Abstract
Otolith shape variation in the Ornate goby, Istigobius ornatus (Teleostei: Gobiidae), collected along the intertidal coasts of the Persian Gulf and Oman Sea, was analysed using Wavelet transform technique in the ShapeR package to determine population differentiation and structure. There were significant differences (P < 0.001, ANOVA) among geographically distant populations based on the variation in the anterior and posterior otolith rims. However, otolith shapes of neighbouring populations were more similar to each other (P > 0.01). Mantel test showed a positive correlation between the Euclidean distance of otolith shape and geographical distances among populations (r = 0.93, P < 0.002). This indicates that levels in otolith shape resemblance between populations are dependent on geographic distance. Different scenarios are discussed to explain the pattern of otolith shape variation and population structure. Among possible key mechanisms responsible for population differentiations are isolation by distance, Late Pleistocene sea level fluctuations, and ecological and geographical differences between the studied locations. This study highlights otolith shape efficiency as an exceptionally convenient morphological marker to study intraspecific-level evolutionary and contemporary phenomena in marine fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Characterizing intraspecific variation is highly important to understand distributional range, migration behavior, ecology, systematics and genealogy of a species (Bohlen 2008; Libungan et al. 2015a). Many fish species show considerable intraspecific variation in their morphology, both among and within populations, which often is environmentally induced or behaviorally influenced (Turan 2006; Kocovsky et al. 2013).
Otoliths are hard structures, functionally associated with hearing and the sense of balance (Popper and Coombs 1982; Ramcharitar et al. 2006; Schulz-Mirbach et al. 2019), acoustic communication (Popper and Lu 2000; Cruz and Lombarte 2004), feeding strategy (Lombarte et al. 2010), swimming (Volpedo and Echeverría 2003; Volpedo et al. 2008) and spatial distribution (Gauldie and Crampton 2002; Lombarte and Cruz 2007; Sadighzadeh et al. 2014). Otolith analysis has made significant contributions to the understanding of evolution and phylogenetic relationships of different teleosts (e.g. Gaemers 1983; Nolf 1985; Reichenbacher et al. 2007; Reichenbacher et al. 2009a, b; Teimori et al. 2012a, b; Nolf 2013; Teimori et al. 2019), interpretation and explanation of historical fisheries (Van Neer et al. 2002; Limburg et al. 2008), ages and growth rates (e.g. Campana et al. 1985; Green et al. 2009), migration and duration of larval phases (e.g. Lord et al. 2010; Avigliano et al. 2014), as well as several other topics (see Vahed et al. 2018; Motamedi et al. 2019; Vahed et al. 2019). Otoliths can be found in plenty as fossils and are used to reconstruct ancient teleost fish diversity, zoogeography and evolution (e.g. Nolf 1995; Girone and Nolf 2009; Bratishko et al. 2015).
Sagittae, the largest otoliths in most teleosts, have been used extensively in taxonomic (e.g. L’Abée-Lund and Jensen 1993; Ponton 2006; Tuset et al. 2006) and phylogenetic studies in several groups of teleosts (e.g. Gaemers 1983; Nolf 1985; Monteiro et al. 2005; Lombarte et al. 2018; Teimori et al. 2019). Additionally, morphological variability of otoliths is a useful tool to measure fish population structure and to discriminate stocks (Burke et al. 2008; Lord et al. 2011; Reichenbacher and Reichard 2014; Libungan et al. 2015b; Libungan et al. 2016; Wujdi et al. 2017; Rashidabadi et al. 2019). The otolith shape is usually species specific, but intraspecific geographic variation may occur due to environmental factors (Mosegaard et al. 1988; Campana and Thorrold 2001; Reichenbacher et al. 2009a; Libungan et al. 2015a, b). While environmental factors essentially modify the otolith growth rate, which in turn alters the general otolith shape (mainly the length/height ratio), genetically induced changes may locally change otolith shape (Mosegaard et al. 1988; Cardinale et al. 2004; Vignon and Morat 2010; Berg et al. 2018). The extent to which these variations are genetically or environmentally stimulated usually remained unexplored (Cardinale et al. 2004; Annabi et al. 2013).
Three methods to study otolith shape have been applied traditionally: morphometry, landmarks and outline (Rohlf 1990). The outline method using the ShapeR package was first developed and applied for otolith analysis in the Atlantic herring Clupea harengus Linnaeus, 1758, but also has been recommended for other teleost fishes (Libungan et al. 2015a, b). Here, we used and tested this method on a gobiid species from the Persian Gulf and Oman Sea.
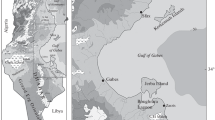
The Ornate goby, Istigobius ornatus (Rüppell, 1830) is widely distributed throughout the tropical Indo-West Pacific including the Persian Gulf and Oman Sea (Maugé 1984; Randall et al. 2003). It is a benthic spawner species that inhabits lower estuaries, mangroves, silty areas and crevices or interstices in rocky substrate with rubble or muddy substrate and, like other reef gobies, is detritivorous in addition to carnivorous (Cole 1990; Chiu et al. 2018). Due to cryptic behavior, its population structure, phylogeography and ecology along the coasts of the Persian Gulf and the Oman Sea largely remained unexplored. The Persian Gulf and Oman Sea, due to their known paleoclimatic history (Lambeck 1996) and high levels of both ecological and geographical heterogeneities (see Briggs 1974; Reynolds 1993; Carpenter 1997; Manilo and Bogorodsky 2003; Swift and Bower 2003; Béarez et al. 2008; Yao 2008; Thoppil and Hogan 2010a) offer a unique setting for studying the population structure of marine fish species.
The objectives of this work were to (i) evaluate otolith shape variation using an outline method based on a new function in the program R, named ShapeR, and (ii) to evaluate whether the spatial distribution of otolith shape showed a population discrimination meaning in the Ornate goby, Istigobius ornatus, in the Persian Gulf and Oman Sea.
Materials and methods
Study area and fish collection
In the intertidal zone, at the northern coasts of the Persian Gulf and the Oman Sea, 187 specimens of Istigobius ornatus (23.88–49.56 mm SL, mean: 37.89 mm) were collected at 10–20 cm water depth from November 2012 to September 2017 using hand net (1.30 mm mesh size) (Fig. 1, Table 1). After anesthesia with Quinaldine sulphate (Ross and Ross 2008), the specimens were documented photographically and fixed in 10% formaldehyde; subsequently stored in 70% ethanol for long-term storage. All the collected specimens are deposited in the Zoological Museum of Shiraz University, Collection of Biology Department, ZM-CBSU. Fishes were classified according to sex based on the shape of the genital papilla (Chiu et al. 2018). To reduce the possible effects of ontogenetic variations on otolith morphology data, only sexually mature specimens were used for analysis. Standard length (SL) (in mm) was measured from median anterior point of the upper lip to posterior end of the hypural plate for each specimen using an Asimeto digital caliper (Miller 1988). Fishes were dissected under a stereomicroscope (Zeiss Stemi SV6) and the left sagittal otolith of each specimen was extracted and cleaned from organic remaining tissue of the macula and the vestibule using fine tweezers and incubation in 5% KOH solution for a few minutes. Otoliths were then washed in distilled water for 30 min and allowed to dry in room temperature before storage in plastic trays.
