statistical analyses
All results are means of three replicates. One-way analysis of variance (ANOVA) in randomized complete block design was performed to check the variability of data and validity of the results; and the data were analyzed with SAS software system (SAS Institute Inc.1994). Differences at p < 0.05 were considered significant. The percent increase or decrease over the control of physiological parameters under Cu stress by liquid cultures was calculated with Microsoft Excel 2003.
Results
The effect of Cu on the concentrations of photosynthetic pigments
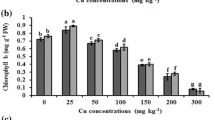
The concentrations of Chl-a, Chl-b, Carotenoids and total chlorophyll (ChlT) significantly increased (p < 0.05) at 15.7 μmol L−1, 78.7 μmol L−1 Cu treatment in the leaves of P. fortunei and significantly decreased at 157 μmol L−1 treatment (Table 2). The concentrations of Chl-a, Chl-b, Carotenoids and ChlT under 157 μmol L−1 dropped 88, 87, 88 and 86% compared to the control, respectively. The concentrations of Chl-a/Chl-b in all treatments were not significantly altered.
The effect of Cu on the concentrations of proline and solute sugars electric conductivity
The impacts of Cu concentrations on proline and solute sugars in leaves of P. fortunei were showed in Table 3. The concentrations of proline in the leaves of P. fortunei were significantly increased (p < 0.05) as the Cu concentrations elevated after 14 days of Cu exposure. With the treatment of 157 μmol L−1 Cu concentration, the proline concentration was 8.3 times higher than that of the control. The increases of the concentrations of soluble sugars in the leaves of P. fortunei were appeared a similar trend as proline concentrations. The concentrations of soluble sugars significantly increased as the Cu concentrations increased after 14 days of Cu exposure and the soluble sugars concentration treated with 157 μmol L−1 Cu concentration was 5.8 times higher than that of the control.
The effect of Cu on the activities of antioxidative enzymes
The activities of SOD were induced to show a dose-dependent increase in the leaves of P. fortune under Cu stress and the activity under 157 μmol L−1 Cu stress was 2.9 times higher than that of control (Table 4). The POD activities in the leaves of P. fortunei under Cu treatment was significantly increased at 78.7 μmol L−1 Cu treatments, but significantly decreased at 157 μmol L−1 Cu stress. In contrast, CAT activities decreased at 15.7 μmol L−1 Cu treatment and then significantly increased as the Cu concentrations increased. The activities of CAT treated with 157 μmol L−1 Cu were 1.9 times higher than that of control.
Cu concentrations of P. fortunei growing in the liquid cultures
The results also suggested that Cu concentrations in the roots, stems and leaves of P. fortunei would significantly increase as the Cu levels increase for 14 days of exposure (Fig. 1). Cu concentrations in roots of of P. fortunei increased sharply to 1911 μg g−1 DW at 157 μmol L−1 Cu treatment, while the concentrations in the stems and leaves increased to 101 and 93 μg g−1 DW at 157 μmol L−1 Cu treatment, respectively. As the ratios of Cu concentrations in the stems and leaves to the roots at 0 μmol L−1 Cu treatment (control) were 1.1 and 0.5, respectively, the ratio of Cu concentrations in the stems and leaves to the roots were sharply decreased to 0.053 and 0.049, respectively, at 157 μmol L−1 Cu treatment. The result appeared that the translocation of Cu from roots to stems and leaves in P. fortunei with higher Cu concentration treatments was lower than that in lower Cu concentration treatments.
Biomass of plants growing in the Cu mine tailing
The effects of different treatments of acetic acid and EDTA on the seedling biomass of P. fortunei after 60 days of Cu mine tailing culture were shown in Fig. 2. The dry weights (DWs) of roots and stems of P. fortunei grown in Cu tailings under the treatments of 0.5 μmol L−1 acetic acid and the DWs of stems under the treatment of 2 μmol L−1 acetic acid increased significantly (p < 0.05) compared with that of the control. However, the DWs of roots, stems and leaves of P. fortunei decreased under both levels of EDTA. Due to the Cu tailings were poor in nutrition (total N content only 0.025%, Table 1), the seedlings of P. fortunei grew weakly in all treatments and the old leaves of the seedlings dropped off and were lost.
