Abstract
Pyrethroid insecticides containing deltamethrin provide broad spectrum insect control that can adversely affect food supplies of insectivorous birds. I hypothesized that this could result in lowered nest survival for a ground-nesting insectivorous bird, the Mountain Plover (Charadrius montanus), which preferentially nests on prairie dog colonies. I studied Mountain Plover nest survival in 2003–2010 at a small cluster of black-tailed prairie dog (Cynomys ludovicianus) colonies in north-central Montana. Three colonies were treated with deltamethrin to control fleas and limit the spread of plague; four untreated colonies served as controls. I monitored 412 plover nests during the 8 year study (264 on treatment colonies and 148 on control colonies) and found a strong negative effect of deltamethrin treatments on nest survival (βDust = −1.24, 95 % CI was −2.00 to −0.48) in the years following the actual treatment (2004–2006). I conclude that the observed treatment effect most likely occurred because of changes in insect (food) availability for the plover, and this in turn lowered nest survival because adults spent more time off nests or switched to less desirable insect prey. These results lend support to the need to consider the indirect effects of insecticide treatments on non-target species and suggest a potential conflict in current plague management strategies for prairie dogs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impacts of commercial insecticides on target species have been well studied, in contrast to the relatively few studies of indirect impacts to non-target species. Many popular insecticides are highly effective, affect a range of insect Orders, and can have effects that last beyond the actual treatment through disruption of population cycles (Karhu and Anderson 2000; Moreby et al. 2001). There is a long history of the use of organocholrine, organophosphate, and carbamate insecticides to control insects; evidence of direct toxicity to birds includes the well-known effects of DDT (Douthwaite 1995). To reduce toxicity to non-target vertebrates, including birds, a class of pyrethroid insecticides was developed with substantially lower toxicity levels compared to organophosphates and carbamates (Extoxnet 1995; Perry et al. 1998). The LD50 levels of these of pyrethroid insecticides for birds are generally between 1,000 and 10,000 mg kg−1 of body weight (Elliot et al. 1978; Hudson et al. 1984; Extoxnet 1995), which is substantially higher than those for many organophosphate and carbamate insecticides. The pyrethroid insecticide deltamethrin is a broad spectrum insecticide that is highly toxic to a wide range of terrestrial insects and aquatic organisms (Extoxnet 1995). Its broad control of terrestrial insects has led to concerns about indirect effects to insectivorous birds, whose food supplies may be depleted by deltamethrin treatments (Pascual and Peris 1992; Martin et al. 1998; Moreby et al. 2001; Pendleton and Baldwin 2007). Insecticides containing deltamethrin were linked to lower hatching success in Chestnut-collared Longspur nests in Alberta (Martin et al. 1998) but had no effect on reproductive output by the same species in a different study (Martin et al. 2000), declines of Gray Partridge in England (Rands 1985), and local declines in some insectivorous birds in Botswana (Pendleton and Baldwin 2007).
In the North American Great Plains, black-tailed prairie dog (Cynomys ludovicianus) colonies provide habitat for other species as a result of burrowing activity and vegetation change (Miller et al. 2007). The prairie dog is sometimes described as a keystone species (Stapp 1998; Kotliar 2000) and its activities are an important source of soil and vegetation disturbance. Prairie dogs are susceptible to plague, a flea-borne disease caused by the bacterium Yersinia pestis (Gage and Kosoy 2005; Collinge et al. 2005). Plague mortality in infected prairie dogs can exceed 99 % (Cully 1997; Cully and Williams 2001) and result in widespread loss of colonies across large regions. Loss of prairie dog colonies poses a threat to many species of high conservation concern such as the black-footed ferret and may pose greater threats to the Great Plains ecosystem as a whole (Biggins and Kosoy 2001). On the basis of these threats, several methods have been investigated to control the spread of plague in prairie dogs. One of the most promising involves the application of commercial insecticides directly to active prairie dog burrows, where it is transferred to the occupants to control fleas. Early applications involved carbaryl (Barnes et al. 1972) or permethrin (Beard et al. 1992; Karhu 1999) dust, both of which were highly effective at killing fleas but had relatively short half-lives (<3 months). The desire for longer-lasting flea control led to the development of DeltaDust (0.05 % active ingredient deltamethrin; Aventis Environmental Health, Montvale, NJ), which is waterproof and lasts up to 10 months post-application (Seery et al. 2003; Biggins et al. 2010). Such a product is appealing because a single application can provide effective flea control to limit the spread of disease for an entire transmission season (Seery et al. 2003).
