Abstract
Cholinesterase (ChE) enzyme activity measurements are widely applied in aquatic organisms for water quality monitoring, especially for pesticide contamination in agricultural watersheds. These biomarkers are amenable to measurement in a variety of species, and are therefore useful for examining effects in model organisms relevant to the ecosystem of interest. However, extensive variation in ChE biochemistry exists among tissues and species. This variation is rarely characterized and may lead to biases in the interpretation of activity determinations. We optimized ChE activity measurement parameters and characterized ChE biochemistry in Sacramento sucker (Catostomus occidentalis), a widely distributed fish native to watersheds of the Central Valley of California. Acetylcholinesterase (AChE) was the predominant ChE present in C. occidentalis brain and muscle, and muscle AChE was most sensitive to diazinon inhibition. Field caging experiments indicated that exposures to ChE-inhibiting pesticides were insufficient to induce neurotoxic effects. However, pesticide usage in the Central Valley is highly variable among years, and long-term monitoring of in-stream effects would be necessary to evaluate trends in pesticide contamination. Recent changes to the State Water Code require agricultural landowners to participate in a regional water quality monitoring plan. As with most regional monitoring plans, measurements of in-stream effects, and effects in resident species, are not scheduled to be included. We suggest that inclusion of biomarker measures would lend important information to the monitoring process, and propose these procedures as a template for adapting ChE activity measurements into region-specific monitoring programs to assess in-stream effects of pesticide contamination on native species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cholinesterase (ChE) enzyme activity determinations in aquatic organisms are an important tool for monitoring and characterizing exposure to agricultural pesticide runoff. Despite application in diverse species (Zinkl et al., 1991; Fulton and Key, 2001), few studies optimize ChE enzyme activity assay parameters or characterize ChE biochemistry in the organism of focus. Butyrylcholinesterase (BuChE; EC 3.1.1.8) and acetylcholinesterase (AChE; EC 3.1.1.7) are two ChE enzymes found in vertebrates. Fish species vary in ChE biochemistry at several levels, including enzyme-specific variance in sensitivity and tissue-specific distributions. Uncharacterized differences in enzyme composition in tissues and species may lead to biases in the interpretation of activity determinations (Sturm et al., 1999; Sturm et al., 2000). Accordingly, species and tissue-specific characterization of enzyme composition and sensitivity to ChE inhibition will lead to strategic selection of measurement endpoints, thereby improving experimental design, preventing biases in the interpretation of results, and allowing for clearer inferences between studies.
Pesticide contamination in the agriculturally-dominated Central Valley watershed of California presents an opportunity for incorporating ChE activity determinations into a regional water quality monitoring program. Contamination by ChE-inhibiting insecticides is of long standing ecotoxicological concern in this watershed due to their widespread use (over 7 million pounds organophosphate and carbamate active ingredient applied in the Central Valley in 2000) and potent toxicity to aquatic life, and accordingly has raised management concerns. The measured concentrations of these pesticides in Central Valley rivers are known to be toxic to standard test organisms such as Ceriodaphnia dubia and Pimephales promelas (Kuivila and Foe, 1995; Werner et al., 2002). In order to enforce the California State Water Code, the Central Valley Regional Water Quality Control Board has recently required agricultural land owners to participate in a regional watershed-scale water quality monitoring plan (Fulton, 2003). Monitoring will include laboratory-based toxicity testing using standard bioassay organisms and analytical chemistry. Although currently proposed monitoring will contribute more thorough bioassay data, the effects of watershed contamination by pesticides on native species, in either the field or the laboratory, are not proposed and have not been examined. Since in-stream effects and effects on resident organisms are not often monitored, the regional plan described above is one example of water quality monitoring in agricultural areas that would benefit from the additional data gained through implementation of biomarker measures. Although the power of biomarkers may often be overstated, they are the best tools available to assess in-stream effects in non-standardized test organisms including native and threatened species. However, in order for biomarkers to be adopted into water quality monitoring programs, standard measures need to be optimized for appropriate model organisms.