Otolith imaging and data analysis
The left sagittal otolith of each specimen was placed on a dark plate and digital images with high resolution at a same magnification of 4x were captured using a 14MP Industrial Microscope Camera 180x equipped with an S-EYE 1.2.4.128 image processing system (Fig. 2). The images were stored in JPEG format (*.jpg). Orientation of otolith images with their posterior rim to the left and manipulation on their contrast and brightness (Fig. 2) were implemented using ImageJ 1.50i (https://imagej.nih.gov/ij/index.html) and ACDSee Ultimate 9 (https://acdid.acdsee.com/). In addition, drawing of the otolith shape was prepared using the CorelDRAW Graphics Suite X8 (https://www.coreldraw.com/). For the terminology of otolith, Gierl et al. (2013) and Schwarzhans (2014) were followed (Fig. 2). Statistical analyses were done in the software R 3.6.3 (Team 2020) using the packages ShapeR 0.1–5 (Libungan and Pálsson 2015), Vegan 2.5–6 (Oksanen et al. 2013), Ipred 0.9–9 (Peters et al. 2019) and MASS 7.3–51.5 (Ripley et al. 2020).
Shape analysis
Here we focus on the outline analysis using the ShapeR package (Libungan and Pálsson 2015). To quantify otolith shape variation between populations we used several statistical functions provided in the program R (Team 2020). The otolith images were read into the software R. The ShapeR package analyzes otolith shape by extracting outlines from digital images of the otoliths and elimination of pixel noise (Fig. 2b) (see Libungan and Pálsson 2015).
Following the rotation of all otoliths horizontally along their longest axis, a matrix of x and y coordinates from all otolith outlines was estimated. Equally spaced radii were drawn from the centroid of the otolith to the otolith outline. The length of these radii acts as a univariate shape descriptor. By using the Wavelet and Fourier transformation on the equally spaced radii, the Wavelet and Fourier coefficients, respectively, were extracted from the digital images using the Wavethresh 4.6.8 package (Libungan and Pálsson 2015; Nason 2016). By using a normalization technique based on regression to remove the allometric growth effect on otolith shape, the Wavelet and Fourier coefficients were scaled with SL and then those coefficients which showed significant interaction between populations and SL (P < 0.05), were omitted automatically from the analysis (Longmore et al. 2010; Libungan et al. 2015a). As an output of the ShapeR package, the remaining coefficients were directly imported into the statistical packages in the software R (see Libungan and Pálsson 2015). To get more detailed information of the shape differences between populations at angles of the otolith outline, Wavelet transform has proven to be more useful than Fourier transform (Libungan et al. 2015a).
Mean otolith shape analysis
For visual assessment of otolith shape variation among populations, the average otolith shape of each population was plotted using the Wavelet and Fourier coefficients (Fig. 3). To evaluate which areas of the otolith shape indicated the most variation between populations, mean shape coefficients and their standard deviation of all combined otoliths were plotted against the angle of the outline using Fourier or Wavelet transform by the package gplots 3.0.3 (Warnes et al. 2020; Libungan and Pálsson 2015) (Fig. 4). Since the proportion of variation among groups (the intraclass correlation) provides more information about the partition of the variation along the outline of the otolith, to measure the differences between populations, intraclass correlation was calculated along the outline. Also using the Pearson correlation test, the correlation of the length of each radius that shows the most differences among populations was calculated. Since the plots based on Wavelet and Fourier were similar, solely the outcome of the Wavelet method was used for further analyses.
Mean otolith shapes based on Wavelet reconstruction for the I. ornatus populations from five (a) and four (b) sampling sites (CH = Chabahar, DA = Bushehr (Dayyer), KA = Kandaloo, PA = Parsian, QE = Qeshm). The numbers 0, 90, 180 and 270 indicate angle in degrees (°) on the outline (see Fig. 4)
Mean and standard deviation of the Wavelet coefficients for all combined otoliths and the proportion of variance between I. ornatus populations or the intraclass correlation (black solid line). The horizontal axis shows angle in degrees (°) as unit based on polar coordinates where the centroid of the otolith is the center point of the polar coordinates (see also Fig. 3)
ANOVA-like permutation test
Using an ANOVA-like permutation test using the package Vegan 2.5–6 (Oksanen et al. 2013), the length of the radii as a univariate shape descriptor was used to examine the significance of differences between populations based on 1000 permutations. To recognize if there is a significant difference between otolith shape of male and female and also if it is possible to combine the samples of two sexes, sexual stability in otolith shape was analyzed between sexes within each sampling locality (Table 2).
Canonical analysis of principal coordinates (CAP)
To evaluate the variation in otolith shape among the populations, the standardized Wavelet coefficients were transformed into principal coordinates and subjected to canonical analysis (CAP: Canonical Analysis of Principal coordinates) (Anderson and Willis 2003). The outcome was compared among populations using the function capscale with the package Vegan 2.5–6 (Oksanen et al. 2013). In addition, Wavelet coefficients were used for visualizing the clustering of the CAP results in two discriminating axes CAP1 and CAP2. Also, a dendrogram based on the CAP outcome was derived by the Squared Euclidean Dissimilarity Distance performed in PAST 3.03 (Hammer et al. 2001).
Correct estimation of individuals to their sampling origin
The classification success into groups was evaluated using a leave-one-out cross-validation estimation. In this regard, the Linear Discriminant Analysis (LDA) on the standardized Wavelet coefficients was applied to show classification of individuals to original populations with cross-validation estimation using the functions errorest and lda in the ipred and MASS packages, respectively (Peters et al. 2019; Ripley et al. 2020)
Isolation by distance
The relationship between otolith shape variations and geographical distances among sampling sites was evaluated using the Mantel test (Mantel 1967) with a Pearson correlation test (1000 permutations) in the package Vegan. Therefore, the matrices of average Euclidean distances based on otolith shape (CAP1 and CAP2) for each population, and the geographical distances between sampling sites (km as unit) were estimated. We used the software Geographic Distance Matrix Generator 1.2.3 (Ersts 2020) to create a geographic distance matrix.
Results
In total, otoliths of 187 individuals (23.88–49.56 mm SL, mean: 37.89 mm) were analyzed. The results of ANOVA-like permutation test (Table 2) using the radii demonstrated no significant differences between sexes within each sampling site (P > 0.05). Likewise, the CAP analysis based on PC1 of the wavelet coefficients detected no differences between the male and female otoliths in each of the five populations (CAP, P > 0.05). Therefore, all the samples (male and female) from each sampling site were combined together for further analyses.
