Abstract
Habitat loss and degradation are causing collapses in freshwater fish in the Mediterranean region, where habitat restoration actions are still hampered by poor understanding of fish habitat selection and fitness. Here, we combined field surveys and laboratorial experiments to investigate how water velocity, body size and intra-specific interactions influence habitat selection and foraging success by the highly endangered Mira chub Squalius torgalensis. Velocity negatively affected habitat selection and fitness of chub via its negative effects on prey capture rate. Small chub occupied lower velocity ranges than large chub, and both captured the most prey at the range of velocities selected in the stream. Size-based intra-specific interactions also affected capture success, with small chub capturing proportionally less prey in the presence of large chub. Our results suggest that, during base-flow conditions, restoring low and moderate velocities up to 26 cm/s will help ensure suitable habitat and improve the fitness of small and large chub occurring in interacting groups. Integrated approaches uncovering factors directly related to habitat selection and individual fitness should guide habitat restoration for fish in Mediterranean streams and may help identify critical habitat features for other endangered species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss and degradation is the main factor leading to biodiversity loss worldwide (Chase et al., 2020), with actions to avoid further habitat deterioration being urgently needed to reverse biodiversity declines (Tickner et al., 2020). The biodiversity crisis is more acute in freshwater ecosystems than anywhere else (Tickner et al., 2020), and the global rise in river degradation has prompted numerous efforts to protect and restore critical habitats for freshwater species (e.g., Sievers et al., 2017; Marttila et al., 2019; Raymond et al., 2019). However, past efforts frequently have fallen short in their outcomes (Palmer & Ruhi, 2019), with species recovery proving to be difficult and restoration failing to halt declines and meet conservation goals (e.g., Nilsson et al., 2015; Sievers et al., 2017; Lorenz et al., 2018). This may be partially because many attempts to quantify critical habitat have focused on physical factors alone (Haase et al., 2013; Hering et al., 2015), largely ignoring other important components of habitat suitability for target species (e.g., competition, predation, territoriality and feeding behavior) (Nestler et al., 2019). Indeed, habitat restoration in rivers traditionally includes a range of actions from watershed to reach scales (Beechie et al., 2010), which in the case of fishes have mostly involved the enhancement or creation of structural elements, such as spawning grounds, instream wood debris, and pools and riffles, with the expectation that fishes will recolonize, and their habitat requirements will be met (Roni et al., 2008; Beechie et al., 2010). Improving habitat restoration outcomes for endangered freshwater species will likely require biologically realistic approaches that include the study of the process of habitat selection and fitness outcomes (Hill & Grossman, 1993; Grossman, 2014; Hale et al., 2019, 2020).
Fitness-based habitat selection models provide a convenient approach to guide habitat management and restoration actions. These models typically quantify habitat selection based on individual fitness proxies, providing insights on the likely suitability of habitat features (Grossman, 2014; Piccolo et al., 2014; Rosenfeld et al., 2014). The inclusion of some measures of fitness or fitness proxies when classifying habitat quality and quantity will more likely lead to the establishment of self-sustaining populations of target species, thus to more effective preservation (Grossman, 2014; Hale et al., 2020). Strong relationships between habitat features and fitness proxies, such as foraging success, growth and survival have been identified for species in diverse river systems (e.g., Grossman, 2014; Bozeman & Grossman, 2019a,b; Polivka et al., 2020). Notably, velocity appears to be a critical component of habitat selection and foraging success by drift-feeding fishes (e.g., Hill & Grossman, 1993; Donaldson et al., 2013; Champion et al., 2018). However, other factors, such as ontogenetic variation in body size and intra-specific interactions, also may affect habitat suitability (Grossman et al., 1998; Petty & Grossman, 2010), particularly for species that change habitat and dietary preferences throughout ontogeny (e.g., Rosenberger & Angermeier, 2003; Ward et al., 2006). Therefore, clarifying how multiple factors affect habitat selection and foraging success might provide insights to improve habitat management and restoration for fish.
Greater insights into habitat selection and how habitat variation relates to fitness are particularly relevant for fish species in strongly modified rivers in the Mediterranean Basin Hotspot, which are among the most threatened species in the world (Freyhof et al., 2020). This region harbors many endemic fish that are seriously threatened by increasing degradation in natural habitats and flow regimes (Freyhof et al., 2020). Despite the growing number of studies devoted to fish-habitat relationships conducted in Mediterranean streams (e.g., Martínez-Capel et al., 2009; Santos et al., 2011, 2018; Martelo et al., 2014; Vardakas et al., 2017), little is known about habitat selection and its relation to individual fitness. Most studies have addressed how physical habitat resources are used both among species and by different size classes within a species (Grossman & de Sostoa, 1994a,b; Santos et al., 2011, 2018; Martelo et al., 2014), but how factors such as foraging success and intraspecific competition affect habitat selection by Mediterranean fishes remains poorly understood.
Here, we used an approach that combines fieldwork and laboratorial experiments to investigate habitat selection and foraging success by Mira chub Squalius torgalensis (Coelho et al., 1998), henceforth chub, a small Mediterranean cyprinid that is chiefly threatened by habitat loss and degradation (Rogado et al., 2005). We addressed the following questions: (1) Do large and small chub display selectivity with respect to velocity and other potentially relevant habitat conditions, namely depth, streambed composition and cover? (2) What is the relationship between individual prey capture success and velocity, body size and intra-specific interactions? (3) How does group size affect these relationship? Finally, we discussed habitat management and restoration recommendations for chub and explored how our approach may help guide and improve restoration efforts for other stream fish.
Methods
Study species
We focused on chub because it is typical of Mediterranean endemic fish and currently is listed as critically endangered (Rogado et al., 2005). This species has a very small distribution range, restricted to the Mira drainage in Southwest Portugal, where it can be locally abundant (Magalhães et al., 2002, 2007; Pires 2012; Pires et al., 2014), occurs in shoals from 2 to more than 10 individuals of similar or disparate sizes and shows aggregated distributions (Martelo et al., 2014). Chub displays small adult size (< 14 cm standard length SL), a relatively short life span (up to 5 years), early maturation (in 1–2 years), spring spawning (Magalhães et al., 2003), and preys upon drift and benthic invertebrates during daylight hours (Martelo et al., 2013). It may move for more than 100 m out of dry season pool refugia, but throughout the wet season movement is uncommon (Pires et al., 2014).