The objectives of this study were to optimize ChE enzyme activity assay parameters and characterize ChE biochemistry in Sacramento sucker (Catostomus occidentalis) in order to assess in-stream neurotoxic effects of agricultural runoff in the Central Valley of California. C. occidentalis are an appropriate model for this system since they are one of only a few native species still abundant in the Central Valley, and our data indicate that they are relatively sensitive to ChE inhibition. Although invertebrate species are often most sensitive, recent evidence indicates that pesticide contamination can have direct sublethal effects in vertebrates (Scholz et al., 2000; Moore and Waring, 2001) including C. occidentalis (Whitehead et al., 2004). We applied ChE activity determinations during field experiments to test whether C. occidentalis were exposed to toxic concentrations of pesticides during rainstorm runoff events following applications to orchards. Due to variability in methodologies of the widely applied Ellman assay, and variation among species in ChE biochemistry, we initially modified assay conditions specifically for C. occidentalis. Tissue homogenate dilution factor and substrate concentration were optimized, ChE biochemistry was characterized in brain and muscle, and laboratory exposures to diazinon contrasted relative sensitivities of brain and muscle to ChE inhibition. The following experiments and procedures provide a template for future application of ChE measurements for environmental monitoring in non-standard fish species.
Methods
Animals and maintenance
Sacramento suckers (Catostomus occidentalis) were selected for use in these experiments because they are a cosmopolitan species native to the Sacramento-San Joaquin watershed (Moyle, 2002). Suckers used in the following exposures were 30–50 mm long (juveniles), and were captured by seining from the upper Russian River watershed (Mendocino county, CA) far above any agricultural inputs. Fish were subsequently maintained at the University of California, Davis, Bodega Marine Laboratory (BML) in aerated freshwater flow-through tanks. Water temperatures ranged from 15 to 17 °C, and the fish were fed (No. 3 crumbles, Rangen Inc, ID) ad libidum daily.
Cholinesterase enzyme activity assay
Determinations of ChE activities were performed according to the Ellman (Ellman et al., 1961) colorimetric assay with modifications for use with an automated microplate reader (Bio-Tek automated microplate reader, Model EL3401). Steps involved in optimizing the assay for application in C. occidentalis muscle and brain included optimizing homogenate dilution factor, optimizing substrate concentration, and determining enzyme identities present. Tissue homogenates of brain and muscle were prepared in a glass homogenizer in lysis buffer (0.1 mM NaPO4, pH 8, 0.5% triton X-100) on ice. All reagents were from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise noted.
In a final volume of 320 μl the reaction mixture consisted of 30 μl sample (tissue homogenate), 30 μl acetylthiocholine (ATCh) substrate, and 10 μl 5,5′-dithiobis-2-nitrobenzoic acid (DTNB, 10.3 mM in 0.1 M pH 7.0 sodium phosphate buffer) in each well of a 96-well plate (polystyrene, flat bottom microtiter plates, Dynatech Laboratories, Inc., Chantilly, VA). ATCh substrate concentrations (in 0.1 M pH 8.0 sodium phosphate buffer) were varied (0.5, 1.0, 2.0, 3.0, 5.0, and 10.0 mM in the reaction mixture), as were tissue homogenate dilution factors (tissue weight to final volume ratios 1:10, 1:100, 1:500, and 1:1000), to determine optimal assay conditions for ChE activity determinations for C. occidentalis brain and muscle. Rate of production of 5-thio-2-nitrobenzoic acid anion (TNB) was monitored in a 96-well microplate reader as absorbance of light (at 412 nm) by the reaction mixture over time. Triplicate measurements were made for each sample, measurements were made every 2 min over 10 min at 25 °C, and ChE activity was calculated as μmol ATCh substrate hydrolyzed per minute per gram tissue wet weight. Substrate blanks (no tissue) and tissue blanks (no substrate) were used to correct measured activities. Solutions of DTNB and ATCh were prepared daily. Total protein was measured using the Lowry assay (Lowry et al., 1951), and final activity determinations in exposure experiments were normalized to protein concentrations (μmol/min/mg protein).
In order to determine the identity of ChE enzymes present in C. occidentalis brain and muscle, tissue homogenates were treated with enzyme-specific ChE inhibitors. BW284c51 is a selective inhibitor of the AChEs, whereas iso-OMPA is a selective inhibitor of the BuChEs (Hoffmann et al., 1999). ChE activities were determined over a dose range of 10−3 to 10–9 M for both inhibitors. From a 10.7 mM stock, 30 μl inhibitor in 320 μl assay volume yielded a 10−3 M final concentration. All other concentrations were prepared by serial dilution with dH2O. Inhibitors were incubated in the reaction mixture for 10 min before substrate was added, then enzyme activities determined as described above.