The Mountain Plover (Charadrius montanus) is one of a suite of vertebrate species that preferentially occupies active prairie dog colonies (Knowles et al. 1982; Knowles and Knowles 1984). Colonies provide important nesting areas because they contain a mix of short vegetation and abundant insects preferred by the plover (Graul 1975; Knopf and Wunder 2006). The plover is a ground nester, has a typical clutch of three eggs, and occupies colonies in Montana during the April to September breeding season (Dinsmore et al. 2002). It is insectivorous and feeds primarily on insects in the Orders Coleoptera and Orthoptera, although it is known to consume insects outside these groups (Baldwin 1971; Knopf 1998). In north-central Montana, the loss of prairie dog colonies to plague poses a serious threat to the plover because of habitat loss (Dinsmore 2000; Dinsmore et al. 2005; Augustine et al. 2008a) and the control of fleas that carry plague is a short-term conservation measure. There, an estimated 74 % of plovers nest on active black-tailed prairie dog colonies at densities approaching 7 birds/km2, which is the highest density recorded in the species’ breeding range (Childers and Dinsmore 2008). Plague history plays an important role in determining how and when plovers utilize prairie dog colonies (Dinsmore and Smith 2010) and could also affect their local dispersal patterns (Skrade and Dinsmore 2010). Thus, plague is an important driver of Mountain Plover population dynamics in this region, and attempts to manage plague (e.g. on-going efforts to develop an oral plague vaccine for prairie dogs) should seek to minimize negative impacts to non-target species, including the plover.
Here I describe the impacts of DeltaDust application to nesting Mountain Plovers at a small cluster of black-tailed prairie dog colonies in Phillips County, Montana. I monitored seven colonies during an 8 year study and measured responses by plovers as the number of nests per colony and nest survival. I hypothesized that DeltaDust might have broader impacts to the insect community that might negatively affect nesting Mountain Plovers.
Methods
Study area
Mountain Plover responses to DeltaDust treatments were studied on a small complex of black-tailed prairie dog colonies in southern Phillips County, Montana, during a 8 year period (2003–2010; Fig. 1). These colonies are located approximately 1 km south of the Dry Fork Road and 26 km east of its junction with U.S. Highway 191. All of the colonies are in public ownership with the Bureau of Land Management (BLM, Malta Field Office). The study site contains habitat typical of this region, including mixed-grass prairie with flat-topped ridges dissected by shallow coulees and sagebrush flats (Knowles et al. 1982; Olson and Edge 1985; Dinsmore et al. 2002).
Study design
DeltaDust treatments were applied to active black-tailed prairie dog colonies as part of an experimental study of enzootic plague effects on black-footed ferrets (Mustela nigripes), a highly endangered mammal that preys on live prairie dogs (Campbell et al. 1987; Augustine et al. 2008b; Matchett et al. 2010). Specifically, DeltaDust is used to control fleas that carry the flea-borne bacterium Yersinia pestis, which causes plague (Collinge et al. 2005). The loss of prairie dogs to plague reduces the food supply and habitat for the ferret, limiting its’ recovery and reducing nesting habitat for the Mountain Plover. This scenario offered a rare opportunity to monitor possible indirect effects of a broad spectrum insecticide on a terrestrial non-target bird, the Mountain Plover. DeltaDust, a pyrethroid insecticide containing 0.05 % deltamethrin active ingredient, was applied in a powdered dust form directly to prairie dog burrows, where it came in contact with the animal’s fur as it passed through the burrow entrance. Although I did not monitor insects, I predicted that DeltaDust would reduce plover food supplies (insects) and negatively affect nest survival, which is an important measure of reproductive output (Martin et al. 1998; 2000). DeltaDust is known to affect insects in the Orders Coleoptera, Hemiptera, Lepidoptera, and Orthoptera, many of which are food items of the plover. The loss of food could translate into lowered nest survival by forcing adults to spend more time foraging (Cooper et al. 1990; Holmes 1998), switch their diet to less preferred prey items (Sample et al. 1993; Martin et al. 1998), or experience a decline in body condition that could reduce egg production or nest attentiveness (Hunter et al. 1984; Whitmore et al. 1993; Holmes 1998). Mountain Plover chicks are precocial and leave the nest within hours of hatching. I was unable to monitor chick survival and acknowledge that this information would add greatly to an understanding of possible indirect effects of DeltaDust on Mountain Plovers.
The experimental design included a balance of three treatment colonies and four control colonies. The six primary study colonies were chosen by first identifying three pairs of colonies of roughly equal size and topography and with similar densities of prairie dog burrows (Matchett et al. 2010). Within each pair of colonies, one was randomly selected for treatment in 2003–2005 and the other served as a control. Colony B-041 (177 ha, treated) was paired with B-072 (171 ha), B-069 (75 ha, treated) was paired with B-043 (58 ha), and B-045 (28 ha, treated) was paired with B-042 (27 ha, colony sizes from GPS mapping in 2004, Fig 1). Colony B-047 (19 ha) was included as another control colony because it did not visibly differ from other study colonies, was located 1,500 m from the nearest treatment colony (B-041), and brought the total area of non-dusted experimental control colonies to 275 ha compared to the 280 ha treated with deltamethrin.