Cu concentrations of P. fortunei growing in the Cu mine tailings
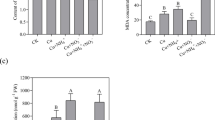
Quantitative analyses of Cu accumulation of P. fortunei revealed that Cu concentration in the roots under all the treatments of acetic acid and EDTA after 60 days exposure increased significantly (p < 0.05) compared with that of the control. Cu concentration in the roots under the treatments of 0.5 μmol L−1 EDTA and 2 μmol L−1 EDTA were 3.2 and 3.4 times higher than that in the control, respectively (Fig. 3). However, the concentration of Cu in the stems and leaves treated with both concentrations of acetic acid did not increase significantly. The Cu concentrations in the stems and leaves of P. fortunei under the treatment of EDTA increased significantly than that of the control, as concentrations of Cu in the stems and leaves under the treatment of 2 μmol L−1 EDTA reached to 189.5 μg g−1 and 763.1 μg g−1 DW, 8.2 and 86.9 times of those of the control, respectively. The ratios of Cu concentrations in the stems and leaves to the roots at 2 μmol L−1 EDTA treatments were 0.39 and 1.58, respectively, while, the ratios at control were 0.16 and 0.06. The experimental results indicated that EDTA could enhance the translocation of Cu from roots to stems and leaves.
Discussion
It is known that, in relation to high metal stress, high Cu absorption causes destruction of chloroplast structure and the decrease of chlorophyll concentration (Wójcik et al. 2009). The decrease in chlorophyll content under stress conditions in plants is considered as direct indicator of damage (Yadav and Mohanpuria 2009). In our investigation, Chl-a, Chl-b, Carotenoids concentrations in the leaves of P. fortunei were enhanced in lower Cu concentration treatments and significantly inhibited by 157 μmol L−1 Cu treatment compared with that of control (Table 2). These findings agreed with other studies in investigating the effects of Cu on different cultivar of Camellia sinensis (Yadav and Mohanpuria 2009; Basak et al. 2001).
Proline is widely considered an osmoregulatory solute and accumulates in plants when exposed to wide variety of environmental stresses and provide stress tolerance (Hare and Cress 1997). Proline is also considered a stabilizer for cellular structures and acts as a free radical scavenger (Hare and Cress 1997). In our experiment, the proline concentration treated with 157 μmol L−1 Cu concentration was 8.3 times higher than that of control (Table 3), which is consistent with that the concentration of proline in Camellia sinensis, increased by 80% under 100 μM CuSO4 exposure (Yadav and Mohanpuria 2009).
The experiment also found that soluble sugar content increased with the increase of Cu concentration (Table 3), suggesting disturbances of carbohydrate regulation. Our result was consistent with earlier study in which Cd was reported to increased soluble carbohydrate concentration and decreased starch concentration in the seedlings (Kim et al. 2003).
The study also found that the activities of the antioxidant enzymes increased to enable plants to protect themselves against the oxidative stress when the plants exposed to stressful conditions, which is evidence that the elevated activity is correlated to increased stress tolerance (Mazhoudi et al. 1997). In our study, activities of the antioxidant enzymes were affected differently in the leaves of P. fortunei (Table 4). The activities of SOD increased significantly as Cu levels increased. The activity of SOD correlated with that of previous studies when enhanced as Cu concentrations increased (Rama Devi and Prasad 1998; Weckx and Clijsters 1996). However, the response of antioxidant enzymes to Cu remains controversial (Mazhoudi et al. 1997). The protective mechanisms adapted by plants to find free radicals and peroxides include several antioxidant enzymes and antioxidant substances (Ke et al. 2007; Zhou et al. 2010; Kováčik et al. 2010b).
