Abstract
Aphanius Nardo, 1827 (Actinopterygii, Cyprinodontidae) is a widely distributed genus in the Mediterranean and Persian Gulf area and includes several endangered species. The otolith morphology in Aphanius is known to represent a valuable tool for the taxonomy, and is also indicative for the genetic diversity of a particular population. The present study focuses on the otoliths of the endangered A. ginaonis (Holly, 1929), which is endemic to the Geno hot spring in southern Iran. The taxonomic status of A. ginaonis has repeatedly been questioned, and some scholars have argued that it merely represents a morphological variation of the widespread A. dispar. We present a comparison of the otolith morphology of A. ginaonis (52 specimens) with that of A. dispar (Rüppell, 1828) from the Mehran River Basin (southern Iran) (17 specimens) and an A. dispar population from the Persian Gulf coast of the United Arab Emirates (32 specimens). Our data obtained from SEM pictures, otolith morphometry and statistical analyses suggest that A. ginaonis represents a valid species. In A. ginaonis individuals with a standard length exceeding 23 mm, the otolith variables length–height and rostrum length represent useful complementary diagnostic characters discriminating this species from other Aphanius species. Besides ontogenetic variation, we found extremely high otolith form variability in A. ginaonis, including some otoliths with a morphology distinctly deviating from the basic morphology type. We hypothesize that these variations may be a result of the artificial introduction of A. dispar into the Geno hot spring during the last years and subsequent hybridisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The toothcarp or killifish Aphanius Nardo, 1827 is widely distributed in euryhaline and freshwater habitats along the Mediterranean Sea, Red Sea, Persian Gulf and Arabian Sea, and also occurs in land-locked ponds and lakes, in small streams and sometimes also in relatively large rivers, e.g., in Turkey and Iran (Villwock 1977; Wildekamp 1993; Coad 2000). Aphanius species typically thrive in environments that are not suitable for other fishes, and thus often lack direct competitors and major predators (Clavero et al. 2007). However, several Aphanius species have low population sizes and/or small areas of occurrence, and are today considered endangered due to drainage, land-use, and pollution around their native habitats (e.g., Fernández-Pedrosa et al. 1995; Moreno-Amich et al. 1999). Moreover, the artificially introduced mosquitofish (Gambusia) may compete with Aphanius, and many native Aphanius populations are seriously threatened by Gambusia in the Mediterranean area, Turkey and the Near East (e.g., Wildekamp et al. 1999; Oliva-Paterna et al. 2006).
The principal methods used to study Aphanius species include crossbreeding experiments, genetic analyses, and comparative analyses of meristic counts, osteology and coloration (Villwock 1977; Sienknecht 1999a, b; Coad and Abdoli 2000; Doadrio et al. 2002; Maltagliati et al. 2003, 2006; Blanco et al. 2006; Hrbek et al. 2006; Tigano et al. 2006; Esmaeili et al. 2008). In addition, otolith morphology represents a useful tool in the identification of Aphanius species, and may also contribute to a better understanding of the genetic diversification (Reichenbacher and Sienknecht 2001; Schulz-Mirbach et al. 2006; Reichenbacher et al. 2007, 2009).
Here, we focus on the otoliths of Aphanius ginaonis (Holly, 1929), which is a species with a very small area of occurrence, and the widespread A. dispar (Rüppell, 1828) (populations from the Mehran River, Hormozgan Province, southern Iran). Aphanius ginaonis occurs exclusively in the Geno hot spring, located to the north of Bandar Abbas in southern Iran (Fig. 1), where it is the only native species (Coad 1998). Aphanius ginaonis is separated from the closely related A. dispar by a single morphological feature (i.e. number of dorsal fin rays). Consequently, the status of A. ginaonis remains controversial. For example, Coad (1980, 1998) refers to A. ginaonis as a separate species, whereas it is viewed as a subspecies of A. dispar by Wildekamp (1993). Molecular data indicate that A. ginaonis is the sister taxon to a geographically close A. dispar population from Hormozgan Province (Hrbek and Meyer 2003).
Aphanius dispar is common in drainages of southern Iran, and also along the Persian Gulf coast (Krupp 1983; Feulner 1998, 2005). This species includes a single generally recognised subspecies (A. d. richardsoni Boulenger, 1907), but there are probably several additional yet undescribed subspecies because several populations strongly differ from A. dispar s.str. with regard to fin size, coloration, and otolith morphology (Wildekamp 1993; Reichenbacher et al. 2009). According to Hrbek and Meyer (2003), A. dispar does not represent a monophylum because the clade also includes A. ginaonis, and thus does not constitute a species in terms of the phylogenetic species concept.
This study addresses the question, as to whether otolith morphology can contribute to a better understanding of the taxonomic status of A. ginaonis. For that purpose, we investigate the otolith morphology of A. ginaonis (specimens caught from a natural population at Geno hot spring), A. dispar from a drainage system close to the Geno hot spring (specimens caught from Mehran River), and A. dispar from a coastal population in the Persian Gulf (specimens caught from Khor Hulaylah, United Arab Emirates; see also Reichenbacher et al. 2009). We also analyze as to whether sexual dimorphism and ontogeny are somehow reflected in otolith morphology. Our data show that otolith morphology and morphometry complement the identification of A. ginaonis, and thus represent a suitable trait for the separation of A. ginaonis from A. dispar. The exceptionally high intraspecific variability among the A. ginaonis otoliths observed by us suggests that the present-day population may contain hybrids that originate from crossbreeding of A. ginaonis with artificially introduced A. dispar.