Univariate shape analysis between populations based on radii
According to the results of ANOVA-like permutation test, the lengths of the three major radii were statistically different between populations of the Oman Sea and the Persian Gulf (P < 0.05, Table 3). The variation in otolith shape between the Chabahar and Dayyer populations was large (F-value = 16.662; P = 0.001; see Table 3). On the other hand, the results of ANOVA-like permutation test indicated no significant difference in the otolith shape among Kandaloo and Qeshm from the Persian Gulf (P = 0.43), so the samples from these localities were combined together as Qeshm (QE). Univariate analysis of otolith shape using the F and P value among populations demonstrated that the otolith shapes of populations correlated with geographical distances between sampling sites.
Differences of mean otolith shapes between populations based on wavelet
Examination of the mean shapes indicated minor differences between the shape of the otoliths from the geographically close populations Kandaloo and Qeshm (Fig. 3a). Therefore, we combined all specimens from these populations together as Qeshm, and the newly calculated mean shape was provided (Fig. 3b). Analysis of the remaining four populations showed that the mean shape of otoliths based on the Wavelet coefficients differed among the studied populations from the Oman Sea (Chabahar) and the Persian Gulf (Dayyer, Parsian and Qeshm), mainly at the posteroventral angle and the posterodorsal and preventral projections (Fig. 3b). This was confirmed by the partition of the variation along the outline of the otolith at 140–200°, 250–270° and 300–360°, respectively (Fig. 4). Most of the variation occurs at 240–270° angles, which confirms that the posteroventral angle was the most varied angle among the populations. Chabahar in the mean shape is in the outermost part of the posterodorsal projection, moving inwards towards the otolith centroid, Qeshm and Parsian are similar, and then in the innermost part is Dayyer. However, at the posteroventral angle, the pattern is reversed, Dayyer in the outermost part has a shape farthest from the centroid, and moving inwards Qeshm and Parsian have a similar shape at the edge, and then Chabahar in the innermost part has a shape closest to the centroid. This shape indicated that the Dayyer and Chabahar populations have the greatest distance from each other.
These results are confirmed by the multivariate analyses. The first two discriminating axes of the CAP analysis based on the Wavelet coefficients explained 91.5% of the variation between the four populations (CAP 1: 78.5%, CAP 2: 12.8%) and demonstrated clear differences between Chabahar (Oman Sea) and Dayyer (Persian Gulf), and Chabahar and Parsian (Persian Gulf) (Fig. 5). A Box’s M test in our data showed that the covariance matrices are homogeneous (P = 0.128), which showed that LDA can be used. The overall classification success (Table 4) with a leave-one-out cross-validation estimation based on all samples from the four populations was 55.9%, the highest classification success was achieved for Chabahar (87%).
A hierarchical cluster analysis based on the values of the CAP1 and CAP2 analysis and using the Euclidean distance as a measure of dissimilarity revealed a dendrogram with two main clusters, the Persian Gulf populations, i.e. Dayyer, Parsian, Kandaloo, Qeshm (Fig. 6a) or Dayyer, Parsian, Qeshm (Fig. 6b) in one cluster, and the Oman Sea population (i.e. Chabahar) in the second cluster.
Isolation by distance
Geographically, the populations found in habitats near to each other were more similar in their otolith shape than populations further apart. The Mantel test based on 1000 permutations also confirmed that there was a significant positive correlation between the otolith shape and the geographical distance matrices (r = 0.93, P < 0.002).
Discussion
The Istigobius ornatus populations are distributed in heterogeneous habitats over a large geographical range along the Iranian intertidal coasts of the Persian Gulf and Oman Sea, and hence, this species is a suitable candidate for population studies. Our results based on radii length as well as based on CAP analysis using the Wavelet coefficients demonstrated no significant sex differences regarding otolith shapes, which is consistent with the results of Yu et al. (2014) on five gobiid species from the northern Chinese coastal seawaters. Generally, significant differences in otolith shape between sexes can occur, as has been shown for the trichiurid Aphanopus carbo from the Portuguese coast based on elliptic Fourier descriptor analysis (Farias et al. 2009), but for gobiids it has not yet been proven.
All analyses revealed significant differences among the studied I. ornatus populations. CAP analyses using the Wavelet coefficients further demonstrated differences among the I. ornatus populations that are correlated with their geographical distances, i.e. the otolith shapes of the geographically close populations of I. ornatus from the Persian Gulf are more similar to each other than to the more distant Chabahar population in the Oman Sea. These results reinforce the outcome of Sadeghi et al. (in review), who detected high gene flow between four populations of I. ornatus along the coast of the Persian Gulf, but a clear separation between these populations and the Oman Sea population using the D-loop marker of mitochondrial DNA. Based on two mitochondrial markers Ghanbarifardi et al. (2018) demonstrated that the populations of Periophthalmus waltoni (Teleostei: Gobiidae) along the northern coasts of the Persian Gulf and Oman Sea are divided into two well-separated clades, the Persian Gulf clade and the eastern clade (i.e. the Strait of Hormuz and Oman Sea clade). Likewise, the studies of Ghanbarifardi et al. (2014) and Ghanbarifardi et al. (2020), who analyzed morphometric data of the Walton’s mudskipper Periophthalmus waltoni Koumans, 1941 and the Indian Ocean slender mudskipper Scartelaos tenuis (Day, 1876), respectively, revealed significant differences between the corresponding Persian Gulf and Gulf of Oman populations. Several further studies have indicated population differentiation between these two regions. Sadighzadeh et al. (2014) showed that the otolith shape in John’s snapper, Lutjanus johnii (Bloch, 1792), exhibits a geographically structured dissimilarity between the two gulfs, which allowed them to consider these populations as separate stocks. According to these authors, the observed dissimilarity in the otolith shape is likely associated with genetic heterogeneity and environmental factors (Sadighzadeh et al. 2014). Similar results have been documented for the Aphanius dispar species group (Teimori et al. 2018) (now Aphaniops, see Esmaeili et al. 2020). Moreover, studies investigating the whole morphology for some other inhabitants of the Persian Gulf and Oman Sea have demonstrated the same geographic structure. An example is the study of Afkhami et al. (2016), who, based on significant morphometric differences among populations of the crab Leptodius exaratus (Brachyura, Xanthidae) from the Persian Gulf and Oman Sea, suggested that the Strait of Hormuz represents a significant biogeographic barrier.
Regarding the otolith shape differences between the I. ornatus populations along the intertidal coasts of the Persian Gulf and Oman Sea, four different scenarios can be put forward to explain the observed population structure. It is likely that some of these factors may have acted together to promote otolith shape differentiation among the Persian Gulf and Oman Sea populations.