Diazinon spiking exposure
Diazinon is one of the most heavily applied organophosphate pesticides in the Central Valley (California Department of Pesticide Regulation Pesticide Use Database, http://www.cdpr.ca.gov/dprdatabase.htm), and was used to characterize the relative sensitivities of C. occidentalis brain and muscle to cholinesterase inhibition. Diazinon (99% pure) was procured from Chem Service Inc., PA. Exposure duration was 96 h, with water changes every 48 h. Fish were exposed to 3.5 l of total solution, individually in 4.0 l glass jars, with eight replicate jars per treatment. Treatments were (1) BML well water (control, 0.0 μg/l diazinon), (2) 5.0 μg/l diazinon, (3) 10.0 μg/l diazinon, (4) 50.0 μg/l diazinon, and (5) 100.0 μg/l diazinon. Water temperature was maintained at 16 °C (±1 °C) in a temperature-controlled water bath. Lighting was ambient and fish were fed daily. Fish were randomized into vessels, and vessels randomized into the bath. Dissolved oxygen, pH, ammonia, and temperature were monitored throughout the experiment. Upon test termination, fish were immediately sacrificed by severing the spinal cord, weighed, and brain and body muscle excised and stored at −70 °C for subsequent enzyme activity determinations. ChE enzyme activities (modified Ellman assay) were measured in muscle and brain.
Enzyme activity data were converted to percent control-treatment activity and subjected to two-way analysis of variance (ANOVA), with diazinon dose and tissue type as factors, and results were determined to be statistically significant if p < 0.05. If significance was determined, the Tukey multiple comparison test was used to distinguish among means. All data passed the test of equal variance (Levene Median test), but not all data were normally distributed (Kolmogorov–Smirnov test). The normality test failed in the two-way ANOVA (p = 0.004) and means were correlated with variances (data not shown). Activity data were therefore log10-transformed, treatment means were recalculated as antilog[mean of transformed variables], and 95% confidence intervals of transformed data were computed and back-transformed according to Sokal and Rohlf (Sokal and Rohlf, 2001). The ANOVA on transformed data passed the normality test (p = 0.131). Analysis of covariance (ANCOVA) was used to test whether slopes of linearized (semi-log transformation) percent activity dose-response curves were significantly different between muscle and brain. This would indicate differences in sensitivity to diazinon-induced ChE inhibition between tissues. Tissue was set as the independent variable, with log10-transformed percent activity as the dependent variable, and dose as the covariable. Interaction between dose and tissue was considered significant if p < 0.05. One-way analysis ANOVA tested whether mean body weights differed between treatment groups.
Field caging exposure
C. occidentalis were caged during January/February 2000 in rivers of the heavily agriculturalized watershed of California’s Central Valley according to the experimental design previously described (Whitehead et al., 2004). Briefly, exposures were timed to coincide with runoff from the first major rainstorm event following winter-season application of insecticides to orchards. Multiple cages with eight replicate fish per cage were deployed at three field sites including the San Joaquin River at Vernalis (SJ; downstream of agriculture; 3 cages), Orestimba Creek at River Road (OD; downstream of agriculture; 2 cages), and the reference site Orestimba Creek at Orestimba Road (RF; upstream of all agriculture; 4 cages). Individual cages were retrieved at different timepoints during the runoff event to capture changing conditions during the rise and fall of pesticide concentrations (Fig. 3). Differences in timing of cage deployment and retrieval between SJ and OD reflect differences in hydrological characteristics of these drainages.
Laboratory exposures to field-collected waters were performed concurrent with field caging experiments. Collection of composite water samples from field sites was timed to coincide with predicted peak pesticide concentrations. The SJ composite was composed of river water collected from February 14 to February 16. The OD composite was collected from late night and early morning of February 13 and 14, respectively. The RF (reference) sample was collected on February 14. Field waters were transported in stainless steel soda kegs to BML and maintained at 15 °C in a temperature-controlled room. C. occidentalis were exposed to field-collected samples for six days at 15 ± 1 °C in order to match temperatures observed in the field, light exposure was maintained on a natural cycle, water changes were conducted every 48 h, and water quality (dissolved oxygen, pH, ammonia, temperature) was monitored daily. Eight replicate fish were included for each treatment, and fish were each exposed individually in 3.5 l of treatment water in 4.0 l glass jars. Treatments included exposure to control water (BML water to which fish were acclimated before all experiments), and OD, SJ, and RF field-collected waters.