Otoliths
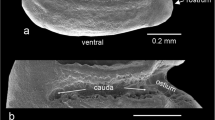
Otoliths are aragonitic mineralizations located in the membraneous labyrinth of the inner ear of bony fishes that play an important role in the senses of hearing and balance (see Popper et al. 2005 for a recent overview). In teleosts, otoliths are arranged in three pairs, each containing a left and right otolith that show mirror symmetry (with the exception of flatfish). According to the position of the otolith pairs in the membraneous labyrinth, otoliths are termed saccular, lagenar, and utricular otoliths (cf. Nolf 1985). The saccular otolith (sagitta) is the largest otolith in most teleosts and the main type that has been studied. Its morphology can be described in terms of the gross contour and a specific complement of traits, e.g., sulcus, rostrum, antirostrum, excisura (Fig. 2). The general morphology of the saccular otolith is usually species specific, and variations in size and contour are used in the discrimination of individual populations or stocks (e.g., Templeman and Squire 1956; Campana and Casselman 1993; Volpedo and Echeverría 2000; DeVries et al. 2002; Cardinale et al. 2004; Stransky 2005; Mérigot et al. 2007; Stransky et al. 2008). In the following text, the term “otolith" refers to the saccular otoliths only.
a Left otolith of Aphanius dispar (Rüppell, 1828) with terminology of otolith characters; male, 35 mm total length, SEM micrograph, BSPG 2004 II 56. b Left otolith of A. dispar (Rüppell, 1828) with measurements of distances and angles used for the statistics; female, 39 mm total length, stereoscope picture, BSPG 2004 II 57 (after Reichenbacher et al. 2009, modified)
Material and methods
Localities, sampling, preparation
Table 1 shows the sampled localities and the number of specimens taken from each site. Fishes with different sizes were sampled from each locality, but it was not possible to sample equal numbers of males and females for each of the size classes (see Table 1). We did not include in this study specimens of less than 20 mm standard length because the otolith morphology in small specimens usually is relatively unspecific. Based on size increments of 5 mm in standard length, the specimens were assigned to size classes (Table 1; note that specimens of the size class 34–38 mm were only available for A. ginaonis). All specimens can be considered as sexual mature due to the typical colour patterns of males and females (the average size at maturity for A. dispar and A. ginaonis varies between 20 and 30 mm standard length; unpublished data of the authors).
Aphanius ginaonis was sampled in February and August 2008 at the Geno hot spring (27°26′77″ N, 56°17′97″ E; altitude 200 m) (Fig. 1). During the last years, the Geno hot spring has become a popular destination for the local people, and the spring water has been directed into many artificial channels, which are each about 1 to 3 m wide. We sampled A. ginaonis from large populations along the margins of the channels in a water depth of ∼30 cm. Water parameters measured include temperature (35°C), pH (7.78), and conductivity (8,000 µS cm−1). The water was greenish and had a strong sulphur odour. The stream bed was composed of stones and pebbles covered by cyanobacteria.
Aphanius dispar from Mehran River was sampled in February 2008 near Dezhgan (26°52′85″ N, 55°16′35″ E; altitude 24 m), Kukherd village (27°4′43″ N, 54°31′30″ E), and Kordan village (27°4′32″ N, 54°33′3″ E) (Fig. 1a). The measured water parameters were as follows: Dezhgan: temperature 17.0°C, pH 8.22, conductivity >20 mS cm−1; Kukherd Village: temperature 18.8°C, pH 8.6, conductivity 59.3 mS cm−1, salinity 39.5 ppt; Kordan Village: temperature 25.3°C, pH 8.29, conductivity 59.3 mS cm−1, salinity 39.7 ppt.
Aphanius dispar from the Persian Gulf coast of the United Arab Emirates was sampled in September 2005 at the coastal site Khor Hulaylah (25°53′55 N, 56°03′23 E, altitude 0 m; measurements on water parameters not available). This sample includes the same specimens that were studied by Reichenbacher et al. (2009).
Fishes were fixed and preserved in 99.9% ethanol. Total length (TL) and standard length (SL) of each individual were measured. Skulls were then opened ventrally and the right and left otoliths removed. Organic residue was removed by incubating the otoliths in 1% KOH solution for 6 h and subsequent rinsing in distilled water for 12 h. For morphometric analysis only left otoliths were used to avoid accumulation of redundant data sets. Dissected specimens and otoliths are deposited in the Bavarian State Collection for Palaeontology and Geology in Munich, Germany, under accession number BSPG 2009 X.
Otolith morphology and morphometry
SEM images of otoliths from each population and available size class were prepared for comparative analysis (Figs. 3, 4). The terminology of otolith morphology follows previous studies (e.g. Nolf 1985; Smale et al. 1995; Tuset et al. 2008). Digital images used for the measurements were taken with a stereoscope with an attached Leica DC 490 digital camera. For photography, the otoliths were oriented with the outer/lateral side down and ventral rim parallel to a horizontal line. Measurements of distances and angles were performed with the Leica IMAGIC software, and functioned as input for the calculation of ten otolith variables according to the method outlined in Reichenbacher et al. (2007; see here Fig. 2b).