Our experimental result suggested that the activity of POD decreased at 15.7 μmol L−1 Cu treatment but significant increased at 78.7 μmol L−1 Cu treatment and then significantly decreased at 157 μmol L−1 Cu treatment compared with that of control (Table 4). The possible reason for the low POD activity at higher Cu concentration treatments may be because of that the plant accumulated higher copper in the leaves where the Cu could inhibit the activity of POD (Mocquot et al. 1996).
Rama Devi and Prasad (1998) reported that CAT activity in Ceratophyllum demersum L. at higher (4 μmol L−1) Cu was lower than 2 μmol L−1, which indicated that CAT activity was inhibited partly by more Cu accumulation. However, in our experiment, the activity of CAT at low Cu concentrations decreased but significantly increased at higher Cu concentrations compared with that of control (Table 4). Higher activities of SOD and CAT in the leaves of P. fortunei indicated that the plants had stronger capacity in scavenging free radicals, and thus could avoid oxidative damage caused by these free radicals. It appears that the P. fortunei can be successfully grown in Cu-enriched areas as we observed at copper mines.
Mining activities have negatively affected lands throughout the world (Plass 2000). As a direct result of the mining operations, soil is destroyed over a considerable area, and the disposal of mined wastes often produces more environmental problems than the mining operations themselves (Fernández-Caliani et al. 2009). The pollutants may be transferred from tailings and waste rock dumps to nearby soils by acid mine drainage and/or atmospheric deposition of wind-blown dust, depending on climatic and hydrologic conditions (Chopin and Alloway 2007). Remediation and re-vegetation of the abandoned mined sites have become major concerns to regional environmental authorities, requiring investment of large financial and human resources.
Because of Cu-limited solubility due to the formation of insoluble precipitates, bioavailable levels of Cu in soils (tailings) are generally low. Compared with the results of Cu concentrations in the roots, stems and leaves of P. fortunei growing in the liquid cultures (Fig. 1) and those in the roots, stems and leaves of P. fortunei growing in the Cu tailings without treatment of acetic acid and EDTA (Fig. 2), it appears that the bioavailable levels of Cu in the tailings were lower than 15.7 μmol L−1. As a result, it is necessary to take steps to increase the bioavailability of Cu for plants’ accumulation and more efficient phyto-remediation.
Organic acids may influence Cu solubility and absorption through their indirect effects on microbial activity, rhizosphere physico-chemical properties, and root growth dynamics, as well as more directly through acidification, complexation, precipitation and oxidation- reduction reactions in the rhizosphere (Cieśliński et al. 1998; Evangelou et al. 2006). Wang et al. (2009) reported that among those eight extractants, the extractable Cu concentrations in soils generally followed the descending order of EDTA > Citric acid > NH4Ac > NH4NO3 > MgCl2 ≈ Tartaric acid > Acetic acid > Oxalic acid. It is also evident that there was a slight correlation between soil Cu concentration obtained by NH4Ac extraction procedures and Cu concentration in the stocks of the wheat plant (Wang et al. 2009).
Although EDTA has been shown in several publications to be effective in enhancing phytoextraction, EDTA and EDTA-heavy metal complexes are toxic to soil microorganisms (Grčman et al. 2001) and to plants because they severely decrease shoot biomass (Epstein et al. 1999). The results in our research showed that lowering acetic acid could promote the growth of the seedlings of P. fortunei and the concentrations of EDTA used in the experiment could inhibit the growth of P. fortunei (Fig. 2). Acetic acid at the experimental levels could promote Cu absorption in the roots of P. fortunei but inhibit the accumulation of Cu in the shoots and leaves of P. fortunei (Fig. 3). Luo et al. (2005) reported that EDTA could significantly enhance Cu accumulation in corn and white bean by severely decreasing the plants’ biomass. We obtained the same results that showed EDTA at the experimental levels significantly enhancing Cu absorption in P. fortunei and translocation into the stems and leaves (Fig. 3). The dry weights of roots, stems and leaves were decreased (Fig. 2).