Following laboratory and field exposures, fish were sacrificed, weighed, and muscle tissue excised and archived at −70 °C for subsequent ChE enzyme activity determinations. For field exposures, two-way ANOVA was applied to test for mean differences in muscle ChE enzyme activities among sites (factor 1), and across timepoints (factor 2). For the laboratory exposure to field-collected waters, one-way ANOVA was applied to test for mean differences in muscle ChE activities between treatments.
Analytical chemistry tested for the presence of 26 pesticides in water samples collected concurrent with caging exposures at all three field sites. Details of water chemistry sampling and analytical chemistry methods have been previously reported (Orlando et al., 2003; Whitehead et al., 2004). Briefly, water samples were collected in 1-l amber glass bottles by mid-channel surface grabs or by pumping, filtered through 0.7 micron glass fiber filters, extracted using C8 solid-phase extraction cartridges, and stored frozen. Upon analysis, cartridges were eluted with 9 ml ethyl-acetate and analyzed using a Varian Saturn GC/MS.
Results
ChE enzyme activity assay optimization
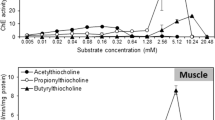
Assay substrate concentration and tissue homogenate dilution factor were optimized for ChE enzyme activity determinations in C. occidentalis brain and muscle. ChE activity was highest at 2.0 mM ATChE substrate in the reaction mixture for both tissues, and this concentration was used for all subsequent ChE activity determinations. Of homogenate dilutions tested, the muscle and brain tissue weight to homogenization buffer volume ratio of 1:500 was within the linear range for substrate hydrolysis rate measurements (data not shown), and was used for all subsequent ChE activity determinations.
Inhibitor assays identified ChE enzymes present in C. occidentalis brain and muscle. The BuChE-specific inhibitor iso-OMPA did not inhibit ChE activity in brain (Fig. 1a) or muscle (Fig. 1b), whereas the AChE-specific inhibitor BW284c51 decreased ChE activity following a dose-response to zero activity. These data indicate that AChE is the predominant ChE present in C. occidentalis muscle and brain. Accordingly, all of the following enzyme activity determinations are described as AChE activity (rather than total ChE activity).
Diazinon spiking exposure
Upon exposure to the highest dose tested (100 μg/l dizainon), 50% mortality was observed within 24 h, with 100% mortality by 96 h. With the exception of one mortality at 48 h in the 50 μg/l dose, all fish in all other treatments survived to experiment termination at 96 h. From these data, the 96-h LC50 for C. occidentalis was 51.6 μg/l. Mean body weights among treatment groups did not differ (p = 0.338).
AChEs from both brain and muscle were inhibited by diazinon in a dose-response manner, and varied in their sensitivity to diazinon inhibition. For each tissue, AChE activities were significantly lower in all treatments relative to the control (Fig. 2). Muscle activities were significantly lower than brain activities above 5 μg/l diazinon. Analysis of covariance detected significant interaction between dose and tissue (F (1,55) = 5.923, p = 0.018), indicating that slopes of log10-transformed percent activity data were heterogeneous among tissues. Although differences between tissues are not large, these data indicate that muscle AChE was more sensitive to diazinon-induced inhibition than brain AChE.
C. occidentalis AChE activities brain (solid line) and muscle (dashed line) in response to four-day diazinon exposure. Data are mean percent of control (0.0 ppm diazinon) brain and liver AChE enzyme activities (μmol/min/mg protein), with associated 95% confidence limits, after back-transformation of log10-transformation of data. Same letter indicates no significant (p < 0.05) differences among mean percent AChE activities determined by the Tukey test following two-way ANOVA.