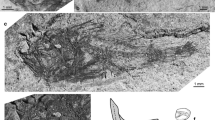
Otoliths of the studied Aphanius ginaonis population, arranged according to the size classes and sorted in males (d, e, i–o, b1–e1) and females; SEM micrographs. All figures are left otoliths in medial view. BSPG 2009 X. Standard length of fish specimens in mm: (a–e) 19, 22.5, 23, 21.5, 22.5; (f–o) 25, 26.5, 28, 25.5, 25.5, 26, 27, 27.5, 28, 28; (p–e1) 30, 30, 31, 31.5, 32, 32.5, 33, 33, 33, 33, 33, 33.5, 29, 29, 32, 33; (f1–i1) 34, 38, 35, 36
Otoliths of the studied Aphanius dispar populations, arranged according to the size classes and sorted in males (e, f, i–k, m–q, s) and females; SEM micrographs. Figures k, r, s are specimens from the coastal site Khor Hulaylah (United Arab Emirates, also figured in Reichenbacher et al. 2009), all other specimens come from the Mehran River in Iran. All figures are left otoliths in medial view. BSPG 2009 X. Standard length of fish specimens in mm: (a–f) 22, 22, 22.5, 23, 23, 23.5; (g–k) 24, 24.5, 25, 27.5, 24.6; (l–s) 32, 29, 29, 29.5, 31, 31, 30.2, 33.1
Statistical analyses were conducted with SPSS 16.00 (SPSS Inc. 2006). The Kolmogorov-Smirnov and Shapiro Wilk tests (p < 0.05) were used to test the normal distribution of the otolith variables. We suspect that the few non-normally distributed variables may represent artefacts resulting from the relatively small sample size; consequently these data were not normalized. The Mann-Whitney-U test and Kolmogorov-Smirnov tests were applied to infer possible sexual dimorphism in the individual otolith variables in A. ginaonis and the two A. dispar populations. One-way ANOVA with post-hoc tests (Tamhane-T2, p < 0.05) was used to compare the individual otolith variables among the size classes. The canonical discriminant analysis (CDA) was used to test whether the otolith variables separate A. ginaonis and the two A. dispar populations from each other; classification success was tested by jackknifed cross validation.
Results
Morphological description of otoliths
The otoliths of the Aphanius samples display uniform sulcus morphology (Figs. 3, 4). The sulcus is consistently subdivided into a small, slightly deepened, funnel-like anterior part (ostium) and a longer posterior part (cauda), which is first straight and then curving downwards (see also Fig. 2a). Based on the observed sulcus shape, all studied specimens satisfy the definition of otolith Group II of Aphanius, which also includes A. sirhani, but not the Mediterranean Aphanius species (see Reichenbacher et al. 2007). In contrast to the sulcus, the overall shape appears to be species-specific:
Aphanius ginaonis otoliths (Fig. 3) are oval-triangular to elliptic-triangular in shape; a few specimens show a small dorsal tip (Fig. 3, m–o, v). In most specimens, the rostrum is distinctly longer than the antirostrum and pointed. A few specimens have a rostrum and antirostrum of equal length with a rounded or blunt tip (Fig. 3, v, y, b1, c1). The antirostrum is well developed, thick, rounded or slightly pointed. The excisura is usually wide, deeply incised, and V- or U-shaped.
Aphanius dispar otoliths (Fig. 4) are oval-triangular in shape. The rostrum is slightly longer than the antirostrum, and the shape of the rostrum tip is variable (pointed, rounded, or blunt). The antirostrum is less pronounced than seen in A. ginaonis, and mostly pointed, the excisura is wide, deeply incised and V-shaped. Slight differences occur between the two populations. The otoliths from the Mehran River sample are slightly more rounded and have a thicker antirostrum with a blunter tip than those from the United Arab Emirates (see Fig. 4); in addition, these otoliths lack a prominent dorsal tip. For additional details on A. dispar from the United Arab Emirates, see Reichenbacher et al. (2009).
Otolith morphometry
Univariate analyses of the otolith variables (One-way ANOVA with post-hoc test, p < 0.05) support and complement the data obtained from the qualitative study of the otoliths. The otolith variables generally show normal distribution (Kolmogorov-Smirnov and Shapiro Wilk tests; p < 0.05). The excisura angle in A. ginaonis, and the antirostrum length in A. dispar from Mehran River, however, are apparently not normally distributed if all size classes are included in the statistical analysis. However, if the smallest size class is removed from the data set, these variables display a normal distribution pattern. Thus, ontogenetic variation and/or the relatively small sample size affect the normal distribution of the excisura angle in A. ginaonis, and the antirostrum length in A. dispar from Mehran River. As a result, we did not normalize our samples.
Sexual dimorphism
Dimorphism of otolith variables between the sexes were tested with the Mann-Whitney U-test and Kolmogorov-Smirnov tests (p < 0.05) for each sample. No dimorphism was found in A. ginaonis. A tendency towards dimorphism is visible in both A. dispar samples and concerns the posterior angle (lower values in females from Mehran River, but higher values in females from the United Arab Emirates).
