Abstract
Amazonian floodplain lakes, which are of different types based on their proximity to the main river channels, are subjected to a strong seasonal hydrologic cycle and have different habitat types within them. We hypothesized that these two spatial scales (lake type, and habitat type within lakes), and the temporal scale associated with hydrological seasons help explain the high fish diversity of the Amazon basin. We sampled three lake types categorized by proximity and connectivity with the Solimões River (island, coastal, and mainland) in 2011 during both high and low water seasons. Within these lakes, we sampled three habitat types (open water, flooded forest, and macrophyte banks). Although comparisons of diversity indices revealed few differences with respect to overall species numbers, actual species composition, which is not included in calculation of diversity indices, differed markedly at all three scales. Although many species (86) were common to all three types of lakes, each type also had many unique species (11–20), as did within-lake habitat type (10–15) and hydrologic season (26 each). Thus, these temporal and spatial scales are important to the detection and understanding of fish species diversity in this region, and will be important to keep in mind when designing preserves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Amazon River Basin contains the highest fish species diversity of any region on earth (Reis et al., 2003), and is facing anthropogenic threats such as overfishing (Batista et al., 1998; Campos et al., 2015), construction of dams for hydroelectric generation (Finer & Jenkins, 2012; Castello et al., 2013), and droughts exacerbated by global warming (Marengo et al., 2008; Freitas et al., 2013a). In particular, dams and droughts threaten the connectivity among habitats necessary for many fish species to complete their life cycles (Castello & Macedo, 2015; Hurd et al., 2016). It is, therefore, important to understand how to measure and monitor native fish diversity in this region, both to preserve biodiversity and to ensure sustainable levels of fish stocks for harvesting. Lakes situated on riverine floodplains in the neotropics are good habitats for the study of fish community structure on both local and regional scales (Agostinho et al., 2001).
A major environmental factor on the floodplain is the seasonal variation that constitutes an annual hydrological cycle, with changes in water level that can exceed 15 m between high and low water periods that can strongly affect fish assemblages (Junk et al., 1989; Scarabotti et al., 2011). The important factor here is connectivity with the river, which is important for fish migration into and out of the floodplain (Cox-Fernandes, 1997). During the flooding (rising water) period, lakes fill up and are connected to the river, with the consequent addition of a flooded forest habitat around the margins of lakes (Thomaz et al., 2007). This ensures that augmented space and new habitats become available for different fish species. In the receding (falling water) season, the flooded area shrinks, leading to reduced habitat availability and relative or complete isolation from the main river channel. Changes in environmental variables over the hydrologic seasons of the year and across the floodplain landscape are likely to change the relative importance of biotic interactions such as predation and competition, which may increase when low water crowds populations, creating non-random assortments of fish species in these lakes (Fernandes et al., 2009). Abiotic influences such as temperature, oxygen concentration, and transparency also change over the hydrologic cycle and differ among lakes, which can be the basis of habitat selection among fish (Petry et al., 2003a; Freitas et al., 2010a; Miyazono et al., 2010; Wolfshaar et al., 2011).
Given the number of potentially important environmental variables that may influence species composition in Amazonian floodplain lakes, and previously reported differences in fish species diversity between lake types (Freitas et al., 2013b), we designed a study to evaluate the relative influence of components of habitat heterogeneity on diversity and species composition. Specifically, we examined fish diversity and species composition in three general types of lakes that differ in their connectivity with the main river channel over the hydrologic cycle and in their geomorphological origin. Within each of these lake types, we also examined three different habitat types: open water, floating macrophytes, and flooded forest. Finally, we added a temporal component by making our measurements during two different seasons of the hydrological cycle, flooding and receding water. Our null hypothesis was that fish community composition does not differ with respect to any of these variables. Our most likely alternative, assuming we can reject the hypothesis, is that habitat differences at multiple spatial and temporal scales invoke differences in species composition of these floodplain lakes.