Field experiments
Reflective of watershed size and structure, change in the hydrograph following a storm event on February 13th 2000 at Orestimba Creek sites was steep and rapid, whereas change in the hydrograph at the San Joaquin site was comparatively broad. Accordingly, the pesticide profiles at OD were steep and short, while profiles at SJ were comparatively shallow and broad (Fig. 3b and c). At OD, exposure of caged fish to ChE-inhibiting pesticides during the storm runoff event was minimal due to low concentrations and short duration of exposure, and minimal at SJ due to even lower concentrations. Chlorpyrifos, diazinon, and methidathion (organophosphate insecticides) were the only measured ChE-inhibiting pesticides detected. Total ChE-inhibiting pesticide concentrations reached a maximum of 277 ng/l for less than three hours at OD, and did not exceed 78.3 ng/l at SJ (Fig. 3). No pesticides were detected at the RF reference site. Other ChE inhibitors measured but not detected include the organophosphates azinphos-methyl, fonofos, malathion, methyl parathion, phosmet, and sulfotep, and the carbamates carbaryl and carbofuran (Orlando et al., 2003; Whitehead et al., 2004).
Muscle AChE enzyme activities from C. occidentalis caged during a storm event. Rainfall (a) is represented in inches. Hatched bars represent mean muscle AChE activity (μmol/min/mg protein) (±SD) in fish caged at (b) Orestimba Creek at River Road (OD), and (c) San Joaquin River near Vernalis (SJ). Solid bars represent AChE activities in fish caged at the RF reference site. All cages were deployed on February 13 and retrieved at various times during the runoff event. Line graph indicates total concentration (ng/l) of ChE-inhibiting pesticides detected in water samples from (a) OD and (b) SJ. Same letter indicates no significant (p < 0.05) differences among mean AChE activities determined by the Tukey test following two-way ANOVA.
Muscle AChE enzyme activities from C. occidentalis caged during laboratory exposures to field-collected water. Bars represent mean muscle AChE activity (μmol/min/mg protein) (±SD) in fish exposed to water in which the fish were maintained at BML (control water, CON) and composite field-collected water samples from the field reference site (RF), Orestimba Creek at River Road (OD), and San Joaquin River near Vernalis (SJ). Triangles indicate total concentration (ng/l) of ChE-inhibiting pesticides detected in each water sample.
Lower muscle AChE enzyme activities were detected in caged fish exposed to peak pesticide concentrations in Orestimba Creek (OD) and the San Joaquin River (SJ) compared to the reference site (RF) (Fig. 3). Immediately following peak organophosphate pesticide concentrations of 277 ng/l at OD, AChE activities were significantly lower (p = 0.002) compared to fish caged at the RF reference site (Fig. 3b). Six days after peak pesticide concentrations there was no significant difference in enzyme activities among sites (p = 0.073). Immediately following peak pesticide concentrations of 78.3 ng/l at SJ, AChE activities were significantly lower (p < 0.001) compared to fish caged at the RF reference site (Fig. 3c). At four and ten days following peak pesticide concentrations there was no significant difference in enzyme activities among sites (p = 0.191, and p = 0.984, respectively). Mean body weights among treatment groups did not differ (p = 0.239). Mean activities did not vary among treatments (p = 0.445) included in laboratory exposures to field-collected waters (Fig. 4).
Discussion
Biomarkers in general present several advantages over other monitoring approaches, such as offering a measure of exposure integrated over space and time, and indicating the bioavailable portion of exposed dose reaching the target site for toxicity (Huggett et al., 1992). If monitoring goals include real-world characterization of exposure to pesticide inputs, or characterization of exposure to specific groups of organisms such as at-risk species, then biomarkers applicable to non-standard organisms in the field also offer important benefits not available to other approaches such as bioassays. Since the ChEs are fundamental components of animal nervous systems and are the primary targets of toxicity for organophosphate and carbamate insecticides, ChE activity measures offer widespread applicability for field studies involving field-relevant non-standard test organisms. However, application of ChE activity measures requires species-specific biochemical characterization and assay optimization.
Assay optimization
Initial experiments optimized the modified Ellman assay for application to C. occidentalis brain and muscle. Since AChE may be inhibited at high substrate concentrations, enzyme activity was tested over an acetylthiocholine substrate concentration range of 0.5–10.0 mM, and 2.0 mM provided for highest activity in both brain and muscle of C. occidentalis. Most studies do not report assay optimization, suggesting that such manipulations may not have been performed. Of those studies that have reported the effects of substrate concentration on ChE activities, our results are similar. Sandahl and Jenkins (Sandahl and Jenkins, 2002) tested ATCh substrate concentrations in the range of 0.1–20.0 mM, and found AChE activity in Oncorhynchus mykiss brain to peak between 2.0 and 5.0 mM, and Sturm et al. (1999) selected the substrate concentration of 1.0 M for activity determinations after testing substrate ranges from 0.05 to 12.0 mM.