(i) According to the result from different studies (e.g. L’Abée-Lund and Jensen 1993; Cardinale et al. 2004; Pothin et al. 2006; Vignon 2012; Teacher et al. 2013; Yu et al. 2014; Libungan et al. 2015a), the formation of the otolith shape may be under the control of genetic processes. This was further investigated for I. ornatus populations from the Persian Gulf and Oman Sea by Sadeghi et al. (in review/preparation a, b) using the mitochondrial D-loop marker and morphological characteristics. The plasticity in the larval stage duration between geographical populations of a species might correspond to the expression of selected strategies, which are defined as genetically determined life histories or behaviors (Robinet et al. 2007; Reveillac et al. 2008). Some authors have pointed out that the shape of otolith is associated to the traits of early life history during ontogeny, which may reflect the genetic factors (Volpedo and Echeverrı́a 2003; Lord et al. 2011).
(ii) Ghanbarifardi et al. (2018) speculated that the phylogeographic break between the Persian Gulf and Gulf of Oman could be related to the geo-hydrological history of the area during the Late Pleistocene, when inconstancies of habitat areas due to sea-level fall and rise considerably affected fish populations (Fairbanks 1989). From approximately 63,000 until 18,000 years ago the western basin of the Persian Gulf has been free of water and from the Last Glacial Maximum (LGM) at about 18,000 until 14,000 years ago the Persian Gulf has been free of marine influence out to the Biaban Shelf edge (Randall 1995; Lambeck 1996). The dryness of the Strait of Hormuz during these times probably contributed to the isolation between the Persian Gulf and Gulf of Oman populations of several species, as has been demonstrated for the gobiids Periophthalmus waltoni (Ghanbarifardi et al. 2018), P. argentilineatus (Polgar et al. 2014), I. ornatus (Sadeghi et al. in review and this study), and the blenniid Istiblennius pox (Mehraban et al. 2020).
(iii) Marine environments are often regarded as open habitats in which isolation by distance is the main mechanism that may promote differentiation among populations (Palumbi 1994), and patterns of isolation by distance (Wright 1943; Slatkin 1993) has been well-documented in some marine fish species (e.g. Gold et al. 1994; Pogson et al. 2001; Ashe et al. 2015; Barnes et al. 2016; Gonzalez et al. 2016; Drinan et al. 2018). Since our study populations are distributed along a west-east axis at the northern coasts of the Persian Gulf and Oman Sea, we expected geographic isolation to contribute to otolith shape affinities, which was confirmed (Mantel test, see Results). It thus appears that isolation by distance is one of the main mechanism that promoted otolith shape differentiation in I. ornatus. Possible reasons are that the fertilized eggs of I. ornatus are adhesive and demersal (Patzner et al. 2012; Chiu et al. 2018), and that members of I. ornatus seem to avoid the open water, i.e. they often accumulate during the low tide at the margin of water, and in the high tide they hide in the submerged burrows to avoid being attacked by predatory fish (Murdy and Hoese 1985). For these reasons, adults of I. ornatus probably do not move between the Persian Gulf and Oman Sea. On the other hand, their larvae have a planktonic life stage (Patzner et al. 2012; Chiu et al. 2018); therefore, passive transport of larvae via currents within the Persian Gulf and/or the Oman Sea might also have contributed to the dispersion of I. ornatus, keeping the geographically close populations similar in their general morphological and genetic features. Similary, Ghanbarifardi et al. (2014) concluded that the main factor to connect P. waltoni populations from different sites within the Persian Gulf or within the Oman Sea and keep them similar, morphologically and probably genetically, is likely to be dispersal of larvae.
(iv) In general, fishes demonstrate greater variances in morphological traits both within and between populations than any other vertebrates (see Hossain et al. 2010; Brraich and Akhter 2015), which could be (amongst others) a result of physical and ecological habitat characteristics (e.g. water temperature, salinity, depth, substrate, diet and niches) (McElroy and Douglas 1995). Therefore, geographically or ecologically separated populations of a species that occupy different habitats may display unique otolith shape throughout their lifetime (Yu et al. 2014). Accordingly, differences in the ecological settings within the Persian Gulf as well as between the Persian Gulf and Oman Sea may explain the here observed population differentiations in I. ornatus. Due to its unique oceanographic conditions, the Persian Gulf should be regarded as a distinct ecosystem (Carpenter 1997; Manilo and Bogorodsky 2003) and the Oman Sea should be considered as a zoogeographic boundary between the Western Indian Ocean and the Indo-Polynesian provinces (Briggs 1974; Béarez et al. 2008). This distinctness affects aquatic organisms. The waters of the Persian Gulf and the Oman Sea have markedly different physical and chemical characteristics. The Persian Gulf is a relatively closed and very shallow sea (average 35 m) connected to the Oman Sea through the narrow Strait of Hormuz (Swift and Bower 2003; Thoppil and Hogan 2010b). In comparison, the Oman Sea is deeper than the Persian Gulf (in most cases deeper than 1000 m), has more stable physical and chemical conditions and an open direct connection to the Indian Ocean (Reynolds 1993). In addition, the Persian Gulf experiences wide seasonal temperature fluctuation of its surface water, low rainfall and high evaporation rates and has a higher level of salinity than the Oman Sea (Yao 2008; Thoppil and Hogan 2010a). Moreover, environmental factors such as temperature and salinity are different in the western part of the Persian Gulf and its other parts, most probably caused by the discharge of major rivers (i.e. Heleh, Zohrehand Arvand) into the western part (Ghanbarifardi et al. 2014). The dendrogram of fig. 6 showed two sub-clusters within the Persian Gulf cluster, the western sub-cluster (Dayyer) and the eastern sub-cluster (Parsian, Kandaloo and Qeshm). Therefore, we might conclude that the observed otolith shape differences between the western and eastern population of I. ornatus along the coast of the Persian Gulf may be due to ecological differences.
Conclusion
This study demonstrated the high efficiency of otolith shape analysis (in comparison to otolith morphometry and landmarks analysis) using the Wavelet transform technique to discriminate populations of I. ornatus along the northern coasts of the Persian Gulf and Oman Sea. Our results indicated significant variations in otolith shape among the I. ornatus populations. Possible reasons for these differences include genetic factors, paleoclimatic history of the study area, isolation by distance, and different ecological settings. It is likely that some of these factors have contributed together to promote otolith shape differences between the Persian Gulf and Oman Sea populations. However, further investigations including habitat and phylogeographic analyses are required to complement these results and evaluate the role of each of these factors. This study could provide a basis for further extensive researches to be done on the otolith shape variation and implementing this highly efficient morphological marker to investigate population structure of gobiid species.
Data availability
Specimens used in the present study are deposited in the Zoological Museum of Shiraz University, Collection of Biology Department, ZM-CBSU.
