Abstract
Studies on assemblages of freshwater fishes along elevational gradients of rivers are lacking, even in Europe. In this paper we have explored the entire range of elevational gradients existing in the European part of Russia. We analyzed how fish biodiversity (species richness, abundance, diversity indices) at 435 river sites differed by elevation. The impact of elevation on the distribution of freshwater fish species was analyzed using regression and ordination methods. For the first time for a large area of Eastern Europe, optimum points and niche breadth for fish species along altitude gradients were estimated. Our analyses showed: (1) species richness and Shannon index decreased in the upper part of the gradient; fish abundance showed a unimodal response to elevation; highest numbers were found at elevations between 250 and 500 m; (2) ordination analysis demonstrated an upstream-downstream gradient of the fish assemblages; (3) regression analysis showed significant preferences for elevation by 19 species, all of which were monotonic; (4) optimum and niche breadth (tolerance) were highly variable between species; only five species (brown trout, grayling, common minnow, bullhead and stone loach) were encountered at elevations above 650 m; and (5) in our region, the habitat of grayling was higher in the mountains, and its abundance (numbers) at extreme elevations was greater, than brown trout. These results show how fish assemblages differ with elevation. Our findings identify the data that can be used for regional environmental monitoring of the state of small rivers and for aquatic conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, studies of changes in animal and plant communities along elevation gradients have been of global interest to ecologists. Change in species diversity along altitude gradients has been one of the most documented patterns in terms of the spatial variation of biota, and studies have covered a large number of both countries and terrestrial organisms (e.g., Sanders and Rahbek 2011). Although numerous studies have been carried out on elevational patterns of species richness in plants, mammals and birds, fewer studies have been conducted on such patterns in fish (but see, e.g., Bhatt et al. 2012; Carvajal-Quintero et al. 2015), perhaps because of the need for labour-intensive trapping in the latter. In particular, studies on assemblages of freshwater fish along elevation gradients at the eastern edge of Europe are lacking (Askeyev et al. 2015).
A study of a whole region allows for assessment of a wide range of environmental conditions: i.e., small headwater streams in mountain or hill areas to large lowland channels downstream (Vannote et al. 1980). In riverine systems, the values of many environmental factors, such as elevation, usually correspond to a longitudinal upstream-downstream gradient, along which fish can easily disperse among preferred habitats. Zonation of fish species has been described along these elevation and longitudinal gradients (Huet 1959; Sheldon 1968). Zonation is characterised by successive replacements of species from mountains to lowland types of rivers.
Research of fish assemblages that use total species richness have often reported a tendency for richness to decrease linearly as elevation increases (a low-elevation peak) (Fu et al. 2004; Jaramillo-Villa et al. 2010; Askeyev et al. 2015). However, there are studies that show either a unimodal (i.e. mid-elevation optimum) or nonlinear (i.e. non-constant response to elevation) pattern (Grenouillet et al. 2004; Fu et al. 2004; Li et al. 2009), or an increase in species richness in high altitude zones (Carvajal-Quintero et al. 2015). In addition, studies on the influence of the altitudinal gradient on the change in number of fish are very rare.
Carvajal-Quintero et al. (2015) stated that studies on elevational changes cannot be based on altitudinal gradient changes in fish populations in one area, rather it is necessary to describe the whole range of existing altitudinal gradients in the region. For example, the elevation range in Tatarstan is limited and represents only a small part of the range that occurs across Eastern Europe. Therefore, we have expanded on our earlier work to a broader geographic area with a wider range of environmental parameters (for example, the South Ural region where there is a large range of elevation).
Most fish species on the eastern edge of Europe show distribution limits with elevation or lower abundance at higher elevations (Askeyev et al. 2014, 2015), most likely due to adverse climates. However, many hill and mountain ecosystems in this region of Europe remain pristine or only mildly influenced by humans, and often harbour rare species. In this context we specifically studied changes in fish populations along an elevation gradient in the far east of Europe. The ichthyofauna of rivers of the Middle Volga and South Ural regions live entirely in the catchment of the largest rivers in Europe, and despite the proximity of these regions to the margins of Eastern Europe, the fish fauna remains largely derived from European ichthyofauna. However, the fish assemblages of small and mid-sized rivers in the Volga and Ural catchments are poorly understood because of limited distributional information. These rivers and assemblages may represent good examples of others in Eastern Europe owing to their similar history. The main objectives of the study were: to (1) describe species richness and abundance in relation to elevation in small and mid-sized rivers on the eastern edge of Europe, (2) investigate changes in fish assemblages along elevation gradients, and (3) establish species optimum points and niche breadths in relation to elevation.