Species-specific characterization
C. occidentalis were relatively sensitive to diazinon toxicity compared to other fish species. Although a greater dose range is preferable for LC50 calculation, our data indicate that the 96-h LC50 for C. occidentalis was between 50 and 100 ppb. In contrast, acute LC50 values for other fish range from 90 ppb for Oncorhynchus mykiss to 3,100 ppb for Channa punctatus (World Health Organization, Environmental Health Criteria Monographs; http://www.inchem.org/pages/ehc.html). Rangefinding experiments are helpful for characterizing relative sensitivity when selecting model organisms from species available for study in the watershed of interest. Such experiments also identify sensitivity differences among tissues in order to focus resources and efforts.
Patterns of ChE enzyme composition among tissues are not consistent between fish species, and sufficient characterization for species-specific applications is lacking in the literature, especially considering the widespread application of activity measurements as biomarkers of exposure. Our results indicate that AChE is the predominant ChE enzyme in C. occidentalis brain and muscle, and that BuChE does not contribute to overall ChE activity in either tissue. AChE is typically the predominant ChE enzyme present in brains of most fish species including Oncorhynchus mykiss (Sandahl and Jenkins, 2002) and three species of marine teleosts (Sturm et al., 1999). In contrast to brain, both AChE and BuChE contributed to ChE activity in muscle of the same three marine teleosts. Both BuChE and AChE contribute to ChE activity in muscle of many marine fish species, and BuChE can be much more sensitive to inhibition from organophosphates than AChE (Sturm et al., 1999; Sturm et al., 2000). However, while the importance of AChE in neurotransmission is well established, the physiological role of BuChE remains unclear (Hoffmann et al., 1999). Sturm et al. (Sturm et al., 1999) emphasize that although BuChE activity may be a sensitive biomarker of exposure, inhibition may offer only a weak relation to toxic effects. This reinforces the importance of characterizing ChE biochemistry in the species and tissue of study, thereby preventing bias in the interpretation of results.
Decisions to focus resources of an experiment on the most sensitive tissue, or on the most ecotoxicologically relevant tissue, depend on the nature of the question. Some evidence indicates that ChE inhibition in muscle is a better predictor of induced mortality than in brain, but that brain inhibition may be a better predictor of behavioral effects (Fulton and Key, 2001). However, for studies aiming to monitor trends in pesticide contamination of watersheds, it may be more appropriate to include measurements in the tissue most sensitive to inhibition. Muscle AChEs were more sensitive to inhibition by diazinon than brain AChEs in C. occidentalis. In four other fish species, muscle has also been identified as the most sensitive tissue (Sturm et al., 1999; Sturm et al., 2000), but high sensitivity was attributable to differences in BuChE content among tissues in these species. Since muscle was most sensitive in C. occidentalis, this tissue was most likely to detect exposure to low concentrations of organophosphates and carbamates in the field.
Field experiments
AChE enzyme activity data from field exposures in 2000 indicated that, immediately following peak organophosphate concentrations, enzyme activity was lower in fish caged at both SJ and OD relative to those caged at the reference site (Fig. 3). Enzyme activities in fish retrieved from all other timepoints at SJ and OD were not statistically different from activities in fish from the reference site. One interpretation of these data is that the pesticide pulse induced inhibition of enzyme activity in recently exposed fish, and activities recovered in fish retrieved several days later. However, upon consideration of the entire data set, this interpretation should be treated with caution. Enzyme activities did not drop then “recover” following exposure back up to reference levels. Rather, variability among timepoints at the RF reference site resulted in no significant differences between exposed-site versus reference-site activity at later timepoints. Furthermore, AChE enzyme inhibition was not induced in fish exposed in the laboratory to field-collected waters.