References
Afkhami M, Schubart CD, Naderloo R (2016) Morphometric differentiation among populations of Leptodius exaratus (H. Milne Edwards, 1834)(Brachyura, Xanthidae) from the Persian Gulf and the Gulf of Oman. Crustaceana 89:259–271. https://doi.org/10.1163/15685403-00003525
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525. https://doi.org/10.1890/0012-9658
Annabi A, Said K, Reichenbacher B (2013) Inter-population differences in otolith morphology are genetically encoded in the killifish Aphanius fasciatus (Cyprinodontiformes). Sci Mar 77:269–279. https://doi.org/10.3989/scimar.03763.02A
Ashe JL, Feldheim KA, Fields AT, Reyier EA, Brooks EJ, O’Connell MT, Skomal G, Gruber SH, Chapman DD (2015) Local population structure and context-dependent isolation by distance in a large coastal shark. Mar Ecol Prog Ser 520:203–216. https://doi.org/10.3354/meps11069
Avigliano E, Martinez CFR, Volpedo AV (2014) Combined use of otolith microchemistry and morphometry as indicators of the habitat of the silverside (Odontesthes bonariensis) in a freshwater–estuarine environment. Fish Res 149:55–60. https://doi.org/10.1016/j.fishres.2013.09.013
Barnes TC, Junge C, Myers SA, Taylor MD, Rogers PJ, Ferguson GJ, Lieschke JA, Donnellan SC, Gillanders BM (2016) Population structure in a wide-ranging coastal teleost (Argyrosomus japonicus, Sciaenidae) reflects marine biogeography across southern Australia. Mar Freshw Res 67:1103–1113. https://doi.org/10.1071/MF15044
Béarez P, Kerneur S, Gabsi Z (2008) New record of a surgeonfish from Oman with notes on some uncommon rays. Cybium 32:355–358
Berg F, Almeland OW, Skadal J, Slotte A, Andersson L, Folkvord A (2018) Genetic factors have a major effect on growth, number of vertebrae and otolith shape in Atlantic herring (Clupea harengus). PLoS One 13:e0190995. https://doi.org/10.1371/journal.pone.0190995
Bohlen J (2008) First report on the spawning behaviour of a golden spined loach, Sabanejewia vallachica (Teleostei: Cobitidae). Folia Zool 57:139–146
Bratishko A, Schwarzhans W, Reichenbacher B, Vernyhorova Y, Ćorić S (2015) Fish otoliths from the Konkian (Miocene, early Serravallian) of Mangyshlak (Kazakhstan): testimony to an early endemic evolution in the eastern Paratethys. Paläontol Z 89:839–889. https://doi.org/10.1007/s12542-015-0274-4
Briggs JC (1974) Marine zoogeography. McGraw Hill Book Company, New York
Brraich OS, Akhter S (2015) Morphometric characters and meristic counts of a fish, Crossocheilus latius latius (Hamilton-Buchanan) from Ranjit Sagar wetland, India. Int J Fish Aquat Stud 2:260–265
Burke N, Brophy D, King PA (2008) Otolith shape analysis: its application for discriminating between stocks of Irish Sea and Celtic Sea herring (Clupea harengus) in the Irish Sea. ICES J Mar Sci 65:1670–1675. https://doi.org/10.1093/icesjms/fsn177
Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can J Fish Aquat Sci 58:30–38. https://doi.org/10.1139/f85-127
Campana SE, Neilson JD, Sciences A (1985) Microstructure of fish otoliths. Can J Fish Aquat Sci 42:1014–1032. https://doi.org/10.1139/f85-127
Cardinale M, Doering-Arjes P, Kastowsky M, Mosegaard H (2004) Effects of sex, stock, and environment on the shape of known-age Atlantic cod (Gadus morhua) otoliths. Can J Fish Aquat Sci 61:158–167. https://doi.org/10.1139/f03-151
Carpenter KE (1997) Living marine resources of Kuwait, eastern Saudi Arabia, Bahrain, Qatar, and the United Arab Emirates. FAO, Rome
Chiu PS, Leu MY, Meng PJ (2018) Year-round natural spawning, early development, and the effects of temperature, salinity and prey density on captive ornate goby Istigobius ornatus (Rüppell, 1830) larval survival. Aquac Res 50:173–187. https://doi.org/10.1111/are.13880
Cole KS (1990) Patterns of gonad structure in hermaphroditic gobies (Teleostei Gobiidae). Environ Biol Fish 28:125–142. https://doi.org/10.1007/BF00751032
Cruz A, Lombarte A (2004) Otolith size and its relationship with colour patterns and sound production. J Fish Biol 65:1512–1525. https://doi.org/10.1111/j.0022-1112.2004.00558.x
Drinan DP, Gruenthal KM, Canino MF, Lowry D, Fisher MC, Hauser L (2018) Population assignment and local adaptation along an isolation-by-distance gradient in Pacific cod (Gadus macrocephalus). Evol Appl 11:1448–1464. https://doi.org/10.1111/eva.12639
Ersts PJ (2020) Geographic distance matrix generator (version 1.2.3). American Museum of Natural History, Center for Biodiversity and Conservation. http://biodiversityinformatics.amnh.org/open_source/gdmg. Accessed 27 April 2020
Esmaeili HR, Teimori A, Zarei F, Sayyadzadeh G (2020) DNA barcoding and species delimitation of the Old World tooth-carps, family Aphaniidae Hoedeman, 1949 (Teleostei: Cyprinodontiformes). PLoS One 15:e0231717. https://doi.org/10.1371/journal.pone.0231717
Fairbanks RG (1989) A 17,000-year glacio-eustatic sea level record: influence of glacial melting rates on the younger Dryas event and deep-ocean circulation. Nature 342:637–642. https://doi.org/10.1038/342637a0
Farias I, Vieira AR, Gordo LS, Figueiredo I (2009) Otolith shape analysis as a tool for stock discrimination of the black scabbardfish, Aphanopus carbo Lowe, 1839 (Pisces: Trichiuridae), in Portuguese waters. Sci Mar 73:47–53. https://doi.org/10.3989/scimar.2009.73s2047
Gaemers PA (1983) Taxonomic position of the Cichlidae (Pisces, Perciformes) as demonstrated by the morphology of their otoliths. Neth J Zool 34:566–595. https://doi.org/10.1071/MF15052
Gauldie R, Crampton J (2002) An eco-morphological explanation of individual variability in the shape of the fish otolith: comparison of the otolith of Hoplostethus atlanticus with other species by depth. J Fish Biol 60:1204–1221. https://doi.org/10.1111/j.1095-8649.2002.tb01715.x
Ghanbarifardi M, Aliabadian M, Esmaeli HR (2014) Morphometric variation of Periophthalmus waltoni (Teleostei: Gobiidae) in the Persian Gulf and Gulf of Oman. Iran J Anim Biosyst 10:137–144