Methods
Study area
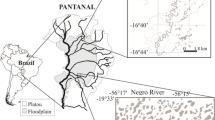
The study area comprised a set of nine floodplain lakes, located in the Solimões River between the mouth of the Purus River and the confluence of the Solimões and Negro rivers (Fig. 1) at Manaus, Amazonas, Brazil. Whitewater rivers, such as the Solimões, carry a high load of transported sediments and nutrients from pre-Andean headwaters. These rivers are highly productive and support high fish species diversity and productive fish stocks for human exploitation (Lowe-McConnell, 1999; Freitas et al., 2010b). The lakes we included in the present study were selected because they represent three different categories of connectivity with the river. In the descriptions below, lake area in km2 is given for typical high water periods for comparison because lake area is highly variable over the hydrologic cycle, and between years.
Coastal lakes (CT) are common on the banks of the Solimões River, in shallow depressions. The water in this type of lake originates from the water table or from the overflow of the river during the high water season, or from canals connecting other lakes. In our study, coastal lakes were represented by lakes Padre (−3,19661S, −59,93148W; 0.35 km2), Camaleão (−3,664323S, −60,909816W; 1.26 km2), and Cacauzinho (−3,668614S, −60,871198W; 0.16 km2).
Island lakes (IL) are located on islands in the main channel of the river that are formed by the accumulation of sediments of the Solimões over the years. They are also supplied by groundwater and by the river during high season, and lose their connections to the main river in the low water season. They are represented by lakes Central on Marchantaria Island (−3,253823S, −59,970098W; 0.42 km2), Sacambú on Paciência Island (−3,306744S, −60,243298W; 0.27 km2), and Camboa on Linda Nova Island (−3,584226S, −60,844022W; 1.63 km2).
Mainland lakes (MD) are located in lowland areas more distant from the river than CT lakes, close to the nearby higher ground called Tertiary Plateau (Soares, 1977), showing dendritic conformation due to occurrence of streams (igarapés) flowing into the floodplain. These lakes receive waters from the ground water table, from the main river during high water, and from the surrounding igarapés. The three lakes representing this category are Calado (−3,313896S, −60,580843W, 2.12 km2), Santo Antonio (−3,245241S, −60,244719W; 0.27 km2), and Laguinho (−3,393994S, −60,244719W; 0.23 km2).
Within these lakes, there are three distinct habitat types: open water (OW) in the central areas that vary from 0.5 to 10.0 m deep according to the season of the hydrologic cycle, floating macrophyte banks (MA) nearer to the shorelines, and the flooded forest (FF), consisting of trees that are partially or entirely submerged when the water is high enough. Although there are at least 388 species of aquatic macrophytes recorded (Junk & Piedade, 1997), the plant species that were most abundant in MA for all of our lakes were Pistia stratiotes L., Eichhornia crassipes (Mart.) Solms., Salvinia auriculata Aublet, Echinochloa polystachya (Kunth) A.S. Hitch., Paspalum repens Berg., and Paspalum fasciculatum Wild. Ex Flügge. These floating plant assemblages are important sources of food and refuge for many fish species (Junk & Robertson, 1997; Sánchez-Botero & Araújo-Lima, 2001). The FF habitat is available to fish when the water is high enough to permit entry, and as with MA, this habitat provides shelter as well as food from allochthonous sources such as seeds, fruit, and insects from the trees. This habitat was structurally similar for all of our lakes, with the most abundant tree species being Cecropia sp., Hevea spruceana (Benth.) Müll. Arg., Alchornea schomburgkiana (Miq.) Baill., Piranha trifoliate Baill., and Eschweilera sp.