Study area
Our study was conducted in the Torgal (37° 38′ N, 8° 39′ W), a largely undisturbed stream that provides adequate conditions for investigating fish habitat requirements to inform conservation and restoration actions. The Torgal flows for 28 km and drains about 238 km2 of siliceous igneous rocks, slates and greywackes, discharging at about sea level into the Mira estuary. Human settlement is sparse, and land cover comprises mainly cork oak Quercus suber L. woodlands and eucalyptus Eucaliptus globulus Labill plantations, interspersed with pasture and dry cereal fields. The riparian galleries are well developed and dominated by alder Alnus glutinosa (L.) Gaertn. and ash Fraxinus angustifolia Vahl, with an understory of Mediterranean scrub. The stream is largely free from urban pollution, impoundments, angling and other recreational activities.
The climate is Mediterranean, with annual rainfall varying markedly from year-to-year (129–1121 mm); about 80% of the annual rain occurs in October–March and virtually none in the hot, dry months (July–August). Mean monthly temperature ranges from 11 °C (December) to 24 °C (August). Flow regime is highly dependent on rainfall patterns. Headwaters are ephemeral, while downstream reaches typically dry to isolated pools during summer-early fall. In dry years, there are no significant floods, the drying period is extended, and surface water is restricted to the deepest pools. Conversely, in wet years there are major floods, and flows may persist through summer-early fall in downstream reaches.
Habitat use and selection were analysed in an 80-m long reach with intact riparian vegetation that encompassed a shallow run (< 80 cm deep in spring) with clear water, bounded by deep pools and shallow rapids with boulders. This reach had persistent flow, which enabled us to avoid the potentially confounding effects of emigration and recolonization processes that may weaken fish-habitat relationships (Angermeier & Schlosser, 1989). As derived from electrofishing and snorkelling, chub dominated the local fish assemblage (87%), co-occurring only with Southwestern arched-mouth nase Iberochondrostoma almacai (Coelho et al., 2005) (12%) and exotic pumpkinseed Lepomis gibbosus (Linnaeus, 1758) (1%) from which it was easily distinguishable as juveniles and adults (Martelo et al., 2014).
Habitat selection
We quantified physical habitat selection by chub via underwater observations, which enable the exact location of individual fish (Almeida & Grossman, 2012), and generated focal data that might be further related to individual fitness traits (Grossman, 2014 and references therein). Previous studies demonstrated this method to be adequate for quantifying habitat selection by fish in other Mediterranean streams (Grossman & de Sostoa, 1994a,b; Martínez-Capel et al., 2009).
We conducted surveys during base-flow conditions, in late spring 2010, to avoid peak flows and biases associated with fish reproduction and dispersal. Underwater observations of fish were conducted during daylight hours so that habitat use by chub corresponded to foraging habitat (Martelo et al., 2013). Habitat conditions during the study were typical for the stream, as rainfall during October 2009 to September 2010 was close to the long-term annual median (658 versus 690 mm from 1931 to 2010; www.snirh.pt).
We quantified habitat use by chub using the methods of Grossman & de Sostoa (1994a, b). We first entered the reach from downstream and slowly moved upstream to minimize disturbance. Upon sighting an undisturbed chub, we marked its exact position using a numbered weight and visually estimated its standard length (SL, cm, ± 1 cm) (Fig. 1). Fish less than 5 cm were excluded because they were not reliably identifiable to species underwater. Although our focus was on velocity selection, we also quantified depth, streambed composition and cover that typically are correlated with velocity (Donaldson et al., 2013). Velocity (Global Water FP101 electronic velocity meter, ± 3 cm s−1) and depth (meter stick, ± 1 cm) were measured at the exact localization of the fish. We visually estimated proportions of substrata and cover in a 20 × 20 cm quadrat also at the fish’s location. We categorized substrata based on maximum linear dimension as: mud (≤ 0.2 cm), gravel (0.3–2.5 cm), small cobble (2.6–15 cm), large cobble (16–30 cm) and boulders (> 30 cm). Cover was categorized as roots, debris and aquatic vegetation.
To quantify habitat availability, we established a 1 × 1 m grid over the wetted channel of the study reach using transect ropes and numbered spikes (Freeman & Grossman, 1993; Martelo et al., 2014; Fig. 1). Spikes were driven into the banks at the ends of transect lines and marked with coloured tape. Habitat availability was quantified in approximately 10% of quadrats, resulting in a relatively similar number of used and available quadrats (51 versus 55), as recommended for minimization of modelling bias (Hosmer & Lemeshow, 1989). We stratified quadrats by location, selecting 80% of quadrats in the mid channel and 10% along each margin. Stratified random sampling was used to obtain a statistically reliable sample of habitat availability (Grossman & Skyfield, 2009). Velocity and depth were measured at the centre of each quadrat. Velocity was measured at 60% depth in quadrats ≤ 75 cm deep and at 20% and 80% depth in quadrats > 75 cm deep. The remaining habitat variables described above were estimated in a 20 × 20 cm quadrat in the centre of the 1 × 1 m quadrats.
In total, we observed 73 small chub (mean ± SD = 6 ± 1 cm; min–max = 5–7 cm) and 11 large chub (9 ± 1 cm; 8–10 cm) in 51 quadrats, and quantified habitat availability in 55 quadrats. Small and large chub corresponded chiefly to juveniles and adults, respectively (Magalhães et al., 2003).
Prey capture success
Fish collection and maintenance
Fish used in experiments were collected between March 2009 and July 2010 across the Torgal stream, using minnow traps set for 3 h, and transported to animal care facilities at Faculty of Sciences, University of Lisbon, in aerated coolers with stream water at ambient temperature. In total, we collected 216 small chub (6.4 ± 0.3 cm; 6.0–7.0 cm) and 216 large chub (8.3 ± 0.4 cm; 8.0–9.0 cm). None of the fish showed evidence of physiological stress or injuries.