Ghanbarifardi M, Aliabadian M, Esmaeili HR (2018) Phylogeography of Walton’s mudskipper, Periophthalmus waltoni Koumans, 1941 (Perciformes: Gobiidae), from the Persian Gulf and Gulf of Oman. Zool Middle East 64:207–218. https://doi.org/10.1080/09397140.2018.1470300
Ghanbarifardi M, Aliabadian M, Esmaeili H (2020) Shape variation of the Indian Ocean slender mudskipper, Scartelaos tenuis (day, 1876) from the Persian Gulf and Oman Sea (Gobioidei: Gobiidae). Iran J fish Sci 19:612–622. https://doi.org/10.22092/ijfs.2018.119368
Gierl C, Reichenbacher B, Gaudant J, Erpenbeck D, Pharisat A (2013) An extraordinary gobioid fish fossil from southern France. PLoS One 8:e64117. https://doi.org/10.1371/journal.pone.0064117
Girone A, Nolf D (2009) Fish otoliths from the Priabonian (late Eocene) of North Italy and south-East France–their paleobiogeographical significance. Rev Micropaleontol 52:195–218. https://doi.org/10.1016/j.revmic.2007.10.006
Gold JR, Richardson LR, Furman C, Sun F (1994) Mitochondrial DNA diversity and population structure in marine fish species from the Gulf of Mexico. Can J Fish Aquat Sci 51:205–214. https://doi.org/10.1139/f94-306
Gonzalez EB, Knutsen H, Jorde PE (2016) Habitat discontinuities separate genetically divergent populations of a rocky shore marine fish. PLoS One 11:e0163052. https://doi.org/10.1371/journal.pone.0163052
Green BS, Mapstone BD, Carlos G, Begg GA (2009) Tropical fish otoliths: information for assessment, management and ecology. Springer Netherlands, Dordrecht. https://doi.org/10.1007/978-1-4020-5775-5
Hammer Ø, Harper DA, Ryan PD (2001) Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hossain MA, Nahiduzzaman M, Saha D, Khanam MUH, Alam MS (2010) Landmark-based morphometric and meristic variations of the endangered carp, Kalibaus Labeo calbasu, from stocks of two isolated rivers, the Jamuna and Halda, and a hatchery. Zool Stud 49:556–563. https://doi.org/10.17576/jsm-2017-4605-03
Kocovsky PM, Sullivan TJ, Knight CT, Stepien CA (2013) Genetic and morphometric differences demonstrate fine-scale population substructure of the yellow perch Perca flavescens: need for redefined management units. J Fish Biol 82:2015–2030. https://doi.org/10.1111/jfb.12129
L'Abée-Lund JH, Jensen AJ (1993) Otoliths as natural tags in the systematics of salmonids. Environ Biol Fish 36:389–393. https://doi.org/10.1007/BF00012418
Lambeck K (1996) Shoreline reconstructions for the Persian Gulf since the last glacial maximum. Earth Planet Sci Lett 142:43–57. https://doi.org/10.1016/0012-821X(96)00069-6
Libungan LA, Pálsson S (2015) ShapeR: an R package to study otolith shape variation among fish populations. PLoS One 10:e0121102. https://doi.org/10.1371/journal.pone.0121102
Libungan L, Óskarsson G, Slotte A, Jacobsen J, Pálsson S (2015a) Otolith shape: a population marker for Atlantic herring Clupea harengus. J Fish Biol 86:1377–1395. https://doi.org/10.1111/jfb.12647
Libungan LA, Slotte A, Husebø Å, Godiksen JA, Pálsson S (2015b) Latitudinal gradient in otolith shape among local populations of Atlantic herring (Clupea harengus L.) in Norway. PLoS One 10:e0130847. https://doi.org/10.1371/journal.pone.0130847
Libungan LA, Slotte A, Otis EO, Pálsson S (2016) Otolith variation in Pacific herring (Clupea pallasii) reflects mitogenomic variation rather than the subspecies classification. Polar Biol 39:1571–1579. https://doi.org/10.1007/s00300-015-1882-x
Limburg KE, Walther Y, Hong B, Olson C, Storå J (2008) Prehistoric versus modern Baltic Sea cod fisheries: selectivity across the millennia. Proc R Soc B 275:2659–2665. https://doi.org/10.1098/rspb.2008.0711
Lombarte A, Cruz A (2007) Otolith size trends in marine fish communities from different depth strata. J Fish Biol 71:53–76. https://doi.org/10.1111/j.1095-8649.2007.01465.x
Lombarte A, Palmer M, Matallanas J, Gómez-Zurita J, Morales-Nin B (2010) Ecomorphological trends and phylogenetic inertia of otolith sagittae in Nototheniidae. Environ Biol Fish 89:607–618. https://doi.org/10.1007/s10641-010-9673-2
Lombarte A, Miletić M, Kovačić M, Otero-Ferrer JL, Tuset VM (2018) Identifying sagittal otoliths of Mediterranean Sea gobies: variability among phylogenetic lineages. J Fish Biol 92:1768–1787. https://doi.org/10.1111/jfb.13615
Longmore C, Fogarty K, Neat F, Brophy D, Trueman C, Milton A, Mariani S (2010) A comparison of otolith microchemistry and otolith shape analysis for the study of spatial variation in a deep-sea teleost, Coryphaenoides rupestris. Environ Biol Fish 89:591–605. https://doi.org/10.1007/s10641-010-9674-1
Lord C, Brun C, Hautecoeur M, Keith P (2010) Insights on endemism: comparison of the duration of the marine larval phase estimated by otolith microstructural analysis of three amphidromous Sicyopterus species (Gobioidei: Sicydiinae) from Vanuatu and New Caledonia. Ecol Freshwater Fish 19:26–38. https://doi.org/10.1111/j.1600-0633.2009.00386.x
Lord C, Morat F, Lecomte-Finiger R, Keith P (2011) Otolith shape analysis for three Sicyopterus (Teleostei: Gobioidei: Sicydiinae) species from New Caledonia and Vanuatu. Environ Biol Fish 93:209–222. https://doi.org/10.1007/s10641-011-9907-y
Manilo LG, Bogorodsky SV (2003) Taxonomic composition, diversity and distribution of coastal fishes of the Arabian Sea. J Ichthyol 43:75–149
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Maugé LA (1984) Gobiidae. In: Daget J, Gosse JP, Thys van den Audenaerde DFE (eds) Check-list of the freshwater fishes of Africa, Cloffa (Vol 2). Royal Museum for Central Africa (Musée royal de l'Afrique Centrale), Paris, pp 358–388
McElroy DM, Douglas ME (1995) Patterns of morphological variation among endangered populations of Gila robusta and Gila cypha (Teleostei: Cyprinidae) in the upper Colorado River basin. Copeia 1995:636–649. https://doi.org/10.2307/1446760
Mehraban H, Esmaeili HR, Zarei F, Ebrahimi M, Gholamhosseini A (2020) Genetic diversification, population structure, and geophylogeny of the Scarface rockskipper Istiblennius pox (Teleostei: Blenniidae) in the Persian Gulf and Oman Sea. Mar Biodivers 50:20. https://doi.org/10.1007/s12526-020-01045-z