Sampling
Fish collections with gillnets were carried out in 2011 during the flooding season (F) in May and in the receding season (R) in August. In each lake, the collections were taken in mixed macrophyte banks (MA), in the open water (OW) of the lake, and in the flooded forest area (FF) surrounding the lake. We used floating gillnets that were 150 m long by 2 m high, composed of 10 segments of 15 m each with different mesh sizes: 30, 40, 50, 60, 70, 80, 90, 100, 110, and 120 mm. They were deployed at 0600 (daybreak) and left for 48 h at each collection site, and fish were collected from them every 6 h. The location of gillnets in each habitat was moved across the lake at the end of 24 h, in order to reduce the damage done by predators attracted to the trapped fish. Each fish was initially identified in the field, fixed in 10% formalin and later identified by specialists at the Instituto Nacional de Pesquisas da Amazônia (INPA) and Universidade Federal do Amazonas (UFAM). Specimens of special interest were preserved in 70 % alcohol and deposited in the UFAM fish reference collection. Sampling effort was held constant throughout this study, so different numbers and biomass of fish caught over time and space represent differences in catch per unit effort (CPUE).
Data analysis
We used rarefaction curves to determine sampling efficacy of obtaining representative samples of species numbers in each type of lake, habitat, and hydrological season. The diversity of fish assemblages was measured as species richness (S), Shannon’s diversity (H′), and Pielou’s evenness (J′). Complementarity measures proposed by Colwell & Coddington (1994) were used to estimate the distinctness of fish assemblages sampled by lake types (CT, IL, MD), habitats (OW, MA, FF), and hydrological seasons (F, R). Venn diagrams were constructed using presence/absence data of species to highlight how many species were unique to, or shared among, habitats (OW, MA, FF) within each type of lake.
To test the effect of hydrologic season (flooding or receding water) and spatial scale (type of lake and habitat within the lake) on the diversity of fish assemblages, we applied a factorial ANOVA (3 × 3 × 2) where the response variables are S, H’, and J’. The Hommel’s method to adjust P values was employed since it reduces type I errors (Hommel 1988). Tukey’s HSD test was used when the null hypothesis of no differences was rejected. This statistical analysis was accomplished using R Statistical Software (R Development Core Team, 2012). Assumptions of normality, linearity, and homoscedasticity were graphically inspected employing the residuals before running these three ANOVAs.
We evaluated species composition of fish assemblages in the two spatial scales: types of lakes and habitats through non-metric multidimensional scaling (NMDS) based on a matrix of Bray-Curtis distance (Borcard et al., 2011). After testing the multivariate homogeneity of groups (permdisp), this same distance matrix was employed to run a two-way PerMANOVA, with 5000 permutations, to test the null hypothesis of the same species composition between types of lakes and between habitats, and of no interaction effects between types of lakes and habitats (Anderson, 2001). Where the null hypothesis was rejected, a pairwise comparison was done using PerMANOVA. Both NMDS and PerMANOVA were done using the Vegan Package (Oksanen et al., 2011) of R Statistical Software (R Development Core Team, 2012).
We employed the package Betapart (Baselga et al. 2013) to estimate the multiple-site dissimilarity measures proposed by Baselga et al. (2007) and Baselga (2010) as measures of β diversity due to their ability to identify the predominance of species loss or species turnover. We used the Sorensen-based multiple-site dissimilarity (β SOR), Simpson-based multiple-site dissimilarity (β SIM), and Nestedness-resultant multiple-site dissimilarity (β NES) (Baselga, 2010).
Results
A total of 18,783 fish were caught, equivalent to 1406 kg, distributed among 167 species, 27 families, and nine orders (Supplementary material Appendix I). Characiformes and Siluriformes were the orders with the greatest abundance and richness. The families Characidae and Curimatidae constituted groups with the highest numerical abundance, while the highest species richness was observed in the families Characidae, Doradidae, and Cichlidae. The most abundant species were in the family Curimatidae, Psectrogaster rutiloides (Kner, 1858) and Potamorhina latior (Spix & Agassiz, 1829), which together accounted for 22% of numerical abundance and 16% of biomass, followed by the piranha, Pygocentrus nattereri (Kner, 1858) with 11% and 8% in number and biomass, respectively (Supplementary material Appendix I).