For acclimation to the laboratory, fish were held for three days in a 900 L fiberglass recirculating tank with water conditions similar to those in the experimental tank. This period allowed fish to recover from collection stress and exhibit normal feeding. The holding tank had a submersible pump (4500 L/h) connected to a biofilter (Eheim Professionel) and chiller unit (Hailea HC-500C), and was supplied with aged tap water. Half of the water in the tank was renewed every two days and water conditions maintained at 15–17 °C, pH 7, and ammonia, nitrite and nitrate levels < 0.25 ppm. Water conditions were measured daily, and adjustments were performed whenever needed. A photoperiod of 12 h:12 h was used. Fish were fed red chironomid larvae ad libitum once daily. We used red chironomid larvae because they are comparable to chironomids preyed by endemic cyprinids in the wild (Magalhães, 1993), and were readily visible and consumed by chub in the laboratory.
After acclimation, individuals were anaesthetised with clove oil, measured (fork length, FL, cm, ± 1 mm) and weighted (g, ± 0.3 g). We then tagged chub with coloured plastic discs posterior to the dorsal fin (Hazelton & Grossman, 2009). To prevent infections from tagging, fish were treated with a prophylactic dose of the antibiotic MyOxin. After tagging, we kept fish for three additional days in the holding tank to assure they recovered and regained balance and swimming. Prior to experiments, fish were moved into a separated chamber of the holding tank for a 48-h non-feeding period to ensure all individuals were motivated to feed.
Experiments
The primary aim of prey capture experiments was to quantify the effects of velocity, body size and intra-specific interactions between small and large chub on prey capture success. Because chub occur in shoals of varying sizes in the stream (Martelo et al., 2013, 2014), we conducted experiments at three densities (see below). Test velocities and densities were within the values recorded in the stream (Martelo et al., 2014). It was not possible to test single individuals because they would not feed in the experimental tank.
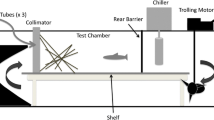
Following the methods of Hazelton & Grossman (2009), we used an experimental fiberglass tank with 2.0 × 1.0 × 0.6 m (length × width × height), in which a 55Lbs thrust electric trolling motor generated flow, and a chiller maintained water temperature (Hailea HC-1000 A) (Fig. 2). The experimental chamber, with 1.5 × 0.6 × 0.5 m, was enclosed at each end with mesh screens that acted as collimators to produce semi laminar flow and had a 2 cm layer of small cobble. We marked the experimental chamber at 1 cm intervals on the bottom and sides of the front wall, and placed 1 cm2 plastic quadrats across its bottom, at 10 cm intervals, to facilitate location of individuals. Temperature was maintained at 15–17 °C, pH near 7, and 12 h:12 h photoperiod, which represented the average conditions at the Torgal stream during the study period (Martelo et al., 2013).
Experiments included trials with single-size and mixed-size fish groups. In both groups, prey capture success was quantified by measuring individual capture rate at four average water velocities (2, 8, 12 and 26 cm s−1), and three fish densities (two, four and six individuals). In mixed-size groups we tested one large and small chub, two large and small chub, and three large and small chub.
Before a trial began, fish were allowed three hours to acclimate to the experimental tank and return to active feeding. During acclimation, velocity was increased gradually, at a rate of 2 cm s−1 every 10 min, until the treatment level was reached. Velocities were measured using an electronic velocity meter (Global Water FP101 ± 3 cm s−1) at 60% depth and mapped in 10 × 10 cm grids at three cross-sections of the tank (length x width x height). Velocity across the tank varied less than 2 cm s−1 for 2, 8 and 12 cm s−1 treatments, but for 26 cm s−1 the range was between 4 and 6 cm s−1.
During each trial, fish behaviour was observed and videotaped from behind a black plastic blind. Prey were released at approximately two minutes intervals, through one of three randomly selected (random number generator) silicone tubes at the front of the experimental chamber. Typically, a trial consisted of 20 releases of individual prey, however, if fish did not react to the first, second and the third prey released, a corresponding number of prey was added at the end of the trial. This occurred in 6% of the trials. Fish were classified as reacting to prey if they oriented directly towards the prey. Prey were either captured or missed. A capture represented a fish grasping a prey, regardless of whether it was swallowed. A miss represented a prey that was not captured, but retained in the mesh screens of the experimental chamber, thus not recirculating in the tank.
For each fish group (small-only, large-only and mixed-size), a minimum of three replicates per combination of velocity and fish density treatments were conducted. Experiments totalized 108 independent trials with individual fish being used in only one trial.
After experiments, fish were kept in holding aquaria and individuals in good conditions and showing no signs of contamination were released into the Torgal stream, approximately three to four weeks after capture, following the Portuguese legislation and the recommendations of ASAB for realising field-trapped animals (Vitale et al., 2018).
Data analysis
Prior to statistical analyses data were plotted and checked for outliers. We transformed skewed variables to approach normality and reduce the influence of peak values, using the angular transformation for proportional data and the log10 transform for continuous variables.
Habitat selection
We described the main gradients of variation in habitat availability and use by conducting a Principal Components Analysis (PCA) because data were strongly correlated (|r|> 0.50). This approach inherently considers the interrelation structure of habitat variables, and therefore it is more appropriate for the analysis of habitat than, for instance, multiple regression (Ahmadi-Nedushan et al., 2006). Because size-related variation in habitat use is common among Mediterranean stream fishes (e.g. Grossman & de Sostoa, 1994a, b; Santos & Ferreira, 2008), we conducted separate analyses for small and large chub. We built frequency distributions of principal component scores for habitat availability and use (Grossman & Freeman, 1987; Grossman & de Sostoa, 1994a, b), using the Sturges’ rule to define the number of bins in the histograms. We used Chi-square goodness of fit tests to contrast frequency distributions for (i) availability and use by small and by large chub, to assess habitat selection, and (ii) use by small and large chub, to assess size-related differences in habitat selection. Significance of statistical testing was assessed at 0.05 and analyses were conducted using the STATISTICA 10.0 software.
Prey capture success
Prey capture success was quantified for each fish as the ratio between the number of captures and the total prey released in each trial. We quantified relationships between capture success and velocity, fish size (i.e., small and large) and fish group (i.e., single- and mixed-size) using Generalized Linear Models with binomial distribution and logit links. Models were built separately for each density to avoid artificial effects associated with the use of constant prey numbers throughout densities.
We started by quantifying the effects of velocity and fish size on capture success in single-size groups. We next combined data from single- and mixed-size groups to quantify the effect of velocity and intra-specific interactions on the capture success of small and large chub. To produce balanced data sets, we randomly selected 65% of the observations from single-size groups. The number of observations in single-size groups was 16, 28 and 36 and in mixed-size groups was 12, 24 and 36 for two, four and six fish densities, respectively.