DeltaDust was applied to treatment colonies during the weeks of 4 August 2003, 19 July 2004, and 17 July 2005. Treatments consisted of a direct application of 4–6 g of DeltaDust per burrow. Treatments were applied using special pressurized dusters (Technicide, Inc.) mounted to ATVs. Each treatment colony was covered in 30 m wide strips, with a follow-up pass on each to ensure complete burrow coverage. DeltaDust was applied to all burrows and as far into the burrow entrance as possible. In part, the timing of dusting in late summer was to reduce impacts to nesting birds, including the plover, and dusting should not have affected nests during the year it was applied because most plover nesting activity is complete by mid-July (Dinsmore et al. 2002). However, I hypothesized that the residual effects of the treatment would affect nesting plovers in the succeeding nesting season(s). Seery et al. (2003), Biggins et al. (2010) documented reduced flea populations at least 10 months post-treatment with deltamethrin, so I assumed that the late summer DeltaDust treatments had a carryover effect the next year and that the first meaningful treatment effect (from a plover perspective) occurred during the 2004 nesting season. This carryover effect assumes that the treatment reduced insect populations such that they did not fully recover until almost a year later. I chose to include 2003 as a pre-treatment year, but did not include earlier years because sample sizes of plover nests were generally low (Table 1). The 2000–2002 nesting seasons had unusually low nest totals for these colonies, and only colonies B-041 and B-072 exceeded three nests in any of these years. Thus, I limited my pre-treatment analysis to a single year (2003) that contained good samples of nests.
Plover and prairie dog monitoring
I monitored plover nesting activity annually between mid-May and late July at each colony from 2003 to 2010. Prairie dog colony boundaries were mapped after the plover nesting season (July–October) with a Trimble Geo XT® handheld GPS unit running TerraSync software (Trimble Navigation Limited Sunnyvale, CA). To do this, I connected straight-line distances between active burrows along the colony perimeter. I later differentially corrected field data using Pathfinder Office Software (Trimble Navigation Limited Sunnyvale, CA) in Montana State Plane (NAD83) units and converted mapping data to shapefiles to calculate the area of each colony in hectares. I used mapping data and field observations of prairie dog activity to infer whether a colony suffered from a plague outbreak during each year of the study.
I visited each colony ≥3 times between mid-May and late July each year to monitor Mountain Plover activity (Dinsmore et al. 2002). On each visit I systematically searched for nests, which I located by following behavioral cues of incubating adults. If I located a nest I returned at 3–5 day intervals to monitor its fate. I floated eggs on each visit to determine nest age (Westerskov 1950; Dinsmore et al. 2002) and trapped and color banded the tending adult. I collected contour feathers from the nest-tending adult and sent them to Avian Biotech International (Tallahassee, FL) to determine gender using molecular techniques described by Dinsmore et al. (2002); sex is not otherwise readily distinguishable in the field in this monomorphic species.
Statistical analyses
I used the nest survival model (Dinsmore et al. 2002) in Program MARK (White and Burnham 1999) to model Mountain Plover nest survival in response to DeltaDust treatments. This model has no goodness-of-fit test, so I relied on model assumptions (Dinsmore et al. 2002) to use this approach; this included assumptions about correctly ageing nests and determining their fates, the independence of nest fates, and minimizing the effects of nest discovery and subsequent visits on nest fate. Previous work with plovers in Phillips County (Dinsmore et al. 2002) found that nest survival was affected by the sex of the nest-tending adult (sex; male-tended nests survive better), daily nest age (age; older nests have greater daily survival), and within-season variation (TT; a quadratic trend was the best fitting model, with survival dropping to a low in mid-June). I combined these factors additively in a baseline model, to which I later added three additional effects, singly and additively:
-
(1)
A dusting effect (Dust). I included this as a nest- and year-specific binary covariate for whether or not the nest was on or off a colony that was dusted. The baseline model was one where this covariate was used in the years 2004–2006 because the treatments were applied after the plover nesting season in 2003–2005. I also considered variations of this dusting effect by including models where the covariate applied to the actual years of the treatments (2003–2005) and to dusted colonies in all years of the study (2004–2010).
-
(2)
Colony effects (Colony). Inasmuch as the sample of colonies was small (n = 7 total), I wanted to also consider possible colony-specific patterns in plover nest survival. Such differences could arise from many factors including subtle habitat differences, variable food resources for a plover, differences in the risk of nest predation (e.g. the presence of black-footed ferrets), and possibly others.
-
(3)
A year effect (Year). I included this effect because many studies have shown annual variation in nest survival of birds, although an earlier 6 year study of Mountain Plover nest survival at this site did not find evidence for year effects (Dinsmore et al. 2002). In the present study, year was important because of possible temporal effects in nest survival arising from the DeltaDust treatments.