Material and methods
Study area and environment
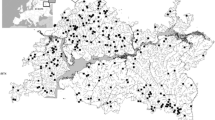
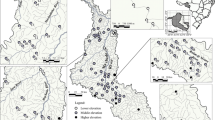
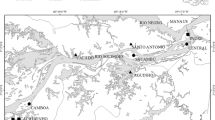
Our research was conducted in the eastern part of the Ponto-Caspian biogeographic region. This region as defined by Reyjol et al. (2007) includes biggest in Europe rivers - the Volga, Kama, Belaya, Ural, Don, Dnepr and Dniestr. This area (Fig. 1) is located in the extreme east of Europe and lies within the Tatarstan and Bashkortostan Republics of Russia. Historically, this area was considered to be within the Middle Volga and South Ural provinces of European Russia. These two republics comprise two natural zones, forest and forest-steppe, containing various habitats (sub-taiga coniferous deciduous mixed forest, montane coniferous mixed forest (South Ural), broad-leaved woods, farmland, steppe landscapes, rivers, lakes, and town and villages). The relief in the Tatarstan Republic is mostly flat or undulating lowland with hills [53–382 m a.s.l. (above sea level)] and mountains (350–1600 m a.s.l.) occur in the eastern part of the Bashkortostan Republic. The continental climate of the region is typical of Eastern Europe; mean annual temperature is c. 2.0 to 4.0 °C and monthly mean temperatures range from −12 to −15 °C in January, to 18 to 21 °C in July. The lowest temperature recorded in the past 200 years was −52 °C, and the maximum was 40 °C. Average annual precipitation is c. 500–700 mm and snow cover lies for 140–200 days. The main rivers, the Volga (3530 km), Ural (2420 km), Kama (1880 km), Belaya (1430 km) and Vyatka (1314 km), are among the 10 largest by length in Europe. Our study of fish populations covered almost the entire territory of the Tatarstan Republic and the mountainous part of the Bashkortostan Republic.
We focused on the study of fish assemblages of small rivers (length up to 500 km). We excluded rivers strongly influenced by large reservoirs. Fish sampling was conducted at 475 sites, but only the 435 sites containing fish were included in analyses. In 30 of the sites without fish, heavy oil water pollution and a strong odour were noted during sampling and are likely the cause of the absence of fish. In addition, 10 rivers above 700–900 m a.s.l. were also without fish, probably because mineral water or harsh climatic conditions. The occupied sites were located between 53 and 720 m a.s.l. with a maximum depth and width of 1.7 and 70 m, respectively. We also recorded other environmental variables (Askeyev et al. 2015) but they are not considered further in the current paper.
Fish assemblage data
Fieldwork was carried out from May to October in the years 2005–2015 during reduced summer flows. Fish samples were collected during active wading catches of similar length (60–70 min). In smaller rivers, four people actively fished by seining and dip netting across the full width of the river. Two people, one at each end, pulled the seine through the water until they reached the shore, beaching the seine on dry ground. We used three different lengths of the nets, depending on the width of the river. Three seining net were 5–15 m in length, with 1.2–1.5 m heights, mesh in the wings 5 × 5 mm, in bags 3 × 3 mm. Dip nets were with diameter of 50–70 cm, with mesh 4 × 4 mm. Lengths of between 200 m (smaller rivers) and 400 m (larger river) of the river sites were sampled. We follow to the recommendations, determining the length of the site, for catching fish by FAME CONSORTIUM (2004). Caught fish were placed in a plastic basin and identified, counted and measured at the end of each catch session, after which ≥90% of fish were returned to the water; the remainder kept for museum specimens (preserved in alcogol) or for food. Both presence-absence and abundance data of fish at each site were used in subsequent analyses.