Rarefaction curves (Fig. 2a–c) reached asymptotes for types of lakes, habitats and seasons of the hydrologic cycle, indicating that our collections were representative with regards to species richness. Total species richness was exactly the same in both seasons, and the proportions of abundance and biomass were not substantially different during receding water than flooding (Table 1). There were no marked differences in the distribution of species richness among lake types or habitats, and the majority of fish species were common to all three types of lakes. However, each lake type had an appreciable number of unique species (i.e., found in only one type, whether abundant or rare), and island and coastal lakes were more similar between them than in comparison with mainland lakes (Table 1). Mainland lakes had about twice the number of unique species as the other two types. In all, about one-third of all species caught were found in only one type of lake, while about one-quarter were shared by only two lake types. Most species unique to coastal or island lakes belonged to the order Siluriformes, while Characiformes were dominant among species unique to mainland lakes (Appendix I). The three habitats shared the majority of species; however, only a few species were common to both the open area and the aquatic macrophytes. Similarly, few were found in both the open area and the flooded forest. About four times as many of fish species were found both in aquatic macrophytes and the flooded forest as were shared among any other pair of habitats. The highest complementarity was observed between samples taken in macrophyte and flooded forest habitats (Table 1). Also, there were no marked differences in the species composition found in the two hydrologic seasons, with a complementarity measure of approximately 31%.
The numbers of species shared by the three habitats were almost the same for all three types of lakes (Fig. 3). However, the number of unique species per habitat shows different patterns for each type of lake. In coastal and island lakes, the flooded forest was the habitat with the highest number of unique species. In coastal lakes, the open water area showed more than twice the number of unique species as at island lakes, and an opposite pattern was observed for macrophytes. Mainland lakes exhibited a more equitable pattern with approximately equal numbers of unique species in each habitat. Seventy-seven species, including 43 from the family Characiformes, 26 Siluriformes, four Perciformes, three Clupeiformes, and one Osteoglossiformes, were common to all type of lakes, habitats, and both hydrologic seasons (Supplementary material Appendix I).
The graphical check for normality, linearity, and homoscedasticity showed no violations for all three response variables. There were differences in species richness only among habitats (Table 2), with greater richness in flooded forest and aquatic macrophytes than in open water (P < 0.01 and <0.001, respectively), and no differences between aquatic macrophytes and flooded forest (P = 0.47). There were detectable effects of types of lakes and habitats with diversity measured by Shannon’s index (Table 2), with lower values in coastal than in island lakes (P = 0.03) and no differences either between coastal and mainland lakes (P = 0.09) or between island and mainland lakes (P = 0.86). Higher values of Shannon’s index were obtained for flooded forest and aquatic macrophytes in comparison to open water (P < 0.001 and P < 0.01, respectively) and no differences between flooded forest and aquatic macrophytes (P = 0.14). There were differences only among habitats when using J′ as the response variable (Table 2), with greater evenness in flooded forest than in open water (P < 0.01) and no differences between flooded forest and aquatic macrophytes (P = 0.12) or between flooded forest and aquatic macrophytes (P = 0.19).
The NMDS stress value for data clustering types of lakes and habitats was 0.04 (non-metric R 2 = 0.99) suggested an excellent representation by a two dimensional solution. There were clear differences among types of lakes, both for biomass and numerical abundance. Mainland lakes were ordered for the right/upper part of the graph and the other two types of lakes were grouped together on the left (Fig. 4).