I first added the effects of year and colony, and then built three models to account for possible dusting effects last. Competing models were ranked by AIC (Akaike 1973), adjusted for small sample sizes (AICc; Burnham and Anderson 2002). I report model-averaged estimates of effects in the results to account for possible model selection uncertainty (Burnham and Anderson 2002), and then used these estimates to predict nest survival responses.
Results
Mountain Plovers occupied all treatment and control prairie dog colonies before and after treatment with DeltaDust, although not all control colonies were continuously occupied post-treatment. The size and occurrence of plague on treated and control prairie dog colonies differed during the study period. All of the treated colonies slowly increased in size (Fig 2A) while all of the control colonies suffered plague events and had recovered to variable degrees by 2010 (Fig 2B). Suspected plague events occurred on colonies B-072 (2005), B-047 (2005), B-042 (2006 and 2008), and B-043 (2008) during the study.
During the 8 year period I monitored 412 Mountain Plover nests that were usable for nest survival analyses on the seven study prairie dog colonies (Table 1). Most nests were on treatment (n = 264) rather than control (n = 148) colonies. Nests were tended by male (n = 193), female (n = 187), or unknown sex (n = 32) plovers across all years. In general, plovers nested more often but with lower apparent hatching success on treated prairie dog colonies than on control colonies (Table 1).
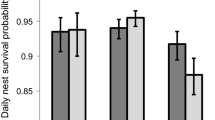
The best model explaining patterns of plover nest survival in response to dusting included the effects of sex of the tending adult, nest age, year, a quadratic seasonal pattern within year, colony, and a dusting treatment on three colonies in 2004–2006 (Table 2). Models that included a dusting effect for all but the treatment year (2004–2010; ΔAICc = 2.98) or the actual years of the treatments (2003–2005; ΔAICc = 3.17) were not well supported. Model-averaged coefficients suggested that there was a strong negative effect of dusting on nest survival (βDust = −1.24, 95 % CI was −2.00 to −0.48) and a weak sex effect (βMale = −0.16, 95 % CI was −0.32 to 0.01) where male-tended nests had lower survival than female-tended nests. There was also evidence of colony variation in nest survival with colonies B-041 and B-043 having lower survival than all other colonies (Table 1). Using model-averaged coefficients for each effect, I predicted the yearly probability that male- and female-tended Mountain Plover nests initiated on 1 June (the median long-term nest initiation date; Dinsmore et al. 2002) would survive the 29 day incubation period on treated and control colonies (Fig. 3). Figure 3 illustrates the lack of a pre-treatment effect in 2003, the strong treatment effect in 2004–2006, and the patterns of yearly variation in nest survival.
Predicted probabilities of a Mountain Plover (Charadrius montanus) nest surviving the 29 day incubation period (1–29 June) on treated (a) and untreated (b) black-tailed prairie dog (Cynomys ludovicianus) colonies in Phillips County, Montana, 2003–2010. The effects are illustrated for colony B-069 (treated colony) and B-072 (control colony). Gray bars indicate male-tended nests and black bars indicate female-tended nests. Error bars indicate the upper limit of symmetrical 95 % confidence intervals
Discussion
In this study there was no evidence for direct toxicity by DeltaDust on nesting Mountain Plovers. The high LD50 levels of pyrethroid insecticides such as DeltaDust on birds (Elliot et al. 1978; Hudson et al. 1984; Extoxnet 1995) suggests that direct toxicity to the plover should be rare or absent, and this was at least partly confirmed because I found no dead adult plovers on or near nests. Plovers could have consumed dying Coleopterans (60 % of diet) or other affected insects, but again I found no evidence that this translated into direct mortality of adults.
The indirect effects of prairie dog flea control on other vertebrates have not been previously studied. After the appearance of sylvatic plague in the Great Plains in the 1980s (Barnes 1982), flea control increased when it was identified as a possible means of reducing the spread of plague and protecting prairie dog colonies (Barnes 1993; Hinkle et al. 1997). Some early control efforts acknowledged the possible negative impacts of deltamethrin to invertebrates (Karhu and Anderson 2000; Moreby et al. 2001), but there was little speculation about how this could impact higher trophic levels such as insectivorous birds.
The few studies of the indirect effects of deltamethrin treatments in insectivorous birds have produced mixed results. Martin et al. (1998) studied Chestnut-collared Longspur nesting success in response to experimental treatments of deltamethrin in Alberta. They found no decline in nesting success in response to deltamethrin treatments, but did find that diet composition of adults changed post-treatment from >85 % grasshoppers to a more diverse diet. In their study, treatments were applied directly to sites with active longspur nests, and nest success included both the incubation and nestling stages in this altricial species. Pendleton and Baldwin (2007) found evidence for declines in some insectivorous birds in Botswana as a result of spraying deltamethrin for tsetse fly control. Other insecticides have also been shown to negatively impact food availability and foraging rates of insectivorous birds (Cooper et al. 1990; Sample et al. 1993) or to have minimal impacts (Holmes 1998). Collectively, these studies illustrate the potential that insecticides, including deltamethrin, have important negative effects on insectivorous birds.