Ontogenetic variation
Univariate analysis (One-way ANOVA with Tamhane post-hoc test, p < 0.05) indicates ontogenetic differences of several otolith variables in A. ginaonis and in A. dispar from Mehran River, which appear to be most distinct in the smallest specimens from size class 1 (see Fig. 5k). In A. ginaonis individuals of size class 1, the values of the excisura angle and medial length are significantly increased, whereas the values of the antirostrum length, and rostrum length are comparatively lowered (Fig. 5a, c, g, h). Aphanius dispar from Mehran River shows the same trend (Fig. 5d, f, i, j), even though the statistical tests only provide support for significant differences with regard to the excisura angle and the length–height (Fig. 5k). The lack of observable ontogenetic variation in A. dispar from the United Arab Emirates most likely is an artefact due to the low number of specimens in the smallest size class (see Table 1).
a–j Ontogenetic variation of otolith variables between the individual size classes (SC) of Aphanius ginaonis and A. dispar from Iran (Mehran River). Box plots showing the median (line within the box), the 25th and 75th percentiles and the data range, open circles refer to outliers within the 100th percentile. k Statistical support for the differences shown
Comparison between species and populations
We used univariate and multivariate statistics for a comparison of the otolith variables between A. ginaonis and the two A. dispar populations. Since the ontogenetic differences primarily affect the otolith variables of the specimens belonging to size class 1, we excluded size class 1 from the following statistical analyses.
Aphanius ginaonis vs. A. dispar
Five out of the ten otolith variables, i.e. posteroventral angle, dorsal length, length–height, medial length, and rostrum length, separate A. ginaonis from both A. dispar populations (Fig. 6c–f, h, k). The excisura angle, posterior angle, and antirostrum height provide additional support for a discrimination between A. ginaonis and A. dispar from the United Arab Emirates (Fig. 6a, b, g, k).
a–j Variation of otolith variables between populations of Aphanius ginaonis and A. dispar from Iran (Mehran River) and the United Arab Emirates (UAE, Khor Hulaylah); individuals from the size class 1 were removed from the data set. Box plots showing the median (line within the box), the 25th and 75th percentiles and the data range for the respective otolith variable, open circles refer to outliers within the 100th percentile. k Statistical support for the differences shown (the sample of A. dispar from UAE was also used in Reichenbacher et al. 2009)
Aphanius dispar (Iran, Mehran River) vs. A. dispar (United Arab Emirates)
Otoliths from Mehran River vary from the other A. dispar population in two variables, i.e. posterior angle and posteroventral angle (Fig. 6b, c, k).
The canonical discriminant analysis (CDA) with stepwise variable selection separates the Aphanius samples with an overall classification success (jackknifed) of 87.1% (Table 2). Misclassification of A. ginaonis is due primarily to confusion with otoliths from the A. dispar population from Mehran River. Separation power of the otolith variables, as selected by the CDA, is highest for the length–height, followed by the posteroventral angle, antirostrum height, excisura angle, and dorsal length (with decreasing separation power).
We then removed the otolith variables posterior angle and posteroventral angle (which are not considered species-specific, see Discussion section) in an additional CDA with stepwise variable selection. The classification success (jackknifed) for A. ginaonis remains unchanged, but displays a considerable decrease for both A. dispar populations (Table 2). Separation power of the otolith variables, as selected by the CDA, is highest for the length–height, followed by the antirostrum height, and antirostrum length (with decreasing separation power).
Correlation of otolith and fish size
Fish standard length and otolith length correlate for each species if all specimens (all size classes) are included (Fig. 7a, Pearson). The correlation is equally good for females and males of both A. dispar samples, whereas it is better for females than for males in A. ginaonis (Fig. 7a). Since our sample sizes were relatively small, we refrained from correlating fish standard length and otolith length for the individual size classes.
a Correlation (r) between otolith length (OL, in mm) and size-classes (SC) in females and males of the studied Aphanius samples (Spearman; *p < 0.05; **p < 0.01). S.D. standard deviation. b Differences in otolith length (mean values) between populations with regard to size classes (the sample of A. dispar from UAE was also used in Reichenbacher et al. 2009)
The measurements of the otolith length for the individual size classes indicate that males of A. ginaonis and A. dispar from Mehran River tend to have comparatively larger otoliths than females in the size classes 1 and 2, whereas females of A. ginaonis tend to have larger otoliths than males in the size class 3 (Fig. 7a). However, statistical tests (Mann-Whitney-U, Kolmogorov-Smirnov, p < 0.05) do not support these differences.
A comparison of the otolith length between the studied samples (without separation of females from males) was conducted using a One-way ANOVA with Tamhane post-hoc-Test (p < 0.05; Fig. 7b). The two A. dispar samples show distinct differences in their otolith dimensions; i.e. otoliths of A. dispar from the United Arab Emirates are consistently larger than those from Mehran River (see Fig. 7a for measurements). In size classes 2 and 3, otoliths of A. dispar from the United Arab Emirates are also larger than those of A. ginaonis. In addition, otoliths of A. ginaonis are larger than otoliths of A. dispar from Mehran River in the size class 2.