However, Wu et al. (2004) found that EDTA could significantly enhance Cu accumulation of Indian mustard without toxicity symptoms. Due to its low biodegradability, EDTA may remain absorbed on soil particles, even after soil cleaning (Wasay et al. 1998). Its prolonged presence in the soil and its non-selective nature dramatically increase the leaching risk of heavy metals (Grčman et al. 2003). Kim and Lee (2010) found that EDTA not only increased the ability of barnyard grass (Echinochloa crus-galli) to take up Cd, Cu and Pb but also resulted in increased soil leaching. Conversely, citric acid induced the removal of Cd, Cu and Pb from soil without increasing the risk of leaching.
Although the seedlings grew weakly in all treatments, P. fortunei was a Cu tolerant plant and had the ability to accumulate Cu in the plant, especially when extractants or chelating agents were added. P. fortunei is widely distributed in south of the Yangtze River of China, Laos and Vietnam. The faster, more vigorous growth and deep root system will be highly advantageous when using P. fortunei to stabilize mine tailing deposits in riparian areas along streams and rivers. In order to select the best way to remediate Cu contaminated areas with P. fortunei, more experiments should be conducted. One project has already demonstrated the use of extractants or chelating agents in an optimal concentration for enhancing the absorption of Cu and other heavy metals by P. fortunei. The next experiment to be conducted will cover the rational utilization of fertilizers and leguminous plants to improve the growing conditions. Improved growing conditions could either promote the growth of plants and increase litter fall and organic matter inputs to the tailings or decrease Cu content in the mine tailings by cleaning up the litter fall without the leaching risk of heavy metals. Meanwhile, studies must be conducted regarding other shrubs or trees, native to Jiangxi province to find species that concentrate Cu in aboveground tissues which would make them better suited for restoration of heavy metal contaminated areas.
References
Basak M, Sharma M, Chakraborty U (2001) Biochemical response of Camellia sinensis (L.) O. Kuntze to heavy metal stress. J Environ Biol 22:37–41
Beauchamp Ch, Fridovich I (1971) Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Anal Biochem 44:276–287
Borkan SC, Gullans SR (2002) Molecular chaperones in the kidney. Annu Rev Physiol 64:503–527
Boyter MJ, Brummer JE, Leininger WC (2009) Growth and metal accumulation of geyer and mountain willow grown in topsoil versus amended mine tailings. Water Air Soil Pollut 198:17–29
Chopin EIB, Alloway J (2007) Distribution and mobility of trace elements in soils and vegetation around the mining and smelting areas of Tharsis, Riotinto and Huelva, Iberian Pyrite Belt, SW Spain. Water Air Soil Pollut 182:245–261
Cieśliński G, van Rees KCJ, Szmigielska AM, Krishnamurti GSR, Huang PM (1998) Low-molecular-weight-organic acids in rhizosphere soils of durum wheat and their effect on cadmium bioaccumulation. Plant Soil 203:109–117
Clemens S, Palmgren MG, Krämer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7:309–315
De Vos CHR, Vonk MJ, Vooijs R, Schat H (1992) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98:853–858
Epstein AL, Gussman CD, Blaylock MJ, Yermiyahu U, Huang JW, Kapulnik Y, Orser CS (1999) EDTA and Pb–EDTA accumulation in Brassica juncea grown in Pb-amended soil. Plant Soil 208:87–94