Links to Mountain Plover nest survival
There are several explanations for the differences in plover nest survival between the treated and control colonies. I acknowledge that the sample of experimental colonies was small and may not be representative of plover nesting habitat, although these seven colonies have accounted for 33 % of the nests found in a long-term study (1995–2011) of plovers in Phillips County, Montana (pers. obs.). Regardless, I found clear patterns in plover nest survival between colonies, as well as a strong difference in nest survival between experimental and control colonies. Models where the treatment effect existed in the actual treatment years (2003–2005) or for all years of the study were poorly supported, further supporting a genuine treatment effect in the years 2004–2006. This is strong evidence that there was an effect of deltamethrin on the nest survival of Mountain Plovers using these colonies. These observed patterns could be the result of food depletion from colony treatments by deltamethrin, or from other causes. Below I discuss the most likely interpretation of my results and contrast it with three alternatives.
The most likely cause of treatment effects in this study is that deltamethrin substantially reduced plover food supplies on treated colonies, somehow resulting in lowered nest survival. The plover is an insectivore with a diverse diet, although it generally feeds on insects in the Orders Orthoptera, Lepidoptera, Hymenoptera, and Coleoptera (Knopf 1998). Foraging occurs during the day, although a recent study in Montana demonstrated that nesting plovers are active and probably feeding at night (Skrade and Dinsmore 2012). Their specific nighttime activities are largely unknown, but the duration of off-bouts (many >1 h) suggest that they were feeding forays and not for predator distraction or courtship. An examination of nighttime video of incubating plovers in Montana revealed that many of the birds simply stepped off the nest and immediately began feeding (P. D. B. Skrade, pers. comm.). The few food habits studies of the plover have not found nocturnal burrowing insects in the diet, but this could be hampered by the timing of data collection (daytime only) and the knowledge that food passes through the digestive system quickly (within a few hours). In the only other nesting season study (Baldwin 1971) about 60 % of the diet was Coleopterans (beetles), which are active at night and take refuge during the day belowground or under litter (pers. obs.). The application of deltamethrin significantly reduced numbers of several families of nocturnal burrowing insects in a Utah study, although other families increased and most showed no change (Biggins et al. 2003). If these insects are important food for the plover, their disappearance could increase foraging time for the adult and result in increased nest predation risk. Deltamethrin treatments were applied directly into prairie dog burrows, and thus are expected to impact only the insect community that utilizes these refugia. Documented nest predators of the plover in Montana include coyote (Canis latrans) and Black-billed Magpie (Pica hudsonia), both of which could locate nests visually and would better cue in on nests where the adults were departing more frequently.
There are at least four possible alternative explanations for my findings, none of which is as plausible as food depletion. One alternative is that there were inherent differences between colonies that caused the appearance of responses to deltamethrin treatments. The potential for this problem is enhanced because of the small sample of colonies. Nest survival did not differ between treatment and control colonies in the pre-treatment year (2003), differed as hypothesized during post-treatment years (2004–2006), and then were similar for the final 4 years of the study. A second alternative is that plover nests on treated colonies were subject to increased predation by reintroduced black-footed ferrets. There is no direct evidence to support this claim, although ferrets did tend to cluster on treated colonies for much of this study because plague had eliminated most of their habitat and prey on control colonies (M. R. Matchett, pers. comm.). The ferret is primarily a predator of prairie dogs, although its diet also includes other mammals (Campbell et al. 1987). Bird’s eggs have not been noted in the ferret’s diet, and it seems unlikely that nest losses could be explained solely by the presence of ferrets on these colonies. Thirdly, deltamethrin may have increased prairie dog survival on treated colonies, resulting in greater densities of prairie dogs that attracted predators that also took plover nests. The principle predators of black-tailed prairie dogs in this part of Montana include the black-footed ferret, badger, coyote, Ferruginous Hawk, and Golden Eagle (pers. obs.). The mammals are all potential predators of plover nests, although it seems unlikely that their low population densities and the lower potential energy gain from a clutch of plover eggs when compared to a prairie dog would provide enough incentive for them to selectively prey on plover nests. There is a potential for density dependent predator–prey interactions that might influence nest predations rates differentially on treated and untreated colonies. For example, if prairie dog density is greater on treated colonies, this could attract more predators like the ferret that might opportunistically take bird’s eggs. However, there are no data on prairie dog density for these colonies, and I have assumed that colony area is more important than prairie dog density per se. Lastly, the treatments of deltamethrin may have altered predator–prey relationships and caused a shift to preying on birds’ eggs. Prairie dogs have been suggested as a potentially important bird nest predator, but published literature of prairie dog diet during summer has shown they feed almost exclusively on plant material,; they will take arthropods and other insects occasionally (Summers and Linder 1978; Uresk 1984). Other rodents such as ground squirrels are important bird nest predators (Sargeant et al. 1987) and may have increased in response to deltamethrin treatments. Other known predators of birds’ nests such as long-tailed weasel (Mustela frenata) and bull snake (Pituophis catenifer), both of which occur in the study area, may have increased in response to changes in prairie dog abundance and taken some plover nests.