Discussion
Based on results from previous studies (Reichenbacher et al. 2007, 2009), we infer that otoliths of Aphanius species have (i) species-specific traits and (ii) traits that are characteristic of a population. The species-specific traits of Aphanius otoliths include the proportion of the maximal length and height, the relative height of antirostrum and rostrum, and the relative length of the rostrum. These traits can be indicated quantitatively by the otolith variables length–height, antirostrum height, rostrum height, and rostrum length. Traits that can be characteristic for a population include the variables excisura angle, posterior angle, medial length, and antirostrum length (for details see Reichenbacher et al. 2007). Here, we assessed whether otolith morphology and morphometry contribute to a sounder definition of A. ginaonis, and we also included analysis of intraspecific sexual and ontogenetic variability in otolith morphology.
Sexual dimorphism and ontogenetic variation
Sexual dimorphism, which may be expressed in otolith morphology (Morales-Nin et al. 1998; Reichenbacher and Sienknecht 2001), was not evident in A. ginaonis, whereas A. dispar exhibited sexual dimorphism with regard to the posterior angle. However, the posterior angle is not consistently high (or low) in females (or males), but rather shows opposite trends (high in females from the United Arab Emirates population, low in females from Mehran River). As differences in the reproductive behaviour between populations of A. dispar are not known, we are unable to explain this pattern of sexual dimorphism at the present time.
Aphanius ginaonis and A. dispar from Mehran River show a clear ontogenetic variation in the form of distinctly different otolith morphology in the smallest (youngest) specimens (size class 1, 19–23 mm SL), whereas such differences were weakly developed or absent in larger specimens (see Figs. 3, 4 and 5). Consistent differences in otolith morphology between juvenile and adult fishes are known for several groups of fishes, i.e. Merluccius and Sciaenids (Lombarte and Castellón 1991; Lombarte et al. 2003; Monteiro et al. 2005), and most likely are due to habitat differences and/or the behavioural differences between young and adult individuals. During our field trip in southern Iran (February 2008), very small (about 10 mm SL) and small individuals (about 20 mm SL) of A. dispar and A. ginaonis were found to live exclusively at the bottom of the habitat, whereas larger specimens often were observed near the water surface. The reduced rostrum length of the small (young) individuals of A. ginaonis and A. dispar (see Fig. 5h, j), for which we assume a demersal lifestyle, fits well with data from literature that a short or lacking rostrum appears in groundfishes, while a long rostrum characterizes pelagic fishes (e.g., Nolf 1985, 1995; Volpedo and Echeverría 2003). We therefore conclude that the short rostrum length and probably also the other ontogenetic differences in A. ginaonis and A. dispar mainly result from different lifestyles, i.e. demersal in small specimens (size class 1) and pelagic in larger specimens.
Separation of A. ginaonis by means of otolith morphometry
We excluded the small specimens of size class 1 from the analyses that focused on a sounder circumscription and definition of A. ginaonis by means of otolith morphology and morphometry. We found that two of the species-specific otolith traits, i.e. length–height, and rostrum length, separate A. ginaonis from both A. dispar samples. The antirostrum height provides additional support for the separation of A. ginaonis from A. dispar from the United Arab Emirates. In all, eight out of the ten available otolith variables differ between A. ginaonis and A. dispar from the United Arab Emirates, while five otolith variables differ between A. ginaonis and A. dispar from Mehran River (see also Fig. 6). As a result, we consider A. ginaonis a valid species, as proposed by Coad (1980, 1998). The otolith traits length–height and rostrum length can be used as complementary traits for species identification if the individuals have a standard length of at least 24 mm.
However, in some of the studied A. ginaonis otoliths we see an atypical development of the species-specific traits, i.e. a reduced rostrum length (e.g. Fig. 3, u, v), and a lower or higher length–height value (e.g. Fig. 3, h, k). The presence of these modified otolith types may indicate that A. ginaonis is characterized by an overall large natural variability in otolith morphology. On the other hand, we cannot exclude the possibility that the “atypical” otolith traits result from hybridisation. Otoliths of killifish hybrids may share characters from both the maternal and paternal lineage (Schulz-Mirbach et al. 2008). Thus, the reduced rostrum length and length–height may represent a heritage from a hybridisation between A. ginaonis and A. dispar. However, as A. ginaonis is the only native species in the Geno hot spring (Coad 1980), the question arises from where A. dispar might have been introduced into the habitat of A. ginaonis. According to Coad (1980) and unpublished observations of the co-authors, the A. ginaonis population was in strong decline during the last years. But the population appeared large when we visited the site in 2008. It is possible that A. dispar specimens were introduced in the waters of the Geno hot spring by local inhabitants to increase the population.