The absence of AChE enzyme inhibition in field-caged fish may be explained by insufficient concentrations or exposure time to organophosphate and carbamate insecticides present in runoff. Elsewhere, field studies have reported ChE enzyme inhibition in test fish exposed to organophosphate and carbamate insecticides in environmental samples at concentration ranges and exposure times higher than we observed (Gruber and Munn, 1998; Sturm et al., 2000). However, differences in observed effect levels are difficult to compare among studies due to variation in sensitivity among species, variation in compositions of pesticide mixtures, and differences in exposure conditions. Given that organophosphate pesticide concentrations of up to 3840 ng/l (diazinon + chlorpyrifos) and 1240 ng/l (diazinon + methidathion + chlorpyrifos) were detected in 1993 at OD and SJ, respectively (Kuivila and Foe, 1995; Domagalski et al., 1997), and given that application of organophosphate insecticides has decreased over 32% from 1995 to 2000 (California Department of Pesticide Regulation Pesticide Use Database, http://www.cdpr.ca.gov/dprdatabase.htm), it is possible that exposures to dormant season runoff events in previous years were sufficient to induce toxic responses in exposed fish, and that decreased application since 1995 has helped mitigate these effects. Current results indicating little or no cholinesterase enzyme inhibition in fish exposed to runoff events could have been more clearly interpreted in relation to mitigation efforts had monitoring been part of a regional plan throughout this period. Field-caging experiments integrated into a regional monitoring program can provide meaningful real-world insights into long-term regional trends in pesticide application and contamination mitigation efforts. Regional agricultural runoff monitoring efforts should incorporate such measures so that in-stream toxicity trends can be predicted and tested, and mitigation efforts evaluated in organisms that are relevant to the system under scrutiny.
Conclusions
ChE activity measurements are highly specific biomarkers for exposure to organophosphate and carbamate insecticides. These measures can be applied in non-standard test organisms making them suitable for incorporation into regional monitoring efforts to assess in-stream effects of agricultural runoff. However, species-specific differences require characterization of different ChE enzymes present in various tissues, characterization of tissue and species-specific differences in sensitivity to ChE inhibition, and require assay parameter optimization. C. occidentalis is a widely-distributed species native to the heavily agriculturalized region of the Central Valley of California, and may therefore be amenable to inclusion in in-stream monitoring efforts. Data presented here indicate that AChE is the primary ChE enzyme present in C. occidentalis brain and muscle, and that muscle AChE is more sensitive to inhibition by diazinon than brain. Field caging of C. occidentalis during the first major runoff event following winter-season pesticide applications indicated that organophosphates and carbamates did not reach sufficient concentrations for a sufficient duration of exposure to induce muscle AChE inhibition. Previous studies at the same field sites have indicated that pesticide contamination can reach higher concentrations (Kuivila and Foe, 1995; Domagalski et al., 1997), and monitoring is needed to evaluate resulting effects. Biomarker measurements, strategically incorporated into a regional monitoring program, could test relationships between changing patterns of pesticide application or runoff mitigation efforts and in-stream toxicity.
References
Domagalski J.L., Dubrovsky N.M., Kratzer C.R., (1997). Pesticides in the San Joaquin River, California: inputs from the dormant sprayed orchards J. Environ. Quality 26:454–65
Ellman G.L., Courtney K.D., Andres V.J., Featherstone R.M., (1961). A new and rapid colorimetric determination of cholinesterase activity Biochem. Pharmacol. 7:88–95
Fulton A. 2003. Ag water and land resource manager. 4
Fulton M.H., Key P.B., (2001). Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects Environ. Toxicol. Chem. 20:37–45
Gruber S.J., Munn M.D., (1998). Organophosphate and carbamate insecticides in agricultural waters and cholinesterase (ChE) inhibition in common carp (Cyprinus carpio) Arch. Environ. Con. Toxicol. 35:391–6