Regardless of the cause, it is clear that there were differences in Mountain Plover nest survival on this prairie dog colony complex in Montana, and that those differences were associated with patterns of DeltaDust treatments. Nest survival was lower on colonies in the years following DeltaDust treatments; such differences were absent from untreated colonies and from treated colonies in years other than post-treatment years.
Conservation implications
The Mountain Plover is a species of conservation concern in the U.S. (Brown et al. 2001; U.S. Department of the Interior 2010, 2011) and is a focal species for conservation efforts in the Great Plains (Dinsmore 2000). In Montana and elsewhere in the Great Plains they nest on active prairie dog colonies (Knowles et al. 1982; Dinsmore et al. 2002) and are sometimes considered a prairie dog associate. Their attraction to active prairie dog colonies may stem from a combination of low vegetation interspersed with bare ground (Knopf and Wunder 2006), abundant insects that comprise a majority of their diet (Baldwin 1971; Knopf 1998), and possibly predator avoidance.
Deltamethrin is a waterproof insecticide that remains active for up to 10 months after application. It is lethal to a wide range of insects, including ants, beetles, and some winged insects, all of which are included in the diet of the plover. The dust is applied directly into prairie dog burrows, which enhances its possible effects on some of these non-target insects. Nothing in the product label suggests it has direct impacts to the plover, but substantial indirect impacts could arise if food sources are depleted. The length of possible impacts to food supplies is unknown, primarily because only short-term lethal effects of deltamethrin have been well studied. The control of fleas to reduce the impacts of sylvatic plague is an important tool to aid recovery of prairie dogs and associated species like the Mountain Plover (Dinsmore et al. 2005; Dinsmore and Smith 2010) and black-footed ferret (Matchett et al. 2010). Left unmanaged, plague threatens to eliminate prairie dogs from portions of their current range. However, colony treatments with insecticides may have negative impacts on non-target species such as the Mountain Plover. This highlights a potential conflict between managing plague with insecticides to preserve viable prairie dog populations and maintaining healthy invertebrate populations on prairie dog colonies that provide benefits to the entire community.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petran BN, Csaki F (eds) Second international symposium on information theory. Akademiai Kiado, Budapest, pp 267–281
Augustine DJ, Dinsmore SJ, Wunder MB, Dreitz VJ, Knopf FL (2008a) Plovers, prairie dogs and plague: response of a declining grassland bird to black-tailed prairie dog colony dynamics in landscapes affected by plague. Landsc Ecol 23:689–697
Augustine DJ, Matchett MR, Toombs TP, Cully JF Jr, Johnson TL, Sidle JG (2008b) Spatiotemporal dynamics of black-tailed prairie dog colonies affected by plague. Landsc Ecol 23:255–267
Baldwin, PH (1971) Diet of the Mountain Plover at the Pawnee National Grassland, 1970–71. U.S. Int Biol Program, Grassland Biome progress report, Colorado
Barnes AM (1982) Surveillance and control of plague in the United States. In: Edwards MA, McDonnell U (eds) Animal disease in relation to animal conservation. Academic Press, New York, pp 237–270
Barnes AM (1993) A review of plague and its relevance to prairie dog populations and the black-footed ferret. In: Oldemeyer, JL, DE Biggins, BJ Miller, R Crete (eds). Management of prairie dog complexes for the reintroduction of the black-footed ferret. U.S. Fish and Wildlife Service Biological Report, vol 13, pp 28–37
Barnes AM, Ogden LJ, Campos EG (1972) Control of the plague vector, Opisocrostis hirsutus, by treatment of prairie dog (Cynomys ludovicianus) burrows with 2% Carbaryl dust. J Med Entomol 9:330–333
Beard ML, Rose ST, Barnes A, Montenieri J (1992) Control of Oropsylla hirsuta, a plague vector, by treatment of prairie dog burrows with 0.5 % permethrin dust. J Med Entomol 29:25–29
Biggins DE, Kosoy MY (2001) Influences of introduced plague on North American mammals: implications from ecology of plague in Asia. J Mammal 82:906–916
Biggins DE, DiTomaso L, Godbey JL (2003) Abundance and diversity of arthropods on prairie dog colonies treated with deltamethrin to control fleas (Siphonaptera). USGS final report, Fort Collins