NMDS plots for numerical abundance and biomass data using units composed of lake x habitat. The first two letters of each labeled point indicate lake type (CT coastal, IL island, MD mainland); the second two letters indicate within-lake habitat type (OW open water, MA macrophytes, FF flooded forest; and the last letter indicates biomass (B) or numerical abundance (N) of fish
The assumption of multivariate homogeneity by groups was accepted for type of lakes (F = 0.63, df = 2, 6, P = 0.56) and for habitat (F = 0.12, df = 2, 6, P = 0.88). The PerMANOVA showed effects of type of lakes (pseudo F = 3.74, df = 1, 5, R 2 = 0.33, P < 0.01), but no effects for habitats (pseudo F = 2.08, df = 1, 5, R 2 = 0.23, P = 0.06) and interactions (pseudo F = 1.67, df = 1, 5, R 2 = 0.15, P = 0.12). The pairwise comparisons between types of lakes showed significant differences between Mainland and Coastal lakes (pseudo F = 3,84, df = 1, 4, R 2 = 0.49, P = 0.04) and between Mainland and Island lakes (pseudo F = 2.31, df = 1, 4, R 2 = 0.37, P = 0.05), but no difference between Coastal and Island lakes (pseudo F = 1.95, df = 1, 4, R 2 = 0.33, P = 0.10).
We found high β diversity, mainly among type of lakes, since the nestedness component, β NES, was much lower than the component accounting for pure spatial turnover, β SIM, for both factors: type of lakes (β SIM = 0.253, β NES = 0.023 and β SOR = 0.276) and habitats (β SIM = 0.165, β NES = 0.056 and β SOR = 0.221).
Discussion
Our study indicates that no single lake type, habitat, or hydrologic season was representative of the species composition of fish assemblages in this floodplain region. The high degree of uniqueness to lake type, habitat type, and hydrologic season, whether attributable to adaptive specialization or merely stochastic variation that may change from 1 year to the next, may be a key reason that species diversity among lakes (β diversity) has been shown to be at least as important as within-lake diversity (α) in characterizing regional diversity (γ) in these floodplain lakes (Freitas et al., 2013b).
Discovery of differences in fish assemblages here was not only a matter of scale but also depended on how the data were analyzed. If we had used just the numbers in Table 1 to describe this system, we would have concluded that spatial and temporal scale of environmental heterogeneity were not very important. Traditional diversity measures based on information theory, Shannon’s diversity (H’), and Pielou’s measure of evenness (J’) detected some differences among habitat types and lake types but not between seasons of the hydrologic cycle. The problem with these indices is that they are static measures, i.e., not always useful in comparing species assemblages over space or time (Cornell et al., 1976). Shannon’s H’ measures the interaction between species richness and evenness and is thus subjected to compensatory changes in these separate values, i.e., a positive change in either one can cancel out a negative effect in the other. Pielou’s J′ measures the evenness of relative abundances in an assemblage independently of species richness but still cannot detect compensations such as a switch in the identity of species occupying a given rank in relative abundance when one species is replaced by another or becomes rare when another one becomes more common. Therefore, there is no substitute for retaining species identity in a dataset if we want an accurate comparison of species composition among communities.
In instances where measures of species diversity showed no difference in these fish assemblages, as was the case between hydrologic seasons, species composition differed markedly: nearly one-third of the 167 species caught, in our study, were found only during one of the two hydrologic seasons. Further, no one habitat type within lakes contained all or nearly all of the fish species in a given lake. Thus, the most important information we obtained in our study were the data on species that were unique or shared among lakes sampled at different spatial scales and over time. Multivariate analysis, NMDS, and PerMANOVA showed effects of type of lakes, clustering island, and coastal lakes apart from mainland lakes. Although we do not suggest that the exact pattern we detected will be obtained every time the lakes are sampled, we do suggest that the kind of heterogeneity we found here, and Freitas et al. (2013b) found, in their study, is more the rule than the exception. This means that representing fish diversity on a regional scale in this system will require sampling on multiple spatial and temporal scales.
From our single-year study, we cannot say how many of the species that we found to be unique to a particular habitat are, in fact, adapted specialists to a particular set of environmental conditions. However, according to the literature, there has been a general expectation that the organization of tropical fish assemblages in floodplains is ecologically and evolutionarily associated with environmental complexity or heterogeneity (Junk et al., 1997; Saint-Paul et al., 2000; Petry et al., 2003b; Súarez et al., 2004; Freitas et al., 2010a). Given this heterogeneity, it is not surprising that so many of the species we found were unique to lake types and specific habitats, as well as to seasons of the hydrologic cycle. This kind of habitat selection can lead to restricted areas of occurrence for many species. Mojica et al. (2009) observed that among the 171 species found in a creek draining into the Amazon River floodplain, 40 were considered unique to the immediate flooded area, while 38 reached only as far as 4 km upstream of this area.