We built and compared the relative fit of five candidate models, including all explanatory variables (i.e., global model), all combinations of explanatory variables and two-way interactions, and an intercept only (i.e., null model). Global models were checked for normally distributed errors by examining residual plots. Candidate models were evaluated with Akaike Information Criteria for small sample sizes (AICc; Burnham & Anderson, 2002). The relative fit of each model was assessed via ΔAICc, with models for which ΔAICc < 2 having substantial support. Only models with Akaike weights > 10% of the model with the best fit were interpreted (Burnham & Anderson, 2002). Model selection uncertainty was quantified from model-averaged coefficient estimates (β), with 95% confidence intervals (Burnham & Anderson, 2002). Coefficients with confidence intervals including zero were not interpreted. Model selection and averaging analysis was conducted using the R software (R Core Team, 2019).
Results
Habitat availability
Velocity in the study reach was 7.7 ± 0.9 cm s−1 (mean ± SE) and ranged from 0.0 to 30.0 cm s−1 (Table 1). Thirty-seven percent of quadrats had zero velocities, with non-zero observations averaging 12.3 ± 0.8 cm s−1 and ranging from 3.0 to 30.0 cm s−1. Depth was 46 ± 2 cm and ranged between 10 and 81 cm (Table 1). Streambed was composed mainly of gravel (34%) and boulder (27%), moderate amounts of large cobble and mud (13–18%), and little quantities of small cobble (8%); debris were found in moderate quantities (11%) and roots and aquatic vegetation in very little amounts (4%) (Table 1).
Habitat selection
The PCA extracted four axes with eigenvalues higher than 1, though the third and fourth axes were not interpreted because they accounted only for 14 and 11% of the total variation, respectively, and after checking appeared to add little relevant information to other components. The first two principal components showed considerable variation in habitat availability and use by small and large chub (Fig. 3). PC1 (25% of the total variance) primarily represented a gradient in velocity and substratum composition and debris (Fig. 3a). PC2 (19% of total variance) represented a substratum gradient from boulder and roots to debris and gravel (Fig. 3b). Both small and large chub displayed habitat selection along PC1 (χ2 = 14.02, df = 7, P = 0.04 and χ2 = 38.29, df = 7, P < 0.0001, respectively) and along PC2 (χ2 = 27.82, df = 7, P = 0.0002 and χ2 = 41.06, df = 7, P < 0.0001, respectively). Moreover, there were significant differences between size classes in habitat use along PC1 (χ2 = 54.14, df = 7, P < 0.0001) and PC2 (χ2 = 68.60, df = 7, P < 0.0001). Small chub selected low to moderate velocity positions with intermediate amounts of small cobble, mud, gravel, and boulder and some debris and roots, whereas large chub selected positions with higher velocities, larger amounts of small cobble and more gravel.
Frequency distributions of principal component analysis scores for habitat available and used by small and by large chub (Squalius torgalensis) in the Torgal stream, during May 2010. a Scores along principal component 1 (25% of total variance) b Scores along principal component 2 (19% of total variance). Habitat variables with loadings higher than |0.50| in each ordination axis are shown. Scores range and class amplitude for principal component 1 were − 2.83 to 1.65 and 0.56, and for principal component 2 were − 2.13 to 2.77 and 0.61
Prey capture success
Velocity affected prey capture success for chub in single-size groups and at all densities (Tables 2, 3). Model averaging further indicated that capture success decreased with velocity and there was little evidence of either a fish size effect or velocity x fish size interaction (Table 3; Fig. 4a–c).
Models of capture success for small and for large chub in both single and mixed-size groups demonstrated that capture success was influenced by velocity, group, and the velocity × group interaction (Tables 4, 5). However, results differed among fish densities. Models for small chub in groups of two and six fish revealed that capture success decreased with velocity and was lower in the presence of large chub, except at high velocities (26 cm/s; Table 5; Fig. 4d, f). Models for groups of four fish also demonstrated a decrease in capture success for small chub (Table 5; Fig. 4e), but in the presence of large chub this effect had limited support, with model-averaged coefficient estimates overlapping zero (Table 5). Additionally, there was weak support for an interaction between velocity and group on capture success by small chub (Table 5). Models for large chub indicated that capture success decreased with velocity and was higher in the presence of small chub, at all fish densities (Table 5; Fig. 4 g–i). The effect of the interaction between velocity and group was weakly supported, with model-averaged coefficient estimates overlapping zero.
Discussion
Our integrated approach coupling descriptive field data on habitat use with experimental data on the relationship between a fitness surrogate (prey capture success) and a habitat feature (velocity) provided insights that can help plan habitat management and restoration for an endangered fish species in Mediterranean streams. Velocity played a key role with respect to both habitat selection and capture success by chub. Small and large individuals occupied distinct velocity ranges, and velocity negatively affected capture success. Experiments also showed that capture success was highest at velocities within the range selected by small and large chub in the stream, suggesting that capture success may be high at the positions occupied by chub. Consequently, these velocities likely represent suitable habitat features required by chub that should be targeted in habitat restoration actions. However, this may not suffice because size-based intra-specific interactions also affected capture success, with small chub capturing proportionally less prey in the presence of large chub, irrespective of velocity. Taken together, our findings suggest that habitat restoration plans for chub might be more successful when restoration of altered stream flows is combined with the assessment of fish size and group composition and accounts for chub foraging patterns.
Underwater observations showed that velocity was important to habitat selection by chub, with small individuals favoring lower velocities while large individuals selected higher velocities. Similar patterns have been reported in a variety of fishes in both Mediterranean streams (Grossman & de Sostoa, 1994a, b; Santos et al., 2004; Santos & Ferreira, 2008; Martínez-Capel et al., 2009) and elsewhere (Hill & Grossman, 1993; Donaldson et al., 2013; Henry & Grossman, 2008). Velocity selection by small and large chub was accompanied by differences in substratum and cover preferences between the two size classes. While small chub selected both fine and coarse substrata covered by debris and roots, large chub preferred fine substrata with no cover. Similarly, other studies have found size-related differences in the use of substrata and cover (Grossman & de Sostoa, 1994a, b; Santos et al., 2004,2018; Santos & Ferreira, 2008). We found no relation to depth, which is typically associated with size-related variation in habitat use in association with predation (e.g., Byström et al., 2003; Vardakas et al., 2017; Santos et al., 2018). However, this was unlikely to be the case here given piscivorous fish were virtually absent in the study reach, and otter Lutra lutra Linnaeus, 1758 mainly prey on crayfish and eels, with cyprinids being a staple prey in winter only (Beja, 1996). Nevertheless, a considerable amount of variation in the data remained unexplained as typically found in habitat selection studies (e.g., Grossman & Ratajczak et al., 1998a; Henry & Grossman, 2008; Santos et al., 2018), probably reflecting the influence of unmeasured environmental and biotic factors, and stochastic events (Grossman & Ratajczak et al., 1998a; Donaldson et al., 2013), which should be further investigated.