Biggins DE, Godbey JL, Gage KL, Carter LG, Montenieri JA (2010) Vector control improves survival of three species of prairie dogs (Cynomys) in areas considered enzootic for plague. Vector Borne Zoonotic Dis 10:17–26
Brown S, Hickey C, Harrington B, Gill R (2001) The U.S. shorebird conservation plan. 2nd edition. Manomet Center for Conservation Sciences, Massachusetts
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Campbell TM, Clark TW, Richardson L, Forrest SC, Houston BR (1987) Food habits of Wyoming Black-footed Ferrets. American Midl Nat 117:208–210
Childers TM, Dinsmore SJ (2008) Density and abundance of Mountain Plovers in northeastern Montana. Wilson J Ornithol 120:700–707
Collinge SK, Johnson WC, Ray C, Matchett R, Grensten J, Cully JF Jr, Gage KL, Kosoy MY, Loye JE, Martin AP (2005) Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landsc Ecol 20:941–955
Cooper RJ, Dodge KM, Martinat PJ, Donahoe SB, Whitmore RC (1990) Effects of diflubenzuron application on eastern deciduous forest birds. J Wildl Manage 54:486–493
Cully JF Jr (1997) Growth and life history changes in Gunnison’s prairie dogs after a plague epizootic. J Mammal 78:146–157
Cully JF Jr, Williams ES (2001) Interspecific comparisons of sylvatic plague in prairie dogs. J Mammal 82:894–905
Dinsmore SJ (2000) Mountain Plover. In: Reading RP, Miller B (eds) Endangered animals: a reference guide to conflicting issues. Greenwood Press, Westport, pp 213–218
Dinsmore SJ, Smith MD (2010) Mountain Plover responses to plague in Montana. Vector Borne Zoonotic Dis 10:37–45
Dinsmore SJ, White GC, Knopf FL (2002) Advanced techniques for modeling avian nest survival. Ecology 83:3476–3488
Dinsmore SJ, White GC, Knopf FL (2005) Mountain Plover population responses to black-tailed prairie dogs in Montana. J Wildl Manage 69:1546–1553
Douthwaite RJ (1995) Occurrence and consequences of DDT residues in woodland birds following tsetse fly spray operations in NW Zimbabwe. J Appl Ecol 32:727–738
Elliot M, Janes NF, Potter C (1978) The future of pyrethroids in insect control. Annu Rev Entomol 23:443–469
Extoxnet (1995) Deltamethrin. http://pmep.cce.cornell.edu/profiles/extoxnet/carbaryl-dicrotophos/deltamethrin-ext.html. Accessed 14 February 2012
Gage KL, Kosoy MY (2005) Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol 50:505–528
Graul WD (1975) Breeding biology of the Mountain Plover. Wilson Bull. 87:6–31
Hinkle NC, Rust MK, Reierson DA (1997) Biorational approaches to flea (Siphonoptera: pulicidae) suppression: present and future. J Agric Entomol 14:309–321
Holmes SB (1998) Reproductive and nest behaviors of Tennessee warblers (Vermivora peregrine) in forests treated with Lepidoptera-specific insecticides. J Appl Ecol 35:185–194
Hudson RH, Tucker RK, Haegele MA (1984) Handbook of toxicity of pesticides to wildlife, 2nd edn. U.S. Fish and Wildlife Service, Washington, DC
Hunter ML, Witham JW, Dow H (1984) Effects of carbaryl induced depression in invertebrate abundance on the growth and behavior of American black duck and mallard ducklings. Can J Zool 62:452–456
Karhu RR (1999) Field trials of technical pyriproxyfen (Nylar) and Pyraperm in black-tailed prairie dog (Cynomys ludovicianus) towns on the Rocky Mountain Arsenal National Wildlife Refuge, Colorado. MS thesis, University of Wyoming, Laramie
Karhu RR, Anderson SH (2000) Effects of pyriproxyfen spray, powder, and oral bait treatments on the relative abundance of non-target arthropods of black-tailed prairie dog (Rodentia: Sciuridae) towns. J Med Entomol 37:612–618
Knopf FL (1998) Food habits of Mountain Plovers wintering in California. Condor 100:382–384
Knopf FL, Wunder MB (2006) Mountain Plover (Charadrius montanus). In: Poole A (ed) The Birds of North America. The Academy of Natural Sciences, Philadelphia, p 211
Knowles CJ, Knowles PR (1984) Additional records of Mountain Plovers using prairie dog towns in Montana. Prairie Nat 16:183–186
Knowles CJ, Stoner CJ, Gieb SP (1982) Selective use of black-tailed prairie dog towns by Mountain Plovers. Condor 84:71–74
Kotliar NB (2000) Application of the new keystone-species concept to prairie dogs: how well does it work? Conserv Biol 14:1715–1721
Martin PA, Johnson DL, Forsyth DL, Hill BD (1998) Indirect effects of the pyrethroid insecticide deltamethrin on reproductive success of Chestnut-collared Longspurs. Ecotoxicology 7:89–97