In our study, mainland lakes had the greatest number of unique species, which may be surprising considering that the annual flood pulse has the potential to homogenize fish species distributions across the region at high water (Hoeinghaus et al., 2003; Freitas et al., 2010a). However, several studies have reported non-random assortments of fish species among floodplain lakes, especially during low water (e.g., Petry et al., 2003b, Arrington & Winemiller, 2006; Fernandes et al., 2009), which supports the hypothesis that at least some species are specialists to lake types and the flood pulse does not obliterate the structure of floodplain fish assemblages. Another factor is that mainland lakes are connected to numerous creeks (igarapés) whose headstreams are in higher terrains flowing into the floodplain, and from which species not found in the main river channel can migrate, adding to species richness in these lakes (Taylor & Warren Jr., 2001). Some species that are both unique to, and typical of, these igarapés are Bryconops caudomaculatus (Günther, 1864) (Mendonça et al., 2008), Gymnocorymbus thayeri (Eigenmann, 1908), Boulengerella maculata (Valenciennes, 1840), and Leporinus agassizii (Steindachner, 1876) (Mojica et al., 2009).
The connection between the floodplain and the main river channel through flooded forests when the water is high creates important habitats for nutrition, growth, reproduction and sheltering from predators (Saint-Paul et al., 2000; Sánchez-Botero & Araújo-Lima, 2001; Claro-Jr et al., 2004; Correa et al., 2008). The forest there supplies allochthonous food for fish during high water, such as fruits, seeds, and invertebrates falling from the canopy (Goulding, 1980; Junk & Robertson, 1997; Araújo-Lima & Goulding, 1998).
The flooded forest of island lakes represents an ecotone both between adjacent lakes and between the lakes and the main channel of the river, which is not the case for forests adjacent to the other two types of lakes. Therefore, the reason for the substantially higher number of species found exclusively in the flooded forest habitat of these lakes probably is due to contributions of species both from the lake and from the river. There is not yet enough information about the basic life cycle of many of the fish we caught in the flooded forest to know how many of them are river-dwelling species that migrate into the flooded forest during high water. We do know that schooling species are constantly moving in and out of lakes when the water is high, becoming common and abundant in the central areas of these lakes (Siqueira-Souza & Freitas, 2004; Martelo et al., 2008). Soares et al. (2009) reported catching many individuals of Potamorhina latior, Triportheus albus (Cope, 1872), and Pygocentrus nattereri, which reach high abundances in open water in floodplain lakes near Manacapuru when the water is high.
For many fish species, population densities are relatively low during high water, and resources are diluted. Conversely, during low water plankton and other food sources become more concentrated for grazing species, and predation pressure from carnivorous species increases as a function of crowding among fish (Soares et al., 2007; Petry et al., 2010). The open water area of the center of a lake does not have the same refuge properties as afforded by macrophytes and the flooded forest, and so prey species are more vulnerable to predators in this habitat (Rodríguez & Lewis, 1997). Even in open water, however, rising water enriches the lakes with organic material from decaying aquatic and semi-aquatic vegetation, and animal remains from the surrounding forest (Goulding, 1980; Forsberg et al., 1993; Junk & Piedade, 1997). Environmental anomalies such as extended drought can enhance this effect: Freitas et al. (2013a) found that 1 year after an extreme drought, in the Amazon basin in 2005, the fish fauna was composed mainly of small algivorous and detritivorous fish. They advanced the hypothesis that the predominance of these trophic groups of fish could be attributed to fertilization from a combination of the carcasses of dead fish stranded in shrinking lakes during drought, and the death and decomposition of grasses that colonized the exposed soil and were submerged when the water level began to rise.