Statistical analysis
We examined the relationships between species richness, total abundance (calculated as total number of fish in a catch), biodiversity indexes (Shannon_H and inverse Simpson Dominance_D) and elevation using regression analysis. Species assemblages were analyzed using Principal Coordinate Analysis (PCоA). Ordination was carried out on presence-absence data. The Bray-Curtis index was chosen as a dissimilarity measure between species because it is non-parametric and therefore has no assumptions about the underlying distribution as well as it is more robust than most other dissimilarity indices (Faith et al. 1987). It is for these reasons that Bray-Curtis index is a popular choice among ecologists for comparing communities. The significance of correlation between variables and axes was assessed by Monte-Carlo permutation tests. Individual species responses to elevation were modeled with the use of binomial generalized linear models (GLM) with a logit link. The hypothesis was that the probability of occurrence of a species increased or decreased monotonically with elevation. Both linear and quadratic forms of elevation were considered because species can have a unimodal response to an environmental gradient. The best explanatory model was selected based on the Akaike information criterion (AIC), where the model with the lowest AIC is the one that best fits the data.
Species optima in terms of fish numbers by elevation were calculated to rank species by habitat preferences. This was done by fitting Gaussian response models to species abundances along the elevational gradient. The fitted parameters are optimum (i.e. average) and niche breadth/tolerance (i.e. standard deviation). The algorithm is based on weighted averaging according to ter Braak and van Dam (1989). All calculations and graphics were done in PAST version 2.17c (Hammer et al. 2001) and XLSTAT 2015.
Results
A total of 71,788 individual fish of 42 species and two hybrids (Alburnus alburnus × Rutilus rutilus) and (Alburnus alburnus × Leuciscus?) were captured (see Table 1 for scientific and common names and abbreviations). English common names are used throughout the remainder of the text. Species richness per site ranged from 1 to 23 species (mean ± S.E. = 5.2 ± 0.15). Total number of fish at each site varied from 1 to 2003 individuals (mean ± S.E = 165.0 ± 10.8). The eight most abundant species (common minnow, gudgeon, stone loach, bleak, chub, sunbleak, dace and roach) comprised 90.0% of all captured individuals (Table 1). Only stone loach, gudgeon and common minnow were present in more than 50% of the catches (Table 1). The fish species, which occurred at less than 14 sites were excluded from analysis to prevent distortion of the results. Gibel was excluded from analysis because there were no good data about its systematic differentiation. Hybrid species were also excluded from analysis. Thus, the data matrix contained 21 species and 435 sites. We did not observe any fish farms that could have supplemented numbers of (native or non-native) fish in our sites. We have found and established habitats of five species listed in the Red Book of the Russian Federation (Table 1).
Relationship of species richness, total abundance and diversity indexes with elevation
Elevation had a significant effect on the fish assemblage parameters (Table 2). Species richness was negatively related to elevation (Fig. 2a). Total fish abundance showed a unimodal response to elevation; highest numbers was found at elevations between 250 and 500 m with a decrease at higher elevations (Fig. 2b). Shannon index decreased with elevation in contrast to Simpson index (Fig. 2c, d).
Fish assemblages and elevation (PCoA)
The first two axes of the PCоA explained 80% of the variability in the fish assemblage (axis 1: 65%, axis 2: 15%; Fig. 3). The first axis of PCoA was positively correlated with elevation (r = 0.81, P < 0.001). Correlations with the second axis were not significant. Generally, the sequence from left to right on the first axis demonstrated a change of fish assemblages along the elevation gradient. At the species level, results suggested that species such as common minnow, grayling, brown trout, bullhead and stone loach were particularly associated with “high” elevations. Other fish species were mainly associated with “lowland” rivers.
Principal Coordinate Analysis (PCоA) biplot of fish assemblege and elevation variables in the eastern edge of Europe. Elevation is shown as a vector (see Table 1 for species abbreviations)
Binomial generalized linear models (GLM) for each of the 21 species
Elevation was the variable that explained most about the distribution of fish in our study area. Binomial regression showed that all but two species (burbot, spirlin) had statistically significant relationships with elevation (Table 3). All the best models were linear according to the AIC criterion. Figure 4 shows the probabilities of occurrence for the 19 significant species against elevation. For brown trout, grayling and bullhead, a positive relationship with elevation was evident but probabilities of occurrence were zero or nearly zero below 130–150 m a.s.l. Common minnows reached a maximum above 500 m. Stone loach and gudgeon occurred with high probabilities across all elevations. Bleak, sunbleak, chub, dace, perch, spined loach and pike occurred over almost the full range of elevation, but the probability of occurrence decreased with increasing elevation and were nearly absent above 500 m. For roach, Volga nase, white-finned gudgeon, common bream and ruffe the probability of occurrence decreased sharply along the elevation gradient, and probabilities of occurrence become zero above 100–200 m a.s.l.