Adding support to the recent hybridisation hypothesis comes from a comparison of our A. ginaonis sample (collected in 2008) with a sample from a collection in 2001 (studied by Reichenbacher et al. 2007). This latter sample is predominantly composed of small specimens fitting size class 1 (n = 13), as well as three specimens of size class 3 and one individual of size class 4. Due to the limited number of larger specimens and the observed ontogenetic variation in the data set of this study (Fig. 5), we only compared the specimens of size class 1. The statistics show that two out of the ten otolith variables, i.e. the antirostrum height and antirostrum length, are different between the samples (Mann-Whitney-U and Kolmogorov-Smirnov tests, p < 0.05), with distinctly higher values in the sample from 2001: 37.5 (±6.5) vs. 28.0 (±5.3) for the antirostrum height, and 12.9 (±5.6) vs. 4.9 (±3.3) for the antirostrum length. It is unlikely that habitat differences in the Geno hot spring between 2001 and 2008 are responsible for these differences. We therefore hypothesize that (i) artificial introduction of A. dispar into the Geno hot spring has led to hybridisation, (ii) that the present-day population of A. ginaonis contains genetic material of the introduced A. dispar, and (iii) that this hybridisation is reflected in the observed deviations in some of the 2008 specimens from the “normal” A. ginaonis otolith morphology. The capability of Aphanius species to generate large numbers of offspring within less than one year (Frenkel and Goren 1997; Leonardos and Sinis 1999), and the ability of A. dispar to produce natural hybrids with other species (A. fasciatus, see Villwock 1985) provide further support for our hypothesis. It would be an interesting topic for a future study as to whether the hypothesized fast hybridization or segregation potential of Aphanius offers a model for explaining present-day species diversity, e.g. in Southwestern Anatolia or in Iran.
Otolith differences among A. dispar populations
The otolith morphology of the Aphanius dispar population from the United Arab Emirates represents the “basic otolith type” of the coastal populations of that species according to Reichenbacher et al. (2009) (see here Fig. 4, k, r, s). The A. dispar otoliths from Mehran River differ slightly from the basic type; i.e. they lack a dorsal tip and are more rounded in shape (Fig. 4, a–j, l–q). The more rounded shape is clearly reflected in the values of the posterior angle and posteroventral angle, which are distinctly lower in A. dispar otoliths from Mehran River than in A. dispar from the United Arab Emirates (Fig. 6b, c, e, f, k). However, the posterior angle is known to vary between populations, and also the posteroventral angle does not represent a species-specific trait (Reichenbacher et al. 2007). As a result, we assume that environmental parameters have affected these otolith traits. This assumption is supported by the size relations, as otoliths of A. dispar from Mehran River are significantly smaller than those of A. dispar from the Persian Gulf (Fig. 7). Thus, slower growth of the otoliths of A. dispar from Mehran River may be assumed. It is known that increased or reduced otolith growth rates most often are the result of changes in water temperature, water depth and diet (e.g., Lombarte and Lleonart 1993; Tuset et al. 2003; Katayama and Isshiki 2007; Mérigot et al. 2007). More elongate otolith contours are produced during increased growth rates, while more rounded otolith contours, as seen in A. dispar from Mehran River, occur if growth is reduced. We conclude that the different values of the posterior angle and the posteroventral angle in the two studied A. dispar populations result from differences in the respective environments, rather than from different genetic information. This assumption is consistent with the observation that none of the species-specific otolith variables (length–height, antirostrum height, rostrum height, rostrum length) differs between these A. dispar populations.
References
Blanco JL, Hrbek T, Doadrio I (2006) A new species of the genus Aphanius (Nardo, 1832) (Actinopterygii, Cyprinodontidae) from Algeria. Zootaxa 1158:39–53
Campana SE, Casselman JM (1993) Stock discrimination using otolith shape analysis. Can J Fish Aquat Sci 50:1062–1083
Cardinale M, Doering-Arjes P, Kastowsky M, Mosegaard H (2004) Effects of sex, stock, and environment on the shape of known-age Atlantic cod (Gadus morhua) otoliths. Can J Fish Aquat Sci 61:158–167
Clavero M, Blanco-Garrido F, Prenda J (2007) Population and microhabitat effects of interspecific interactions on the endangered Andalusian toothcarp (Aphanius baeticus). Environ Biol Fish 78:173–182
Coad BW (1980) A re-description of Aphanius ginaonis (Holly, 1929) from southern Iran (Osteichthyes; Cyprinodontiformes). J Nat Hist 14:33–40
Coad BW (1998) Threatened fishes of the world: Lebias ginaonis (Holly, 1929) (Cyprinodontidae). Environ Biol Fish 51:284
Coad BW (2000) Distribution of Aphanius species in Iran. J Amer Killifish Assoc 33:183–191
Coad BW, Abdoli A (2000) Systematics of an isolated population of tooth-carp from northern Iran (Actinopterygii: Cyprinodontidae). Zool Middle East 21:87–102
DeVries DA, Grimes CB, Prager MH (2002) Using otolith shape analysis to distinguish eastern Gulf of Mexico and Atlantic Ocean stocks of king mackerel. Fish Res 57:51–62
Doadrio I, Carmona JA, Fernández-Delgado C (2002) Morphometric study of the Iberian Aphanius (Actinopterygii, Cyprinodontiformes), with description of a new species. Folia Zool 51:67–79
Esmaeili HR, Ebrahimi M, Saifali M (2008) Karyological analysis of five tooth-carps (Actinopterygii: Cyprinodontidae) from Iran. Micron 39:95–100