Experiments indicated that prey capture success by chub decreased with velocity, a trend that appears to be consistent among cyprinids (Grossman et al., 2002; Hazelton & Grossman 2009; Champion et al., 2018) and possibly reflects both the physical constraints on prey capture as well as the increase in energy expended per prey at high velocities. Moreover, capture success was highest at velocities corresponding to the range selected by small and large chub in the stream, suggesting that foraging success may be high at the positions occupied by chub. Consistent with our findings, other studies have showed that capture success can successfully be used to predict field velocity selection by drift-feeding fishes (Hill & Grossman, 1993; Grossman et al., 2002; Bozeman & Grossman, 2019a, b). Nevertheless, future quantification of effects of higher velocities occurring during winter and flash floods, when capture success might dramatically decrease (Hill & Grossman, 1993; Grossman et al., 2002), and of energetic costs and gains over that range of velocities is required to determine optimal velocities for chub (Hill & Grossman, 1993).
Beyond velocity, body size also affected prey capture success when accounting for interactions between different size individuals. Capture success of small chub was lower in the presence of large chub, suggesting that large individuals may outcompete small individuals, as found for other species (Petty &
Grossman, 2010; Kukuła et al., 2019), possibly due to their greater swimming abilities (Hill & Grossman, 1993; Tracy and Timothy 2009; Silva et al., 2012). Indeed, large chub are mostly found in mixed-size shoals (90%), which are more common than shoals with large chub-only (47% vs. 10%), while small chub frequency in mixed-size shoals is much lower (56%) and they are equally found in single and mixed-size shoals (43% vs. 47%) (Martelo et al., 2014; unpublished data). This suggests that intra-specific interactions between different size individuals may affect habitat suitability through their effect on prey capture success, and that large chub may benefit from being in mixed size shoals.
We failed to detect an effect of body size and of the interaction between body size and velocity on prey capture success in single-size groups. This was unexpected because larger fish generally are more able to maintain position under high velocities than smaller fish (Hill & Grossman, 1993; Tracy and Timothy 2009; Silva et al., 2012), and therefore more likely to capture prey at higher velocities. For example, capture success of medium rosyside and large sized dace (Clinostomus funduloides Girard, 1856; 47–52 mm and 53–70 mm SL, respectively) dropped below 90% at an average of 8 cm/s and 11 cm/s, respectively (Hill and Grossman, 1993; Grossman et al., 2002), and similar patterns have been found for rainbow trout [Oncorhynchus mykiss (Walbaum 1792)] (Hill and Grossman, 1993). In the case of chub, it is possible that this difference may only be expressed among more disparate size individuals and under higher velocities. Although our treatments were within the range of velocities prevailing in the Torgal and other streams in southwest Portugal (Santos & Ferreira, 2008; Pires, 2012), higher velocities are likely to occur during peak flows and major floods. It is possible that size-related differences in capture success might occur during these periods, with large chub being more able to cope with higher velocities than small chub.
We found evidence for the influence of velocity on intra-specific interactions. Capture success of large chub always was higher than that of small chub, except in groups of two and six fish at the highest velocity. Probably, prey capture ability of large chub was somewhat reduced at the highest velocity, allowing smaller individuals to capture more prey. Although many physical factors, including velocity (Warnock & Rasmussen, 2013; Jermacz et al., 2015), but also temperature (Mofu et al., 2019) and habitat complexity (Warfe & Barmuta, 2004), have been recognized as important drivers of interactions among fish species, little is still known about the factors influencing interactions among conspecifics differing in size. Additional studies, in which fish groups and velocity are manipulated and using more replicates, will be needed to understand the interactive effects of size-based intra-specific interactions and velocity on chub foraging success.
Our study provides an approach based on the understanding of habitat selection and prey capture success rather than conventional use of physical habitat features as proxies of habitat suitability that can help guide habitat restoration for chub. However, besides the improvements in capture success experiments mentioned above, the study of chub habitat selection can also be further improved. Specifically, assessing the effects of spatial variation in prey variability should help clarify the factors influencing habitat suitability. Moreover, although chub tend to dominate local assemblages (Magalhães et al., 2002, 2007; Pires 2012; Pires et al., 2014), inter-specific interactions should also be examined because competition often affects habitat selection in stream fishes (e.g., Hazelton & Grossman, 2009; Crow et al., 2010; Petty & Grossman, 2010). Finally, habitat selection by chub should be evaluated over broader environmental contexts, because habitat use by Mediterranean stream fish typically changes in response to spatial, seasonal, and annual variations in habitat availability (Grossman & de Sostoa, 1994a,b; Martelo et al., 2014; Vardakas et al., 2017; Santos et al., 2018).
Our study has important implications for habitat management and restoration for chub during base-flow conditions. We suggest that, during these periods, low to moderate velocities up to 26 cm/s are key components of habitat suitability for chub and will need to be provided in restored habitats to improve fitness outcomes. This should be accompanied by the restoration of other physical habitat features that influence velocity and were important for chub, specifically, mosaics of fine and coarse substrata covered by roots and debris. Finally, fish size and group composition should also be considered in restoration scenarios because intra-specific interactions may influence individual fitness, thus habitat suitability. These recommendations might also be useful for other fish in Mediterranean streams for which knowledge of habitat selection and fitness consequences remains scarce, given that these species frequently display size-related variation in habitat selection and often occur in interaction groups of varying sized individuals (Grossman & de Sostoa, 1994a,b; Santos & Ferreira, 2008; Vardakas et al., 2017; Santos et al., 2018).