Hoffmann W.E., Solter P.F., Wilson B.W., (1999). Clinical enzymology In Loeb W.F., Quimby F.W., (Eds) The Clinical Chemistry of Laboratory Animals 2 Taylor and Francis Philadelphia, PA, USA 399–454
Huggett R.J., Kimerle R.A., Mehrle P.M. Jr., Bergman H.L., (eds) (1992). Biomarkers: Biochemical, Physiological, and Histological Markers of Anthropogenic Stress. SETAC Special Publications Series Lewis Publishers, Boca Raton
Kuivila K.M., Foe C.G., (1995). Concentration, transport and biological effects of dormant spray pesticides in the San Francisco Estuary, California Environ. Toxicol. Chem. 14:1141–50
Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J., (1951). Protein measurement with the Folin phenol reagent J. Biol. Chem. 193:265–75
Moore A., Waring C.P., (2001). The effects of a synthetic pyrethroid pesticide on some aspects of reproduction in Atlantic salmon (Salmo salar L.) Aquat. Toxicol. (Amsterdam) 52:1–12
Moyle P.B., (2002). Inland Fishes of California University of California Press Berkeley
Orlando J.L., Kuivila K.M. and Whitehead A. (2003). Dissolved pesticide concentrations detected in storm-water runoff at selected sites in the San Joaquin River basin 2000–2001. Sacramento, CA, United States Geological Survey, Open File Report
Sandahl J.F., Jenkins J.J., (2002). Pacific steelhead (Oncorhynchus mykiss) exposed to chlorpyrifos: benchmark concentration estimates for acetylcholinesterase inhibition Environ. Toxicol. Chem. 21:2452–8
Scholz N.L., Truelove N.K., French B.L., Berejikian B.A., Quinn T.P., Casillas E., Collier T.K., (2000). Diazinon disrupts antipredator and homing behaviors in chinook salmon (Oncorhynchus tshawytscha) Can. J. Fisheries Aquat. Sci. 57:1911–8
Sokal R.R., Rohlf F.J., (2001). Biometry New York W.H. Freeman and Company
Sturm A., da Silva de Assis H.C., Hansen P.D., (1999). Cholinesterases of marine teleost fish: enzymological characterization and potential use in the monitoring of neurotoxic contamination Mar. Environ. Res. 47:389–98
Sturm A., Wogram J., Segner H., Liess M., (2000). Different sensitivity to organophosphates of acetylcholinesterase and butyrylcholinesterase from three-spined stickleback (Gasterosteus aculeatus): application in biomonitoring Environ. Toxicol. Chem. 19:1607–15
Werner I., Deanovic L.A., Hinton D.E., Henderson J.D., de Oliveira G.H., Wilson B.W., Krueger W., Wallender W.W., Oliver M.N., Zalom F.G., (2002). Toxicity of stormwater runoff after dormant spray application of diazinon and esfenvalerate (Asana®) in a French Prune orchard, Glenn County, California, USA Bull. Environ. Con. Toxicol. 68:29–36
Whitehead A., Kuivila K.M., Orlando J.L., Kotelevtsev S., Anderson S.L., (2004). Genotoxicity in native fish associated with agricultural runoff events Environ. Toxicol. Chem. 23:2868–77
Zinkl J.G., Lockhart W.L., Kenny S.A., Ward F.J., (1991). The effects of cholinesterase inhibiting insecticides on fish In Mineau P., (Eds) Cholinesterase Inhibiting Insecticides: their Impact on Wildlife and the Environment. Vol 2 New York, NY, USA: Elsevier, pp. 255–75, Chemicals in Agriculture
Acknowledgment
Thanks to J. Henderson for assistance in Ellman assay optimizations. K. Marlow, W. Rose, J. Machula, E. Moon, and T. McCrummen were of valuable assistance in executing laboratory and field experiments. K. Kuivila and J. Orlando performed all analytical chemistry and were of great help in the field. Special thanks to two anonymous reviewers for helpful comments on the manuscript. A. Whitehead is grateful for fellowship support from the Natural Sciences and Engineering Research Council (NSERC) of Canada, and the University of California Toxic Substances Research and Teaching Program, Lead Campus Program in Ecotoxicology. This study was supported by a U.S. Environmental Protection Agency (EPA) Science to Achieve Results (STAR) Grant (98-NCERQA-D1) awarded to S. Anderson, B. May, D. Hinton, and K. Kuivila, with additional support from the U.S. Geological Survey Toxic Substances Hydrology Program. Although this study has been funded in part by the U.S. EPA, it does not necessarily reflect the views of the Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whitehead, A., Anderson, S.L., Ramirez, A. et al. Cholinesterases in Aquatic Biomonitoring: Assay Optimization and Species-Specific Characterization for a California Native Fish. Ecotoxicology 14, 597–606 (2005). https://doi.org/10.1007/s10646-005-0010-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-005-0010-z