Evangelou MWH, Ebel M, Schaeffer A (2006) Evaluation of the effect of small organic acids on phytoextraction of Cu and Pb from soil with tobacco Nicotiana tabacum. Chemosphere 63:996–1004
Evangelou MWH, Ebel M, Schaeffer A (2007) Chelate assisted phytoextraction of heavy metals from soil Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 68:989–1003
Fang W, Kao CH (2000) Enhanced peroxidase activity in rice leaves in response to excess iron, copper and zinc. Plant Sci 158:71–76
Fernández-Caliani JC, Barba-Brioso C, González I, Galán E (2009) Heavy metal pollution in soils around the abandoned mine sites of the Iberian Pyrite Belt (Southwest Spain). Water Air Soil Pollut 200:211–226
Foyer CH, Lelandais M, Kunert KJ (1994) Photoxidative stress in plants. Physiol Plant 92:696–717
Grčman H, Velikonja-Bolta S, Vodnik D, Kos B, Leštan D (2001) EDTA enhanced heavy metal phytoextraction: metal accumulation, leaching, and toxicity. Plant Soil 235:105–114
Grčman H, Vodnik D, Velikonja-Bolta S, Leštan D (2003) Ethylenediaminedissuccinate as a new chelate for environmentally safe enhanced lead phytoextraction. J Environ Qual 32:500–506
Hakkou R, Benzaazoua M, Bussière B (2008) Acid mine drainage at the Abandoned Kettara Mine (Morocco): 1. Environmental characterization. Mine Water Environ 27:145–159
Han YL, Yuan HY, Huang SZ, Guo Z, Xia B, Gu JG (2007) Cadmium tolerance and accumulation by two species of Iris. Ecotoxicology 16:557–563
Han YL, Huang SZ, Gu JG, Qiu S, Chen JM (2008) Tolerance and accumulation of lead by species of Iris. Ecotoxicology 17:853–859
Hare PD, Cress WA (1997) Metabolic implications of stress induced proline accumulation in plants. Plant Growth Regul 21:79–102
Huang SZ, Han YL, Yu SL, Gu JG, Zhang LL (2011) The effect of lead and copper on the growth and physiological response of water flower Iris pseudacorus L. Fresen Environ Bull 20:2246–2250
Jouili H, El Ferjani E (2003) Changes in antioxidant and lignifying enzyme activities in sunflower roots (Helianthus annuus L.) stressed with copper excess. C R Biol 326:639–644
Jouili H, El Ferjani E (2004) Effect of copper excess on superoxide dismutase, catalase and peroxidase activities in sunflower seedlings (Helianthus annuus L.). Acta Physiol Plant 26:29–35
Ke WS, Xiong ZT, Xie MJ, Luo Q (2007) Accumulation, subcellular localization and ecophysiological responses to copper stress in two Daucus carota L. populations. Plant Soil 292:291–304
Kim SH, Lee IS (2010) Comparison of the ability of organic acids and EDTA to enhance the phytoextraction of metals from a multi-metal contaminated soil. Bull Environ Contam Toxicol 84:255–259
Kim CG, Power SA, Bell JNB (2003) Effects of cadmium and soil type on mineral nutrition and carbon partitioning in seedlings of Pinus sylvestris. Water Air Soil Pollut 145:253–266
Kováčik J, Klejdus B, Hedbavný J, Bačkor M (2010a) Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci 178:307–311
Kováčik J, Klejdus B, Hedbavný J, Bačkor M (2010b) Tolerance of Silene vulgaris to copper: population-related comparison of selected physiological parameters. Environ Toxicol 25:581–592
Li HS, Sun Q, Zhao SJ, Zhang WH, Chen CL, Hong YZ, Xia K, Wang W, Gong PB (2000) Principles and techniques of plant physiological biochemical experimemt. Higher Education Press, Beijing
López-Bucio J, Nieto-Jacobo MF, Ramírez-Rodríguez V, Herrera-Estrella L (2000) Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci 160:1–13
Lu Y, Li XR, He MZ, Zhao X, Liu YB, Cui Y, Pan YX, Tan HJ (2010) Seedlings growth and antioxidative enzymes activities in leaves under heavy metal stress differ between two desert plants: a perennial (Peganum harmala) and an annual (Halogeton glomeratus) grass. Acta Physiol Plant (Online)