Without actions to recover lost and degraded habitats freshwater fish species will continue to lose critical habitat. We argue that habitat management and restoration for endangered fishes in Mediterranean streams and elsewhere, should include restoring critical habitat features, considering factors directly related to individual fitness. As such, our approach integrating field analysis of habitat selection and foraging success experiments may prove useful in identifying suitable habitats to be restored to sustain multiple endangered species, and ultimately to reduce biodiversity losses.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmadi-Nedushan, B., A. St-Hilaire, M. Bérubé, É. Robichaud, N. Thiémonge & B. Bobée, 2006. A review of statistical methods for the evaluation of aquatic habitat suitability for instream flow assessment. River Research and Applications 22: 503–523.
Almeida, D. & G. D. Grossman, 2012. Utility of direct observational methods for assessing competitive interactions between non‐native and native freshwater fishes. Fisheries Management and Ecology 19: 157-166.
Angermeier, P.L. & I. J. Schlosser, 1989. Species-area relationships for stream fishes. Ecology 70: 1450–1462.
Beechie, T. J., D. A. Sear, J. D. Olden, G. R. Pess, J. M. Buffington, H. Moir, P. Roni & M. M. Pollock, 2010. Process-based Principles for Restoring River Ecosystems. BioScience 60: 209–222.
Beja, P. R., 1996. An analysis of otter Lutra lutra predation on introduced American crayfish Procambarus clarkii in Iberian Streams. Journal of Applied Ecology 33: 1156–1170.
Byström, P., L. Persson, E. Wahlström & E. Westman, 2003. Size‐and density‐dependent habitat use in predators: consequences for habitat shifts in young fish. Journal of Animal Ecology 72: 156-168.
Bozeman, B. & G. D. Grossman, 2019a. Foraging behaviour and optimal microhabitat selection in Yukon River Basin non anadromous Dolly Varden Charr (Salvelinus malma). Ecology of freshwater fish 28: 586-601.
Bozeman, B. & G. D. Grossman, 2019b. Mechanics of foraging success and optimal microhabitat selection in Alaskan Arctic grayling (Thymallus arcticus). Canadian Journal of Fisheries and Aquatic Sciences 76: 815-830.
Burnham K. P. & D. R. Anderson, 2002. Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York.
Champion, J. M., J. S. Rosenfeld & R. Shadwick, 2018. Effects of water velocity and substrate composition on foraging efficiency of an endangered benthic cyprinid, Nooksack dace (Rhinichthys cataractae subsp. cataractae). Hydrobiologia 805: 231–243.
Chase J. M., S. A. Blowes, T. M. Knigth, K. Gerstner & F. May, 2020. Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 584: 238-243.
Coelho, M. M., N. G. Bogutskaya, J. A. Rodrigues & M. J. Collares-Pereira, 1998. Leuciscus torgalensis and L. aradensis, two new cyprinids for Portuguese fresh waters. Journal of Fish Biology 52: 937-950.
Coelho, M. M., N. Mesquita & M. J. Collares-Pereira, 2005. Chondrostoma almacai, a new cyprinid species from the southwest of Portugal, Iberian Peninsula. Folia Zoologica: international journal of vertebrate zoology 54: 201-212.
Crow, S. K., G. P. Closs, J. M. Waters, D. J. Booker & G. P. Wallis, 2010. Niche partitioning and the effect of interspecific competition on microhabitat use by two sympatric galaxiid stream fishes. Freshwater Biology 55: 967–982.
Donaldson, J. A., B. C. Ebner & C. J. Fulton, 2013. Flow velocity underpins microhabitat selection by gobies of the Australian Wet Tropics. Freshwater Biology 58: 1038–1051.
Freeman M. C. & G. D. Grossman, 1993. Effects of habitat availability on dispersion of a stream cyprinid. Environmental Biology of Fishes 37: 121-130.
Freyhof, J., L. Bergner & M. Ford, 2020. Threatened Freshwater Fishes of the Mediterranean Basin Biodiversity Hotspot: Distribution, extinction risk and the impact of hydropower. Radolfzell, Germany: EuroNatur.
Grossman, G. D., 2014. Not all drift feeders are trout: a short review of fitness-based habitat selection models for fishes. Environmental Biology of Fishes 97: 465–473.
Grossman, G. D. & M. C. Freeman, 1987. Microhabitat use in a stream fish assemblage. Journal of Zoology 212: 151–176.
Grossman, G. D. & de Sostoa, 1994a. Microhabitat use by fish in the lower Rio Matarraña, Spain, 1984–1987. Ecology of Freshwater Fish 3: 123–136.
Grossman, G. D. & de Sostoa, 1994b. Microhabitat use by fish in the upper Rio Matarraña, Spain, 1984–1987. Ecology of Freshwater Fish 3: 141–152.
Grossman, G. D. & Jr. R. E. Ratajczak, 1998. Long‐term patterns of microhabitat use by fish in a southern Appalachian stream from 1983 to 1992: effects of hydrologic period, season and fish length. Ecology of Freshwater Fish 7: 108-131.
Grossman, G. D., Jr. R. E. Ratajczak, M. Crawford & M. C. Freeman, 1998. Assemblage organization in stream fishes: effects of environmental variation and interspecific interactions. Ecological Monographs 68: 395-420.
Grossman, G. D., P. A. Rincon, M. D. Farr & Jr. R. E. Ratajczak, 2002. A new optimal foraging model predicts habitat use by drift‐feeding stream minnows. Ecology of Freshwater Fish 11: 2-10.
Grossman, G.D. & J. P. Skyfield, 2009. Quantifying microhabitat availability: stratified random versus constrained focal-fish methods. Hydrobiologia 624: 235-240.
Hale, R., D. T. Blumstein, R. Mac Nally, & S. E. Swearer, 2020. Harnessing knowledge of animal behavior to improve habitat restoration outcomes. Ecosphere 11: e03104.
Hale, R., R. Mac Nally, D. T. Blumstein, & S. E. Swearer, 2019. Evaluating where and how habitat restoration is undertaken for animals. Restoration Ecology 27: 775-781.
Haase, P., D. Hering, S. C. Jähnig, A. W. Lorenz & A. Sundermann, 2013. The impact of hydromorphological restoration on river ecological status: a comparison of fish, benthic invertebrates, and macrophytes. Hydrobiologia 704:475–488.