The effect of elevation range on the probabilities of the occurrence of 19 freshwater fish species in the eastern edge of Europe. The model expected values are in red, and the confidence interval bands are in grey (see Table 1 for species abbreviations)
Species optimum point and niche breadth
Table 4 shows differences between species in their optima and tolerance in relation to elevation gradients. Optimal points by elevation differed from 60.9 m a.s.l. (white-finned gudgeon) to 469.6 m a.s.l. (grayling) and only four species had optimal points above 300 m a.s.l. In addition, only five species (brown trout, grayling, common minnow, bullhead and stone loach) were encountered at elevations above 650–700 m (Table 4). Niche breadth (tolerance) was highly variable between species (Table 4). Volga nase, white-finned gudgeon and roach had a low tolerance, indicating that these were highly specialized with respect to elevation. Ten species had tolerance values between 136.1 and 195.8 and these species had their optimal points between 150 and 400 m elevation.
Discussion
Our study presents the analysis of a large novel data set reflecting the elevational distribution of river fish assemblages on the boundary between Europe and Asia and confirms the existence of such gradients in rivers in the Tatarstan and Bashkortostan Republics.
Total species richness showed a gradual decline with increasing elevation, supporting Rapoport’s rule (Rapoport 1982; Stevens 1992). Our study is also in agreement with studies of fish species in the Yangtze River basin (Fu et al. 2004), in the southern Appalachians (Robinson and Rand 2005), in Himalaya (Bhatt et al. 2012), and in the central Andes (Jaramillo-Villa et al. 2010), which reported that the diversity of freshwater fish decreased gradually with elevation. The opposite was reported in the northern Andes by Carvajal-Quintero et al. (2015) where highest species richness was found at lower elevations between 250 and 1000 m a.s.l. and species richness decreased with increasing elevation up to 1750 m a.s.l., but above 2000 m a.s.l. species richness increased.
Of course, the elevational range in our study area is limited and represents only a small part of the range that occurs across the world. However, we have explored the entire range of elevational gradients existing in the European part of Russia (4,000,000 sq.km). Indeed this allows us to use our data in macroecological studies much more efficiently than data obtained from a restricted range of elevations. In addition, we can rule out changes in species richness on an elevational gradient being caused largely by the presence of endemic species in the samples, since endemism was low in the region. We agree with various authors (Campbell Grant et al. 2007; Finn et al. 2011; Dias et al. 2013) who stated that the conditions of rivers was more complex and fragmented toward their headwaters and can promote isolation in assemblages of freshwater animals. Isolation, in turn, leads to speciation in fish assemblages (Oberdorff et al. 1999; Fu et al. 2004; Tedesco et al. 2012; Carvajal-Quintero et al. 2015) particularly at high elevations. In our case, and indeed in Europe, the proportion of endemic species in areas of high-elevation is very small. For example, in our region there are no endemic species, perhaps the result of the absence of climatic and geographic isolation of these rivers. However, it should be said that we consider the main reason for the lack of endemism is the extremely harsh climatic conditions in the region. Even with geographical conditions for speciation (barriers, distribution limits) and, in our region, the presence of distributional limits for more than 20 fish species, the climate component does not provide conditions at high elevations for this process. In our context climate (winter lasts 6–7 months, temperature dips annually to −40 °C and in some years to −50 °C, and many rivers freeze to the bottom) does not facilitate speciation.