Miller PJ (1988) New species of Corcyrogobius, Thorogobius and Wheelerigobius from West Africa (Teleostei: Gobiidae). J Nat Hist 22:1245–1262
Monteiro LR, Di Beneditto APM, Guillermo LH, Rivera LA (2005) Allometric changes and shape differentiation of sagitta otoliths in sciaenid fishes. Fish Res 74:288–299. https://doi.org/10.1016/j.fishres.2005.03.002
Mosegaard H, Svedäng H, Taberman K (1988) Uncoupling of somatic and otolith growth rates in Arctic char (Salvelinus alpinus) as an effect of differences in temperature response. Can J Fish Aquat Sci 45:1514–1524. https://doi.org/10.1139/f88-180
Motamedi M, Teimori A, Iranmanesh A (2019) Ontogenetic pattern, morphological sexual and side dimorphism in the saccular otolith of a scaleless killifish Aphanius furcatus (Teleostei: Aphaniidae). Acta Zool 100:1–13. https://doi.org/10.1111/azo.12313
Murdy EO, Hoese DF (1985) Revision of the gobiid fish genus Istigobius. Bishop Museum Press, Hawaii
Nason G (2016) Package ‘wavethresh’: wavelets statistics and transforms. CRAN
Nolf D (1985) Otolithi piscium (handbook of paleoichthyology, Vol. 10). Lubrecht & Cramer Ltd, New York
Nolf D (1995) Studies on fossil otoliths—the state of the art. Rec Dev Fish Otolith Res 19:513–544
Nolf D (2013) The diversity of fish otoliths, past and present. Royal Belgian Institute of Natural Sciences, Brussels
Oksanen J, Blanchet FG, Kindt R et al (2013) Package ‘vegan’: community ecology package. CRAN
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Evol Syst 25:547–572. https://doi.org/10.1146/annurev.es.25.110194.002555
Patzner R, Van Tassell JL, Kovacic M, Kapoor BG (2012) The biology of gobies. Science Publishers, Enfield, NH
Peters A, Hothorn T, Ripley BD et al (2019) Package ‘ipred’: improved predictors. CRAN
Pogson GH, Taggart CT, Mesa KA, Boutilier RG (2001) Isolation by distance in the Atlantic cod, Gadus morhua, at large and small geographic scales. Evolution 55:131–146. https://doi.org/10.1111/j.0014-3820.2001.tb01279.x
Polgar G, Zane L, Babbucci M, Barbisan F, Patarnello T, Rüber L, Papetti C (2014) Phylogeography and demographic history of two widespread indo-Pacific mudskippers (Gobiidae: Periophthalmus). Mol Phylogenet Evol 73:161–176. https://doi.org/10.1016/j.ympev.2014.01.014
Ponton D (2006) Is geometric morphometrics efficient for comparing otolith shape of different fish species? J Morphol 267:750–757. https://doi.org/10.1002/jmor.10439
Popper AN, Coombs S (1982) The morphology and evolution of the ear in Actinopterygian fishes. Am Zool 22:311–328. https://doi.org/10.1093/icb/22.2.311
Popper AN, Lu Z (2000) Structure–function relationships in fish otolith organs. Fish Res 46:15–25. https://doi.org/10.1016/S0165-7836(00)00129-6
Pothin K, Gonzalez-Salas C, Chabanet P, Lecomte-Finiger R (2006) Distinction between Mulloidichthys flavolineatus juveniles from Reunion Island and Mauritius Island (south-West Indian Ocean) based on otolith morphometrics. J Fish Biol 69:38–53. https://doi.org/10.1111/j.1095-8649.2006.01047.x
Ramcharitar JU, Higgs DM, Popper AN (2006) Audition in sciaenid fishes with different swim bladder-inner ear configurations. J Acoust Soc Am 119:439–443. https://doi.org/10.1121/1.2139068
Randall JE (1995) Coastal fishes of Oman. University of Hawaii Press, Honolulu, Hawaii
Randall JE, Williams JT, Smith DG, Kulbicki M, Tham G, Mou T G, Labrosse P, Kronen M, Clua E, Mann BS (2003) Checklist of the shore and epipelagic fishes of Tonga. Atoll Res Bull 502:1–35. https://doi.org/10.5479/si.00775630.502.1
Rashidabadi F, Abdoli A, Tajbakhsh F, Nejat F, Avigliano E (2019) Unravelling the stocks structure of the Persian brown trout by otolith and scale shape. J Fish Biol 96:307–315. https://doi.org/10.1111/jfb.14170
Reichenbacher B, Reichard M (2014) Otoliths of five extant species of the annual killifish Nothobranchius from the east African savannah. PLoS One 9:e112459. https://doi.org/10.1371/journal.pone.0112459
Reichenbacher B, Sienknecht U, Küchenhoff H, Fenske N (2007) Combined otolith morphology and morphometry for assessing taxonomy and diversity in fossil and extant killifish (Aphanius, †Prolebias). J Morphol 268:898–915. https://doi.org/10.1002/jmor.10561
Reichenbacher B, Feulner GR, Schulz-Mirbach T (2009a) Geographic variation in otolith morphology among freshwater populations of Aphanius dispar (Teleostei, Cyprinodontiformes) from the southeastern Arabian peninsula. J Morphol 270:469–484. https://doi.org/10.1002/jmor.10702
Reichenbacher B, Kamrani E, Esmaeili HR, Teimori A (2009b) The endangered cyprinodont Aphanius ginaonis (Holly, 1929) from southern Iran is a valid species: evidence from otolith morphology. Environ Biol Fish 86:507–521. https://doi.org/10.1007/s10641-009-9549-5
Reveillac E, Feunteun E, Berrebi P, Gagnaire PA, Lecomte-Finiger R, Bosc P, Robinet T (2008) Anguilla marmorata larval migration plasticity as revealed by otolith microstructural analysis. Can J Fish Aquat Sci 65:2127–2137. https://doi.org/10.1139/F08-122
Reynolds RM (1993) Physical oceanography of the Gulf, strait of Hormuz, and the Gulf of Oman—results from the Mt Mitchell expedition. Mar Poll Bull 27:35–59. https://doi.org/10.1016/0025-326X(93)90007-7
Ripley B, Venables B, Bates DM et al (2020) Package ‘MASS’: support functions and datasets for Venables and Ripley's MASS. CRAN
Robinet T, Feunteun E, Keith P, Marquet G, Olivier JM, Réveillac E, Valade P (2007) Eel community structure, fluvial recruitment of Anguilla marmorata and indication for a weak local production of spawners from rivers of Réunion and Mauritius islands. Environ Biol Fish 78:93–105. https://doi.org/10.1007/s10641-006-9042-3
Rohlf FJ (1990) An overview of image processing and analysis techniques for morphometrics. In: Rohlf FJ, Bookstein FL (eds) Proceedings of the Michigan morphometrics workshop. University of Michigan Museum of Zoology Special Publication 2, pp. 37–60
Ross LG, Ross B (2008) Anaesthetic and sedative techniques for aquatic animals. Blackwell Publishing Ltd. https://doi.org/10.1002/9781444302264