Luo C, Shen Z, Li X (2005) Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59:1–11
Lyubenova L, Nehnevajova E, Herzig R, Schröder P (2009) Response of antioxidant enzymes in Nicotiana tabacum clones during phytoextraction of heavy metals. Environ Sci Pollut Res 16:573–581
Mazhoudi S, Chaoui A, Ghorbal MH, Ferjacu EE (1997) Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum Mill.). Plant Sci 127:129–137
McCready RM, Guggolz J, Silviera V, Owens HS (1950) Determination of starch and amylase in vegetables. Anal Chem 22:1156–1158
Mocquot B, Vangronsveld J, Clijsters H, Mench M (1996) Copper toxicity in young maize (Zea mays L.) plants: effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 182:287–300
Plass W (2000) History of surface mining reclamation and associated legislation. In: Barnhisel RI, Daniels WL, Darmody RG (eds) Reclamation of drastically disturbed lands. American Society of Agronomy, Madison, WI, pp 1–20
Rama Devi S, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138(2):157–165
Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8:221–226
Richmond T (2000) The revegetation of metalliferous tailings. In: Barnhisel RI, Daniels WL, Darmody RG (eds) Reclamation of drastically disturbed lands. American Society of Agronomy, Madison, WI, pp 801–818
SAS Institute Inc. (1994) SAS/STAT user’s guide, version 6, 4th edn. SAS Institute Inc., Cary (4th Printing)
Wang HH, Shan XQ, Liu T, Xie YN, Wen B, Zhang SZ, Han F, van Genuchten MT (2007) Organic acids enhance the uptake of lead by wheat roots. Planta 225:1483–1494
Wang SL, Nan ZR, Liu XW, Li Y, Qin S, Ding HX (2009) Accumulation and bioavailability of copper and nickel in wheat plants grown in contaminated soils from the oasis, northwest China. Geoderma 152:290–295
Wasay SA, Barrington SF, Tokunaga S (1998) Remediation of soils polluted by heavy metals using salts of organic acids and chelating agents. Environ Technol 19:369–379
Weckx J, Clijsters H (1996) Oxidative damage and defense mechanisms in primary leaves of Phaseolus vulgaris as a result of root assimilation of toxic amounts of copper. Physiol Plant 96:506–512
Wenzel WW, Unterbrunner R, Sommer P, Sacco P (2003) Chelate-assisted phytoextraction using canola (Brassica napus L.) in outdoors pot and lysimeter experiments. Plant Soil 249:83–96
Wójcik M, Pawlikowska-Pawlega B, Tukiendorf A (2009) Physiological and ultrastructural changes in Arabidopsis thaliana as affected by changed GSH level and Cu excess. Russ J Plant Physiol 56(6):820–829
Wu LH, Luo YM, Xing XR, Christie P (2004) EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agric Ecosyst Environ 102:307–318
Yadav SK, Mohanpuria P (2009) Responses of Camellia sinensis cultivars to Cu and Al stress. Biol Plant 53:737–740
Zhou YQ, Huang SZ, Yu SL, Gu JG, Zhao JZ, Han YL, Fu JJ (2010) The physiological responses and Sub-cellular location of lead and cadmium in Iris pseudacorus L. Ecotoxicology 19:69–76
Acknowledgments
This project was financially supported by the Natural Science Foundation of China (No. 30771520) and the Natural Science Foundation of Jiangxi Province (No. 2008GZN0064).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, ZF., Huang, SZ., Han, YL. et al. Physiological response of Cu and Cu mine tailing remediation of Paulownia fortunei (Seem) Hemsl. Ecotoxicology 21, 759–767 (2012). https://doi.org/10.1007/s10646-011-0836-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0836-5