Fernández-Pedrosa V, González A, Planelles M, Moya A, Latorre A (1995) Mitochondrial DNA variability in three Mediterranean populations of Aphanius iberus. Biol Conserv 72:251–256
Feulner GR (1998) Wadi fish of the UAE. Tribulus 8:16–22
Feulner GR (2005) Freshwater fishes. In: Hellyer P, Aspinall S (eds) The emirates—a natural history. Trident, London, pp 257–259
Frenkel V, Goren M (1997) Some environmental factors affecting the reproduction of Aphanius dispar (Rüppell, 1828). Hydrobiol 347:197–207
Hrbek T, Meyer A (2003) Closing of the Tethys Sea and the phylogeny of Eurasian killifishes (Cyprinodontiformes: Cyprinodontidae). J Evol Biol 16:17–36
Hrbek T, Keivany Y, Coad BW (2006) New species of Aphanius (Teleostei, Cyprinodontidae) from Isfahan Province of Iran and a reanalysis of other Iranian species. Copeia 2006:244–255
Katayama S, Isshiki T (2007) Variation in otolith macrostructure of Japanese flounder (Paralichthys olivaceus): a method to discriminate between wild and released fish. J Sea Res 57:180–186
Krupp F (1983) Freshwater fishes of Saudi Arabia and adjacent regions of the Arabian Peninsula. Fauna Saudi Arabia 5:568–636
Leonardos I, Sinis A (1999) Population age and sex structure of Aphanius fasciatus Nardo, 1827 (Pisces: Cyprinodontidae) in the Mesolongi and Etolikon lagoons (W. Greece). Fish Res 40:227–235
Lombarte A, Castellón A (1991) Interspecific and intraspecific otolith variability in the genus Merluccius as determined by image analysis. Can J Zool 69:2442–2449
Lombarte A, Lleonart J (1993) Otolith size changes related with body growth, habitat depth and body temperature. Environ Biol Fish 37:297–306
Lombarte A, Torres GJ, Morales-Nin B (2003) Specific Merluccius otolith growth patterns related to phylogenetics and environmental. J Mar Biol Assoc UK 83:277–281
Maltagliati F, Domenici P, Fosch CF, Cossu P, Casu M, Castelli A (2003) Small-scale morphological and genetic differentiation in the Mediterranean killifish Aphanius fasciatus (Cyprinodontidae) from a coastal brackish-water pond and an adjacent pool in northern Sardinia. Oceanol Acta 26:111–119
Maltagliati F, Lai T, Casu M, Valdesalici S, Castelli A (2006) Identification of endangered Mediterranean cyprinodontiform fish by means of DNA inter-simple sequence repeats (ISSRs). Biochem Syst Ecol 34:626–634
Mérigot B, Letourneur Y, Lecomte-Finiger R (2007) Characterization of local populations of the common sole Solea solea (Pisces, Soleidae) in the NW Mediterranean through otolith morphometrics and shape analysis. Mar Biol 151:997–1008
Monteiro LR, Di Beneditto APM, Guillermo LH, Rivera LA (2005) Allometric changes and shape differentiation of sagitta otoliths in sciaenid fishes. Fish Res 74:288–299
Morales-Nin B, Torres GJ, Lombarte A, Recasens L (1998) Otolith growth and age estimation in the European hake. J Fish Biol 53:1155–1168
Moreno-Amich R, Pou Q, Quintana X, García-Berthou E (1999) Efecto de la regulación hídrica en la conservación del fartet (Lebias ibera) en Aiguamolls de L’Empordà: Importancia de los refugios de población. In: Planelles-Gomis M (ed) Peces Ciprinodóntidos Ibéricos Fartet y Samaruc. Generalitat Valenciana, Valencia, pp 115–131
Nolf D (1985) Otolithi piscium. Handbook of paleoichthyology, vol 10. Gustav Fischer, Stuttgart
Nolf D (1995) Studies on fossil otoliths—the state of the art. In: Secor DH, Dean JM, Campana SE (eds) Recent developments in fish otolith research. University of South Carolina Press, Columbia, pp 513–544
Oliva-Paterna FJ, Ignacio Doadrio I, Fernández-Delgado C (2006) Threatened fishes of the world: Aphanius baeticus (Doadrio, Carmona and Fernández-Delgado, 2002) (Cyprinodontidae). Environ Biol Fish 75:415–417
Popper AN, Ramcharitar JU, Campana SE (2005) Why otoliths? Insights from inner ear physiology and fisheries biology. Mar Freshw Res 56:497–504
Reichenbacher B, Sienknecht U (2001) Allopatric divergence and genetic diversity of recent Aphanius iberus and fossil Prolebias meyeri (Teleostei, Cyprinodontidae) from southwest and western Europe, as indicated by otoliths. Geobios 34:69–83
Reichenbacher B, Sienknecht U, Küchenhoff H, Fenske N (2007) Combined otolith morphology and morphometry for assessing taxonomy and diversity in fossil and extant killifish (Aphanius, †Prolebias). J Morphol 268:898–915
Reichenbacher B, Feulner GR, Schulz-Mirbach T (2009) Geographic variation in otolith morphology among freshwater populations of Aphanius dispar (Teleostei, Cyprinodontiformes) from the Southeastern Arabian Peninsula. J Morphol 270:469–484. doi:10.1002/jmor.10702
Schulz-Mirbach T, Reichenbacher B, Yildirim Z, Atalay A (2006) Otolith characteristics of species, subspecies and populations of Aphanius Nardo, 1827 (Teleostei, Cyprinodontiformes) from Anatolia (Turkey). J Nat Hist 40:1687–1705
Schulz-Mirbach T, Scherb H, Reichenbacher B (2008) Are hybridization and polyploidization phenomena detectable in the fossil record?—A case study on otoliths of a natural hybrid, Poecilia formosa (Teleostei: Poeciliidae). N Jb Geol Paläont Abh 249:223–238
Sienknecht U (1999a) Diferencias genéticas a nivel de populación de Lebias ibera (Cuv. et Val. 1846) (Teleostei: Cyprinodontidae). In: Planelles-Gomis M (ed) Peces Ciprinodóntidos Ibéricos Fartet y Samaruc Monografía. Generalitat Valenciana, València, pp 213–223
Sienknecht U (1999b) Kreuzungsgenetische Analyse des Überganges von Paarflossen bis zu fehlendem Extremitätengürtel. Entwickelt am Beispiel der normogenetischen Ventralflossenbildung von Aphanius iberus (Cuv. and Val. 1846) im Vergleich zur Anormogenese seiner Hybriden mit A. apodus (Gervais 1853) (Teleostei: Cyprindontidae). Der Andere Verlag, Osnabrück
Smale MJ, Watson G, Hecht T (1995) Otolith atlas of southern African marine fishes. Ichthyological Monographs 1:1–253
SPSS Inc (2006) SPSS. Ver. 16.0. Base. Chicago, IL: SPSS, Inc.