Sadighzadeh Z, Valinassab T, Vosugi G, Motallebi AA, Fatemi MR, Lombarte A, Tuset VM (2014) Use of otolith shape for stock identification of John's snapper, Lutjanus johnii (Pisces: Lutjanidae), from the Persian Gulf and the Oman Sea. Fish Res 155:59–63. https://doi.org/10.1016/j.fishres.2014.02.024
Schulz-Mirbach T, Ladich F, Plath M, Heß M (2019) Enigmatic ear stones: what we know about the functional role and evolution of fish otoliths. Biol Rev 94:457–482. https://doi.org/10.1111/brv.12463
Schwarzhans W (2014) Otoliths from the middle Miocene (Serravallian) of the Karaman Basin, Turkey. Cainozoic Res 14:35–69
Slatkin M (1993) Isolation by distance in equilibrium and non-equilibrium populations. Evolution 47:264–279. https://doi.org/10.2307/2410134
Swift SA, Bower AS (2003) Formation and circulation of dense water in the Persian/Arabian gulf. J Geophys Res Oceans 108:1–22. https://doi.org/10.1029/2002JC001360
Teacher AG, André C, Jonsson PR, Merilä J (2013) Oceanographic connectivity and environmental correlates of genetic structuring in Atlantic herring in the Baltic Sea. Evol Appl 6:549–567. https://doi.org/10.1111/eva.12042
Team RC (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Teimori A, Jawad LAJ, Al-Kharusi LH, Al-Mamry JM, Reichenbacher B (2012a) Late Pleistocene to Holocene diversification and historical zoogeography of the Arabian killifish (Aphanius dispar) inferred from otolith morphology. Sci Mar 76:637–645. https://doi.org/10.3989/scimar.03635.26C
Teimori A, Schulz-Mirbach T, Esmaeili HR, Reichenbacher B (2012b) Geographical differentiation of Aphanius dispar (Teleostei: Cyprinodontidae) from southern Iran. J Zool Syst Evol Res 50:289–304. https://doi.org/10.1111/j.1439-0469.2012.00667.x
Teimori A, Esmaeili HR, Hamidan N, Reichenbacher B (2018) systematics and historical biogeography of the Aphanius dispar species group (Teleostei: Aphaniidae) and description of a new species from southern Iran. J Zool Syst Evol Res 56:579–598. https://doi.org/10.1111/jzs.12228
Teimori A, Khajooei A, Motamedi M, Hesni MA (2019) Characteristics of sagittae morphology in sixteen marine fish species collected from the Persian Gulf: demonstration of the phylogenetic influence on otolith shape. Reg Stud Mar Sci 29:100661. https://doi.org/10.1016/j.rsma.2019.100661
Thoppil PG, Hogan PJ (2010a) A modeling study of circulation and eddies in the Persian Gulf. J Phys Oceanogr 40:2122–2134. https://doi.org/10.1175/2010JPO4227.1
Thoppil PG, Hogan PJ (2010b) Persian gulf response to a wintertime shamal wind event. Deep Sea Res 57:946–955. https://doi.org/10.1016/j.dsr.2010.03.002
Turan C (2006) The use of otolith shape and chemistry to determine stock structure of Mediterranean horse mackerel Trachurus mediterraneus (Steindachner). J Fish Biol 69:165–180. https://doi.org/10.1111/j.1095-8649.2006.01266.x
Tuset VM, Rosin PL, Lombarte A (2006) Sagittal otolith shape used in the identification of fishes of the genus Serranus. Fish Res 81:316–325. https://doi.org/10.1016/j.fishres.2006.06.020
Vahed NS, Esmaeili HR, Masoudi M, Reichenbacher B (2018) Early otolith development in the critically endangered tooth-carp, Aphanius farsicus (Teleostei: Cyprinodontidae). Environ Biol Fish 101:1309–1317. https://doi.org/10.1007/s10641-018-0778-3
Vahed NS, Esmaeili H, Masoudi M, Reichenbacher B (2019) Ontogenetic Otolith development in an endemic tooth-carp, Aphanius vladykovi (Teleostei: Aphaniidae). J Ichthyol 59:336–343. https://doi.org/10.1134/S0032945219030172
Van Neer W, Ervynck A, Bolle LJ, Millner RS, Rijnsdorp AD (2002) Fish otoliths and their relevance to archaeology: an analysis of medieval, post-medieval, and recent material of plaice, cod and haddock from the North Sea. Environ Archaeol 7:61–76. https://doi.org/10.1179/env.2002.7.1.61
Vignon M (2012) Ontogenetic trajectories of otolith shape during shift in habitat use: interaction between otolith growth and environment. J Exp Mar Biol Ecol 420:26–32. https://doi.org/10.1016/j.jembe.2012.03.021
Vignon M, Morat F (2010) Environmental and genetic determinant of otolith shape revealed by a non-indigenous tropical fish. Mar Ecol Prog Ser 411:231–241. https://doi.org/10.3354/meps08651
Volpedo A, Echeverría DD (2003) Ecomorphological patterns of the sagitta in fish on the continental shelf off argentine. Fish Res 60:551–560. https://doi.org/10.1016/S0165-7836(02)00170-4
Volpedo AV, Tombari A, Echeverría DD (2008) Eco-morphological patterns of the sagitta of Antarctic fish. Polar Biol 31:635–640. https://doi.org/10.1007/s00300-007-0400-1
Warnes G, Bolker B, Bonebakker L et al (2020). Package ‘gplots’: various R programming tools for plotting data. CRAN
Wright S (1943) Isolation by distance. Genetics 28:114–138
Wujdi A, Setyadji B, Nugroho SC (2017) Preliminary stock structure study of skipjack tuna (Katsuwonus pelamis) from south java using otolith shape analysis. Indian Ocean Tuna Commission, pp:19–42
Yao F (2008) Water mass formation and circulation in the Persian Gulf and water exchange with the Indian Ocean. Doctoral dissertation, University of Miami
Yu X, Cao L, Liu J, Zhao B, Shan X, Dou S (2014) Application of otolith shape analysis for stock discrimination and species identification of five goby species (Perciformes: Gobiidae) in the northern Chinese coastal waters. Chin J Oceanol Limn 32:1060–1073. https://doi.org/10.1007/s00343-015-4022-0
Acknowledgments
We thank H. Mehraban, M. Razbanian, Mr. Nasiri and Mr. Tavallai for helping with fish collection and Z. Eslami for helping with ShapeR software.
Funding
Shiraz University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
The research work was approved by Ethics Committee of Biology Department, Shiraz University (SU-9330207).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadeghi, R., Esmaeili, H.R., Zarei, F. et al. Population structure of the ornate goby, Istigobius ornatus (Teleostei: Gobiidae), in the Persian Gulf and Oman Sea as determined by otolith shape variation using ShapeR. Environ Biol Fish 103, 1217–1230 (2020). https://doi.org/10.1007/s10641-020-01015-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-01015-1