Hazelton, P. D. & G. D. Grossman, 2009. The effects of turbidity and an invasive species on foraging success of rosyside dace (Clinostomus funduloides). Freshwater Biology 54: 1977–1989.
Henry, B. E., & G. D. Grossman, 2008. Microhabitat use by blackbanded (Percina nigrofasciata), turquoise (Etheostoma inscriptum), and tessellated (E. olmstedi) darters during drought in a Georgia piedmont stream. Environmental Biology of Fishes 83: 171-182.
Hering, D., J. Aroviita, A. Baattrup-Pedersen, K. Brabec, T. Buijse, F. Ecke, N. Friberg, M. Gielczewski, K. Januschke, J. Köhler, B. Kupilas, A. W. Lorenz, S. Muhar, A. Paillex, M. Poppe, T. Schmidt, S. Schmutz, J. Vermaat, P. F. M. Verdonschot, R. C. M. Verdonschot, C. Wolter & J. Kail, 2015. Contrasting the roles of section length and instream habitat enhancement for river restoration success: a field study of 20 European restoration projects. Journal of Applied Ecology 52: 1518-1527.
Hill J. & G. D. Grossman, 1993. An energetic model of microhabitat use for rainbow trout and rosyside dace. Ecology 74: 685-698.
Hosmer, D. W. J. & S. Lemeshow, 1989. Applied Logistic Regression. Wiley, New York.
Kukuła, K., B. Ortyl & A. Bylak, 2019. Habitat selection patterns of a species at the edge–case study of the native racer goby population in central Europe. Scientific reports 9: 1-11.
Jermacz, Ł., J. Kobak, A. Dzierżyńska, & T. Kakareko, 2015. The effect of flow on the competition between the alien racer goby and native European bullhead. Ecology of Freshwater Fish 24: 467-477.
Lorenz, A. W., P. Haase, K. Januschke, A. Sundermann, & D. Hering, 2018. Revisiting restored river reaches – Assessing change of aquatic and riparian communities after five years. Science of The Total Environment 613: 1185–1195.
Magalhães, M. F., 1993. Feeding of an Iberian stream cyprinid assemblage: seasonality of resource use in a highly variable environment. Oecologia 96:253–260.
Magalhães, M. F., P. Beja, C. Canas & M. J. Collares‐Pereira, 2002. Functional heterogeneity of dry‐season fish refugia across a Mediterranean catchment: the role of habitat and predation. Freshwater Biology 47: 1919-1934.
Magalhães, M. F., I. J. Schlosser & M. J. Collares-Pereira, 2003. The role of life history in the relationship between population dynamics and environmental variability in two Mediterranean stream fishes. Journal of Fish Biology 63: 300–317.
Magalhães, M. F., P. Beja, I. J. Schlosser & M. J. Collares-Pereira, 2007. Effects of multi-year droughts on fish assemblages of seasonally drying Mediterranean streams. Freshwater Biology 52: 1494–1510.
Martelo, J., G. D. Grossman & M. F. Magalhães, 2013. Extrinsic and intrinsic factors influence daily activity of a Mediterranean cyprinid. Ecology of Freshwater Fish 22: 307-316.
Martelo, J., G. D. Grossman, M. Porto & M F. Magalhães, 2014. Habitat patchiness affects distribution and microhabitat use of endangered Mira chub Squalius torgalensis (Actinopterygii, Cypriniformes). Hydrobiologia 732: 93–109.
Martínez-Capel, F., D. García de Jalón, D. Werenitzky, D. Baeza & M. Rodilla-Alamá, 2009. Microhabitat use by three endemic Iberian cyprinids in Mediterranean rivers (Tagus River Basin, Spain). Fisheries Management and Ecology 16: 52-60.
Marttila, M., P. Louhi, A. Huusko, T. Vehanen, A. Mäki-Petäys, J. Erkinaro, J. T. Syrjänen & T. Muotka, 2019. Synthesis of habitat restoration impacts on young-of-the-year salmonids in boreal rivers. Reviews in Fish Biology and Fisheries 29: 513-527.
Mofu, L., J. South, R. J. Wasserman, T. Dalu, D. J. Woodford, J. T. Dick & O. L. Weyl, 2019). Inter‐specific differences in invader and native fish functional responses illustrate neutral effects on prey but superior invader competitive ability. Freshwater Biology 64: 1655-1663.
Nestler, JM, R. T. Milhous, T.R. Payne, D.L. & Smith, 2019. History and review of the habitat suitability criteria curve in applied aquatic ecology. River Research and Applications 35: 1155– 1180.
Nilsson, C., L. E. Polvi, J. Gardeström, E. M. Hasselquist, L. Lind & J. M. Sarneel, 2015. Riparian and in-stream restoration of boreal streams and rivers: success or failure? Ecohydrology 8: 753–764.
Palmer, M. & A. Ruhi, 2019. Linkages between flow regime, biota, and ecosystem processes: Implications for river restoration. Science 365: 6459.
Petty, J. T. & G. D. Grossman, 2010. Giving-up densities and ideal pre-emptive patch use in a predatory benthic stream fish. Freshwater Biology 55: 780–793.
Piccolo, J.J., B. M. Frank, & J. W. Hayes, 2014. Food and space revisited: The role of drift-feeding theory in predicting the distribution, growth, and abundance of stream salmonids. Environmental Biology of Fish 97: 475–488.
Pires, D. F., 2012. Fish distribution and abundance in Mediterranean streams: the role of habitat quality, spatial context, and movement patterns. Ph.D. Thesis, University of Lisbon.
Pires, D. F., P. Beja & M. F. Magalhães, 2014. Out of pools: Movement patterns of Mediterranean stream fish in relation to dry season refugia. River Research and Applications 30: 1269–1280
Polivka, C. M., J. R. Mihaljevic & G. Dwyer, 2020. Use of a mechanistic growth model in evaluating post-restoration habitat quality for juvenile salmonids. PLoS ONE 15: e0234072.
R Core Team, 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/
Raymond, S., J. Koehn, Z. Tonkin, C. Todd, D. Stoessel, G. Hackett, J. O'Mahony, K. Berry, J. Lyon, J. Sharley & P. Moloney, 2019. Differential responses by two closely related native fishes to restoration actions. Restoration Ecology 27: 1463-1472.