Stransky C (2005) Geographic variation of golden redfish (Sebastes marinus) and deep-sea redfish (S. mentella) in the North Atlantic based on otolith shape analysis. ICES J Mar Sci 62:1691–1698
Stransky C, Baumann H, Fevolden SE, Harbitz A, Høie H, Nedreaas KH, Salberg AB, Skarstein T (2008) Separation of Norwegian coastal cod and Northeast Arctic cod by outer otolith shape analysis. Fish Res 90:26–35
Templeman W, Squire HJ (1956) Relationship of otolith lengths and weights in the haddock Melanogrammus aeglefinus (L.) to the rate of growth of the fish. J Fish Res Board Can 13:467–487
Tigano C, Canapa A, Ferrito V, Barucca M, Arcidiacono I, Deidun A, Schembri PJ, Omo E (2006) A study of osteological and molecular differences in populations of Aphanius fasciatus Nardo 1827, from the central Mediterranean (Teleostei, Cyprinodontidae). Mar Biol 149:1539–1550
Tuset VM, Lombarte A, González JA, Pertusa JF, Lorentes MJ (2003) Comparative morphology of the sagittal otolith in Serranus spp. J Fish Biol 63:1491–1504
Tuset VM, Lombarte A, Assis CA (2008) Otoliths atlas for the Western Mediterranean, North and Central Eastern Atlantic. Sci Mar 72S1:7–198
Villwock W (1977) Das Genus Aphanius Nardo, 1827. J dt Killif Gem 9:165–185
Villwock W (1985) Über Naturbastarde zwischen zwei validen Arten der Gattung Aphanius (Nardo, 1827) (Pisces: Cyprinodontidae) aus der Bardawil-Lagune, Nordsinai/Ägypten. Mitt Hamb Zool Mus Inst 82:311–317
Volpedo AV, Echeverría DD (2000) Catálogo y claves de otolitos para la identificación de peces del mar Argentino. Dunken, Buenos Aires
Volpedo A, Echeverría DD (2003) Ecomorphological patterns of the otolith in fish on the continental shelf off Argentina. Fish Res 60:551–560
Wildekamp RH (1993) A world of killies. Atlas of the Oviparous Cyprinodontiform fishes of the World, vol I. American Killifish Association, Mishawaka
Wildekamp RH, Küçük F, Ünlüsayin M, Neer WV (1999) Species and subspecies of the Genus Aphanius Nardo 1897 (Pisces: Cyprinodontidae) in Turkey. Tur J Zool. 23:23–44
Acknowledgments
Financial support was provided by the German Academic Exchange Service (DAAD, Middle East Biodiversity Network) and the Deutsche Forschungsgemeinschaft (Re 1113/14-1). Gary Feulner (Dubai, United Arab Emirates) provided the A. dispar specimens from Khor Hulaylah in the United Arab Emirates, Christine Frosch (Frankfurt, Germany) supplied the A. dispar specimens from Mehran River at Dezhgan, and Kai Borkenhagen (Frankfurt, Germany) provided the information of the habitat at Dezhgan. Nora Dotzler (Munich, Germany) helped with the otolith preparation, digital measurements of otoliths, and SEM images; R. Melzer (Munich, Germany) assisted with SEM. To all, we offer our sincere thanks. For constructive discussion we are grateful to M. Krings (Munich) and F. Krupp (Frankfurt), and for constructive comments on the manuscript we thank Christoph Stransky, an anonymous reviewer and the editor of the EBFi, David L.G. Noakes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichenbacher, B., Kamrani, E., Esmaeili, H.R. et al. The endangered cyprinodont Aphanius ginaonis (Holly, 1929) from southern Iran is a valid species: evidence from otolith morphology. Environ Biol Fish 86, 507–521 (2009). https://doi.org/10.1007/s10641-009-9549-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-009-9549-5