The estimates of β diversity revealed a dominance of spatial turnover on nestedness components for both spatial scales: types of lakes and habitats, slightly higher for the former. A dominance of spatial turnover implies a substitution of some species for others and could represent the effects of environmental differences among sites (Qian et al., 2005). These results corroborate those of a previous study of fish diversity partitioning in Amazonian floodplain lakes (Freitas et al., 2013b) that showed the importance of differences in diversity (β) between types of lakes to regional fish diversity.
Our results are relevant to the growing concern among conservationists about the declining state of the world’s biodiversity. Freshwater biodiversity has decreased more than that of any other major ecosystem (Jenkins, 2003; Pittock et al., 2008). The anthropogenic drivers, including dams, alien species, overfishing, and climate change, have different consequences in terms of species loss, and the combination of these environmental perturbations is potentially catastrophic. In particular, construction of dams obviously will inhibit migration of many species, and global warming may extend droughts beyond the adaptive limits of even non-migratory species (Hurd et al., 2016). Both of these perturbations are likely to change the composition of fish assemblages in floodplain lakes in ways that are currently hard to predict with precision. However, in order to conserve biodiversity, it is first necessary to measure it and understand the biological and physical factors that are important to generating and maintaining it. This will require many more studies that consider the kinds of spatial and temporal heterogeneity we examined, and probably other levels besides that will become evident upon closer examination. This kind of information will be critical in the design of nature preserves, as well as in understanding the basic ecology of this threatened ecosystem.
References
Agostinho, A. A., L. C. Gomes & M. Zalewski, 2001. The importance of floodplains for the dynamics of fish communities of the upper river Paraná. Ecohydrology & Hydrobiology 1: 209–217.
Anderson, M., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
Araújo-Lima, C. A. R. M. & M. Goulding, 1998. So fruitful a fish: ecology, conservation and aquaculture of the Amazon’s tambaqui. Columbia University Press, New York.
Arrington, D. A. & K. O. Winemiller, 2006. Habitat affinity, the seasonal flood pulse, and community assembly in the littoral zone of a Neotropical floodplain river. Journal of the North American Benthological Society 25: 126–141.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19: 134–143.
Baselga, A., A. Jimenez-Valverde & G. Niccolini, 2007. A multiple-site similarity measure independent of richness. Biology Letters 3: 642–645.
Baselga, A., D. Orme, S. Villeger, J. De Bortoli & F. Leprier, 2013. Betapart: Partitioning beta diversity into turnover and nestedness components. v. 1.3. https://cran.r-project.org/web/packages/betapart/betapart.pdf.
Batista, V. S., A. J. Inhamuns, C. E. C. Freitas & D. Freire-Brasil, 1998. Characterization of the fishery in riverine communities in the Low-Solimões/High-Amazon region. Fisheries Management and Ecology 5: 419–435.
Borcard, D., F. Gillet & P. Legendre, 2011. Numerical Ecology with R. Springer, New York.
Campos, C. P., R. C. S. Garcez, M. Catarino, G. A. Costa & C. E. C. Freitas, 2015. Population dynamics and stock assessment of Colossoma macropomum caught in Manacapuru lake system (Amazon Basin, Brazil). Fisheries Management and Ecology 22: 400–406.
Castello, L. & M. N. Macedo, 2015. Large-scale degradation of Amazonian freshwater ecosystems. Global Change Biology. doi:10.1111/gcb.13173.
Castello, L., D. G. McGrath, L. L. Hess, M. T. Coe, P. A. Lefebvre, P. Petry, M. N. Macedo, V. F. Reno & C. C. Arantes, 2013. The vulnerability of Amazon freshwater ecosystems. Conservation Letters 6: 217–229.
Claro-Jr, L., E. Ferreira, J. A. Zuanon & C. A. R. M. Araújo-Lima, 2004. O efeito da floresta alagada na alimentação de três espécies de peixes onívoros em lagos de várzea da Amazônia Central, Brasil. Acta Amazonica 34: 133–137.