Rogado, L., P. J. Alexandrino, P. R. Almeida, M. J. Alves, J. Bochechas, R. V. Cortes, M. I. Domingos, A. F. Filipe, J. Madeira & M. F. Magalhães, 2005. Peixes dulciaquicolas e migradores. In Cabral, M. J., J. Almeida, P. R. Almeida, T. Dellinger, N. Ferrand de Almeida, M. E. Oliveira, J. M. Palmeirim, A. I. Queiroz, L. Rogado & M. Santos-Reis (eds), Livro Vermelho dos Vertebrados de Portugal. Instituto da Conservação da Natureza, Lisboa.
Roni, P., K. Hanson & T. Beechie, 2008. Global review of the physical and biological effectiveness of stream habitat rehabilitation techniques. North American Journal of Fisheries Management 28: 856–890
Rosenberger, A., & P. L. Angermeier, 2003. Ontogenetic shifts in habitat use by the endangered Roanoke logperch (Percina rex). Freshwater Biology 48: 1563–1577.
Rosenfeld, J. S., N. Bouwes, C. E. Wall & S. M. Naman, 2014. Successes, failures, and opportunities in the practical application of drift-foraging models. Environmental Biology of Fishes 97: 551-574.
Santos, J. M. & M. T. Ferreira, 2008. Microhabitat use by endangered Iberian cyprinids nase Iberochondrostoma almacai and chub Squalius aradensis. Aquatic Sciences 70: 272–28.
Santos, J. M., F. N. Godinho & M. T. Ferreira, 2004. Microhabitat use by Iberian nase Chondrostoma polylepis and Iberian chub Squalius carolitertii in three small streams, north-west Portugal. Ecology of Freshwater Fish 13: 223–230.
Santos, J. M., L. Reino, M. Porto, J. Oliveira, P. Pinheiro, P. R. Almeida, R. Cortes, & M. T. Ferreira, 2011. Complex size-dependent habitat associations in potamodromous fish species. Aquatic Sciences 73: 233–245.
Santos, J. M., R. Rivaes, I. Boavida, & P. Branco, 2018. Structural microhabitat use by endemic cyprinids in a Mediterranean-type river: implications for restoration practices. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 26–36.
Sievers, M., R. Hale, & J. R. Morrongiello, 2017. Do trout respond to riparian change? A meta-analysis with implications for restoration and management. Freshwater Biology 62: 445–457.
Silva A.T., C. Katopodis, J. M. Santos, M. T. Ferreira & A. N. Pinheiro, 2012. Cyprinid swimming behaviour in response to turbulent flow. Ecological Engineering 44: 314-328.
Sistema Nacional de Informação de Recursos Hídricos. www.snirh.pt. Accessed April 2011.
Tickner, D., J. J. Opperman, R. Abell, M. Acreman, A. H. Arthington, S. E. Bunn, S. J. Cooke, J. Dalton, W. Darwall, G. Edwards, I. Harrison, K. Hughes, T. Jones, D. Leclère, A. J. Lynch, P. Leonard, M. E. McClain, D. Muruven, J. D. Olden, S. J. Ormerod, J. Robinson, R. E. Tharme, M. Thieme, K. Tockner, M. Wright, & L. Young, 2020. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. BioScience 70: 330–342.
Tracy R. L. & H. B. Timothy, 2009. Relationships among swimming ability, current velocity association, and morphology for freshwater lotic fishes, North American Journal of Fisheries Management 29:72-83.
Vardakas, L., E. Kalogianni, C. Papadaki, T. Vavalidis, A. Mentzafou, D. Koutsoubas, & N. Skoulikidis, 2017. Defining critical habitat conditions for the conservation of three endemic and endangered cyprinids in a Mediterranean intermittent river before the onset of drought. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 1270–1280.
Vitale, A., R. Calisi, C. Carere, T. Carter, J. C. Ha, R. Hubrecht, D. Jennings, N. Metcalfe, A. G. Ophir, J. M. Ratcliffe & T. C. Roth, 2018. Guidelines for the treatment of animals in behavioural research and teaching. Animal Behaviour 135: i-x.
Ward, A. J. W., M. M. Webster & P. J. B. Hart, 2006. Intraspecific food competition in fishes. Fish and Fisheries 7: 231–261.
Warfe, D. M. & L. A. Barmuta, 2004. Habitat structural complexity mediates the foraging success of multiple predator species. Oecologia 141: 171-178.
Warnock, W. G., & J. B. Rasmussen, 2013. Assessing the effects of fish density, habitat complexity, and current velocity on interference competition between bull trout (Salvelinus confluentus) and brook trout (Salvelinus fontinalis) in an artificial stream. Canadian Journal of Zoology 91: 619-625.
Acknowledgements
Thanks are due to António Martins, Amy Oliver and other assistants for field and laboratory support, and to Robert Ratajczak, Peter Hazelton and Duncan Elkins for training and logistical support. The study was conducted under permits of the Portuguese Forest Authority. Funding was granted by the Fundação para a Ciência e Tecnologia through Joana Martelo’s doctoral grant (SFRH/BD/35942/2007), the Centre for Ecology, Evolution and Environmental Change, and the Warnell School of Forestry and Natural Resources. Joana Martelo is currently supported by the Marine and Environmental Sciences Centre.
Funding
Funding was granted by the Fundação para a Ciência e Tecnologia through Joana Martelo’s doctoral grant (SFRH/BD/35942/2007), the Centre for Ecology, Evolution and Environmental Change, and the Warnell School of Forestry and Natural Resources. Joana Martelo is currently supported by the project ISO-INVA (FCT ref. PTDC/CTA-AMB/29105/2017).
Author information
Authors and Affiliations
Contributions
JM, GG, FM conceived and designed the research; JM performed field surveys and experiments; JM, FM performed statistical analyses; JM, GG, FM wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
The study was conducted under permits of the Portuguese Forest Authority. Laboratory procedures conformed to international guidelines (Vitale et al., 2018) and Portuguese legislation regarding animal capture, manipulation, and experimentation for scientific purposes. Fish feeding normally and with no external parasites were returned to the stream after the experiments.
Additional information
Handling editor: Pauliina Louhi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martelo, J., Grossman, G.D. & Magalhães, M.F. Habitat selection and foraging success by an endangered Mediterranean cyprinid: implications for habitat restoration. Hydrobiologia 848, 5187–5202 (2021). https://doi.org/10.1007/s10750-021-04701-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04701-y