We observed that the Shannon index decreased with elevation, i.e. the diversity of common (or typical) species in the fish community decreased on an elevational gradient. Simpson’s index did the opposite, which can be interpreted as increasing the share of the numerically dominant species at higher elevations. Again, these processes were monotonic. The behaviour of these indices suggests that the fish community at high-elevations is dominated numerically by a few species. Only three species (common minnow, grayling and brown trout) dominate above 600 m. This can be interpreted as adaptation of the fish populations to the existing available natural resources (Matthews 1998). In contrast to diversity, total abundance showed a unimodal response to elevation (Fig. 2b). The highest fish numbers were found at elevations between 250 and 500 m. Foremost, it should be noted that the numbers of animals (abundance, density) reflect the quality of the environment which in turn affects the amount of time the reproductive rate has been positive; whereas, the number of species reflects the evolutionary history of the region. So we see that, on the elevational gradient, fish numbers and species richness behaved differently.
Two major environmental gradients determine European fish assemblages: physical characteristics of the river channel, varying along the longitudinal gradient (stream size and slope), and the thermal gradient (Buisson et al. 2008; Logez et al. 2012). In our case, PCоA results and earlier research (Askeyev et al. 2015) indicated that elevation played an important role in determining species composition of fish assemblages. Here it must be said that the gradual change in elevation is strongly correlated with changes in temperature and slope gradients. There is an opinion that, in areas with an abrupt change in elevation, fish communities occur along a stream longitudinal zonation (Matthews 1998). Our results, which were obtained in the extreme east of Europe, are similar to the data collected in Western Europe. In general, the elevation gradient of fish assemblages (fish zones) was similar to that elsewhere in Europe (Huet 1959; Verneaux 1977; Belliard et al. 1997; Oberdorff et al. 2001; Pont et al. 2005; Humpl 2006; Grenouillet et al. 2007; Bergerot et al. 2008; Birzaks 2012). However, it should be said that, in terms of elevational gradient, some cold-water species were arranged a little differently than in Western Europe. For example, grayling and common minnow zones were located above the trout zone. The reasons for this are discussed below.
Our results demonstrated that the response of the typical upstream (cold-water) fish fauna to elevational range differed from that of species inhabiting downstream (warm-water) rivers. Presence-absence data show significant elevational preferences by 19 species at the eastern edge of Europe, all of which were monotonic. Our data are important for two reasons, first we analysed the elevational range of fish species directly, which is rarely done in studies reported in the literature, and second, our study area is at the eastern limit of the majority of European fish species. Therefore, differences in elevational preferences between species in our study and those in Western Europe may be because of the abovementioned reasons. Preference zones in elevation range lie above 500 m for only four species (grayling, brown trout, common minnow, and bullhead); for other species the zones are much lower. Further south in Russia, for example in the Terek-Caspian region the upper boundary of the range of brown trout within the Chechen Republic is 1869 m a.s.l. but the optimal zone lies between 400 and 800 m (Kaimov 2013).
Optimal points and tolerance in environmental gradients can be interpreted as realized niche position and breadth. The 21 studied species were distributed along all elevations according to their optimum points, reported for the first time in this eastern part of Europe. In a previous paper, we acknowledged that the elevation range in Tatarstan is limited and represents only a small part of that found in Eastern Europe (Askeyev et al. 2015). These optima and niche ranges are not directly comparable with those in the literature because the latter were basically estimated using presence-absence data (Pont et al. 2005; Buisson et al. 2008; Logez et al. 2012). It was very interesting that in our region the highest optimum point was for grayling. Below it followed brown trout, common minnow and bullhead. After analysis of the elevational gradient, we detected that the least marginal species with the broadest niche were cold-water species (bullhead, burbot, gudgeon and common minnow), whereas marginal and specialized species were warm-water species (white-finned gudgeon, Volga nase, roach, common bream).
Analyzing the above, we can construct our views on the “fish zone” along an elevational gradient in Eastern Europe. The species ordination along the elevational gradient is basically consistent with the literature. Salmonids, common minnow and bullhead formed the highest zone on the gradient. In the Chechen Republic, brown trout is the only representative of the fish fauna above 900 m a.s.l. (Kaimov 2013). However, in our region, grayling exists higher in the mountains, and its abundance (numbers) at extreme elevations is greater than brown trout. In Europe, grayling zones are lower than the brown trout zone (Huet 1959; Grenouillet et al. 2007; Logez et al. 2012). This difference is probably because of the more extreme conditions observed in our region. Grayling, in contrast to brown trout, is a species of a central Asian and taiga origin and more fitted to a mid-continental climate (with a sharp temperature amplitude). For example, in our region, the amplitude between mean January and July temperature is 30–35 °C. Furthermore, Logez et al. (2012) confirmed that probability of grayling presence increased with increasing thermal amplitude, whereas brown trout decreased along a thermal gradient. The following fish zones are absolutely consistent with the literature. “Warm water” fish species such as roach, ruffe, Volga nase, white-finned gudgeon, common bream, and bleak form a zone in the lowest part of the gradient. Perch, chub, dace, gudgeon, spinned loach, and some other species occupied a middle position between the above two groups.