Martin PA, Johnson DL, Forsyth DJ, Hill BD (2000) Effects of two grasshopper control insecticides on food resources and reproductive success of two species of grassland songbirds. Environ Toxicol Chem 19:2987–2996
Matchett MR, Biggins DE, Carlson V, Powell B, Rocke T (2010) Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in Montana. Vector Borne Zoonotic Dis 10:27–35
Miller BJ, Reading RP, Biggins DE, Detling JK, Forrest SC, Hoogland JL, Javersak J, Miller SD, Proctor J, Truett J, Uresk JW (2007) Prairie dogs: an ecological review and current biopolitics. J Wildl Manage 71:2801–2810
Moreby SJ, Southway S, Barker A, Holland JM (2001) A comparison of the effect of new and established pesticides on nontarget invertebrates of winter wheat fields. Environ Toxicol Chem 20:2243–2254
Olson SL, Edge D (1985) Nest site selection by Mountain Plovers in northcentral Montana. J Range Manage 38:280–282
Pascual JA, Peris SJ (1992) Effects of forest spraying with two application rates of cypermethrin on food supply and breeding success of the blue tit (Parus caeruleus). Environ Toxicol Chem 11:1271–1280
Pendleton FN, Baldwin AH (2007) The effects of spraying deltamethrin for tsetse fly control on insectivorous bird populations in the Okavango Delta, Botswana. African J Ecol 45:566–576
Perry AS, Yamamoto I, Ishaaya I, Perry RY (1998) Insecticides in agriculture and the environment. Springer, Berlin
Rands MRW (1985) Pesticide use on cereals and the survival of grey partridge chicks: a field experiment. J Appl Ecol 2:49–54
Sample BE, Cooper RJ, Whitmore RC (1993) Dietary shifts among song birds from a diflubenzuron-treated forest. Condor 95:616–624
Sargeant AB, Sovada MA, Greenwood RJ (1987) Responses of three prairie ground squirrel species, Spermophilus franklinii, S. richardsonii, and S. tridecemlineatus, to duck eggs. Can Field-Nat 101:95–97
Seery DB, Biggins DE, Montenieri JA, Enscore RE, Tanda DL, Gage KL (2003) Treatment of black-tailed prairie dog burrows with deltamethrin to control fleas (Insecta: Siphonaptera) and plague. J Med Entomol 40:718–722
Skrade PDB, Dinsmore SJ (2010) Sex-related dispersal in the Mountain Plover (Charadrius montanus). Auk 127:671–677
Skrade PDB, Dinsmore SJ (2012) Incubation patterns of a shorebird with rapid multiple clutches, the Mountain Plover (Charadrius montanus). Can J Zool 90:257–266
Stapp P (1998) A reevaluation of the role of prairie dogs in Great Plains grasslands. Conservation Biol 12:1253–1259
Summers A, Linder RL (1978) Food habits of the black-tailed prairie dog in western South Dakota. J Range Manage 31:134–136
U.S. Department of the Interior (2010) Endangered and threatened wildlife and plants: listing the Mountain Plover as threatened. Fed Reg 75:37353–37358
U.S. Department of the Interior (2011) Endangered and threatened wildlife and plants: withdrawal of the proposed rule to list the Mountain Plover as Threatened. Fed Reg 76:27756–27799
Uresk DW (1984) Black-tailed prairie dog food habits and forage relationships in western South Dakota. J Range Manage 37:325–329
Westerskov K (1950) Methods for determining the age of game bird eggs. J Wildl Manage 14:56–67
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139
Whitmore RC, Cooper RJ, Sample BE (1993) Bird fat reductions in forests treated with Dimilin. Environ Toxicol Chem 12:2059–2064
Acknowledgments
I thank Iowa State University (Department of Natural Resource Ecology and Management), the U.S. Bureau of Land Management (Malta Field Office, Montana; annual Challenge Cost Share Grants in 2003–2006), Montana Fish, Wildlife and Parks (Cooperative Agreement in 2006), U.S. Fish and Wildlife Service (Neotropical Migratory Bird Conservation Act Grant #4112), and the World Wildlife Fund (Grant number 3252) for financial support. TM Childers, JJ Grensten, PDB Skrade, and CT Wilcox assisted with fieldwork. I thank FL Knopf, MR Matchett, and two anonymous reviewers for their comments on this manuscript.
Ethical standards
All work conducted as part of this study was approved by the Iowa State University Institutional Animal Care and Use Committee (Protocol #5-06-6129-Q).
Conflict of interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinsmore, S.J. Mountain Plover responses to deltamethrin treatments on prairie dog colonies in Montana. Ecotoxicology 22, 415–424 (2013). https://doi.org/10.1007/s10646-012-1035-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-012-1035-8