In conclusion, we successfully modelled the fish assemblage changes along an elevation gradient, and report the optima and niche width of elevation for 21 fish species in mid-sized and small rivers in the eastern edge of Europe. We also demonstrated that the effect of elevation on fish populations differs in different parts of the world.
The findings of our work identify the data that can be used in regional environmental monitoring on the state of small rivers. Fact sheets on the distribution of rare and endangered fish species may be used in the planning and conduct of economic activity, as well as ecological activities (such as protection, restoration and acclimatization) and to study the biological diversity of aquatic ecosystems.
References
Askeyev A, Monakhov S, Askeyev I, Askeyev O (2014) Rare and endangered fish species distribution in relation to environmental gradients, Tatartstan Republic, Russia. Proceedings of Scientific Works of Institute of Problems Ecology and Mineral Wealth, Kazan, pp 3–15
Askeyev O, Askeyev I, Askeyev A, Monakhov S, Yanybaev N (2015) River fish assemblages in relation to environmental factors in the eastern extremity of Europe (Tatarstan Republic, Russia). Environ Biol Fish 98:1277–1293
Belliard J, Boet P, Tales E (1997) Regional and longitudinal patterns of fish community structure in the Seine River basin, France. Environ Biol Fish 50:13–147
Bergerot B, Lasne E, Vigneron T, Laffaille P (2008) Prioritization of fish assemblages with a view to conservation and restoration on a large scale European basin, the Loire (France). Biodivers Conserv 17:2247–2262
Bhatt JP, Manish K, Pandit MK (2012) Elevational gradients in fish diversity in the Himalaya: water discharge is the key driver of distribution patterns. PLoS One 7(9):e46237
Birzaks J (2012) Occurrence, abundance and biomass of fish in rivers of Latvia in accordance with river typology. Zoology and Ecology 22(1):9–19
ter Braak CJF, van Dam H (1989) Inferring pH from diatoms: a comparison of old and new calibration methods. Hydrobiologia 178:209–223
Buisson L, Blanc L, Grenouillet G (2008) Modelling stream fish species distribution in a river network: the relative effects of temperature versus physical factors. Ecol Freshw Fish 17:244–257
Campbell Grant EH, Lowe WH, Fagan WF (2007) Living in the branches: population dynamics and ecological processes in dendritic networks. Ecol Lett 10:165–175
Carvajal-Quintero JD, Escobar F, Alvarado F, Villa-Navarro FA, Jaramillo-Villa Ú, Maldonado-Ocampo JA (2015) Variation in freshwater fish assemblages along a regional elevation gradient in the northern Andes, Colombia. Ecology and Evolution 5(13):2608–2620
Dias MS, Cornu JF, Oberdorff T, Lasso CA, Tedesco PA (2013) Natural fragmentation in river networks as a driver of speciation for freshwater fishes. Ecography 36:683–689
Faith DP, Minchin PR, Belbin L (1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69:57–68
FAME CONSORTIUM (2004) Manual for the application of the European Fish Index - EFI. A fish-based method to assess the ecological status of European rivers in support of the Water Framework Directive. Version 1.1, January 2005
Finn DS, Bonada N, Murria C, Hughes JM (2011) Small but mighty: headwaters are vital to stream network biodiversity at two levels of organization. J N Am Benthol Soc 30:963–980
Fu C, Wu J, Wang X, Lei G, Chen J (2004) Patterns of diversity, altitudinal range and body size among freshwater fishes in the Yangtze River Basin, China. Glob Ecol Biogeogr 13:543–552
Grenouillet G, Pont D, Hérissé C (2004) Within-basin fish assemblage structure: the relative influence of habitat versus stream spatial position on local species richness. Can J Fish Aquat Sci 61:93–102
Grenouillet G, Roset N, Goffaux G, Breine J, Simoens I, Deleeuw J, Kestemont P (2007) Fish assemblages in European western highlands and Western Plains: a type-specific approach to assess ecological quality of running waters. Fish Manag Ecol 14:509–517
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Huet M (1959) Profiles and biology of western European streams as related to fisheries management. Trans Am Fish Soc 88:155–163
Humpl M (2006) Relating fish assemblage variability to environmental gradients in small and mid-sized streams of the Elbe basin (Czech Republic). Electronic Journal of Ichthyology 1:15–25
Jaramillo-Villa U, Maldonado-Ocampo JA, Escobar F (2010) Altitudinal variation in fish assemblage diversity in streams of the Central Andes of Colombia. J Fish Biol 76:2401–2417
Kaimov MG (2013) Ecology and biological features of brown trout (Salmo trutta morpha fario (Linnaeus, 1758) and (Salmo trutta ezenami. Berg, 1948) in Terek-Caspian region. Dissertation, Dagestan State University
Li J, He Q, Hua X, Zhou J, Xu H, Chen J, Fu C (2009) Climate and history explain the species richness peak at mid-elevation for Schizothorax fishes (Cypriniformes: Cyprinidae) distributed in the Tibetan Plateau and its adjacent regions. Glob Ecol Biogeogr 18:264–272
Logez M, Bady P, Pont D (2012) Modelling the habitat requirement of riverine fish species at the European scale: sensitivity to temperature and precipitation and associated uncertainty. Ecol Freshw Fish 21:266–282
Matthews WJ (1998) Patterns in freshwater fish ecology. Chapman and Hall, Norwell, New York
Oberdorff T, Lek S, Guegan JF (1999) Patterns of endemism in riverine fish of the northern hemisphere. Ecol Lett 2:75–81
Oberdorff T, Pont D, Hugueny B, Chessel D (2001) A probabilistic model characterizing fish assemblages of French rivers: a framework for environmental assessment. Freshw Biol 46:399–415
Pont D, Hugueny B, Oberdorff T (2005) Modelling habitat requirement of European fishes: do species have similar responses to local and regional environmental constraints? Can J Fish Aquat Sci 62:163–173
Rapoport EH (1982) Areography: geographical strategies of species (English edition translated by Drausal B). Pergamon Press, New York
Reyjol Y, Hugueny B, Pont D, Bianco PG, Beier U, Caiola N, Casals F, Cowx I, Economou A, Ferreira T, Haidvogl G, Noble R, De Sostoa A, Vigneron T, Virbickas T (2007) Patterns in species richness and endemism of European freshwater fish. Glob Ecol Biogeogr 16:65–75
Robinson JL, Rand PS (2005) Discontinuity in fish assemblages across an elevation gradient in a southern Appalachian watershed, USA. Ecol Freshw Fish 14:14–23
Sanders NJ, Rahbek C (2011) The patterns and causes of elevational diversity gradients. Ecography 35:1–3
Sheldon AL (1968) Species diversity and longitudinal succession in stream fishes. Ecology 49:193–198
Stevens GC (1992) The elevational gradient in altitudinal range, an extension of Rapoport’s atitudinal rule to altitude. Am Nat 140:893–911
Tedesco PA, Leprieur F, Hugueny B, Brosse S, Durr HH, Beauchard O et al (2012) Patterns and processes of global freshwater fish endemism. Glob Ecol Biogeogr 21:977–987
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Verneaux J (1977) Biotypologie de l’écosystème “eau courante”. Détermination approchée de l’appartenance typologique d’un peuplement ichtyologique. C R Acad Sci (Life Sciences) 284:675–678
Acknowledgements
We thank Professor Tim Sparks for revising the English of the early drafts of the manuscript. Our special thanks go to Vasilya Yanybaeva, the Director of Bashkir Nature Reserve and Rifgat Shagidullin, the Director of the Institute of Problems in Ecology and Mineral Wealth, for their irrepressible support during the field surveys.
We declare that the fish were caught under a survey permit of the Russian Federal Agency for Fisheries.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Askeyev, A., Askeyev, O., Yanybaev, N. et al. River fish assemblages along an elevation gradient in the eastern extremity of Europe. Environ Biol Fish 100, 585–596 (2017). https://doi.org/10.1007/s10641-017-0588-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0588-z