Colwell, R. K. & J. A. Coddington, 1994. Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society B. Biological Sciences 345: 101–118.
Cornell, H. V., L. E. Hurd & V. A. Lotrich, 1976. A measure of response to perturbation used to assess structural change in some polluted and unpolluted stream fish communities. Oecologia 23: 335–342.
Correa, S. B., W. G. R. Crampton, L. J. Chapman & J. S. Albert, 2008. A comparison of flooded forest and floating meadow fish assemblages in an upper Amazon floodplain. Journal of Fish Biology 72: 629–644.
Cox-Fernandes, C., 1997. Lateral migration of fishes in Amazon floodplains. Ecology of Freshwater Fish 6: 36–44.
Fernandes, R., L. C. Gomes, F. M. Pelicice & A. A. Agostinho, 2009. Temporal organization of fish assemblages in floodplain lagoons: the role of hydrological connectivity. Environmental Biology Fishes 85: 99–108.
Finer, M. & C. N. Jenkins, 2012. Proliferation of hydroelectric dams in the Andean Amazon and its implications for Andes-Amazon connectivity. PloS One 7: 1–9.
Forsberg, B. R., C. A. R. M. Araújo-Lima, L. A. Martinelli, R. L. Victoria & J. A. Bonassi, 1993. Autotrophic carbon sources for fish of the Central Amazon. Ecology 74: 643–652.
Freitas, C. E. C., F. K. Siqueira-Souza, A. R. Guimarães, F. A. Santos & I. L. A. Santos, 2010a. Interconnectedness during high water maintains similarity in fish assemblages of island floodplain lakes in the Amazonian Basin. Zoologia 27: 931–938.
Freitas, C. E. C., F. K. Siqueira-Souza, K. L. L. Prado, K. C. Yamamoto & L. E. Hurd, 2010b. Factors determining fish species diversity in Amazonian floodplain lakes. In Rojas, N. & R. Prieto (eds), Amazon basin: plant life, wildlife and environment. Science Publishers, Nova York: 41–76.
Freitas, C. E. C., F. K. Siqueira-Souza, A. C. Florentino & L. E. Hurd, 2013a. The importance of spatial scales to analysis of fish diversity in Amazonian floodplain lakes and implications for conservation. Ecology of Freshwater Fish 23: 470–477.
Freitas, C. E. C., F. K. Siqueira-Souza, R. Humston & L. E. Hurd, 2013b. Assessing drought sensitivity of Amazonian floodplain fish communities. Hydrobiologia 705: 159–171.
Goulding, M., 1980. The fishes and the forest: Explorations in Amazonian natural history. University of California Press, Berkeley, LA.
Hoeinghaus, D. J., C. A. Layman, D. A. Arrington & K. O. Winemiller, 2003. Spatiotemporal variation in fish assemblage structure in tropical floodplain creeks. Environmental Biology of Fishes 67: 379–387.
Hommel, G., 1988. A stagewise rejective multiple test procedure based on a modified bonferroni test. Biometrika 75: 383–386.
Hurd, L. E., R. G. C. Souza, F. K. Siqueira-Souza, G. J. Cooper, J. R. Kahn & C. E. C. Freitas, 2016. Amazon floodplain fish communities: habitat connectivity and conservation in a rapidly deteriorating environment. Biological Conservation 195: 118–127.
Jenkins, M., 2003. Prospects for biodiversity. Science 302: 1175–1177.
Junk, W. J. & M. T. F. Piedade, 1997. Plant life in the floodplain with special reference to herbaceous plants. In Junk, W. J. (ed.), The central amazon floodplain: Ecology of a pulsing system. Ecological Studies. Springer, Berlin: 147–186.
Junk, W. J. & B. Robertson, 1997. Aquatic invertebrates. In Junk, W. J. (ed.), The central amazon floodplain: Ecology of a pulsing system. Ecological Studies. Springer, Berlin: 279–298.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplains systems. In Dodge D. P. (ed.) Proceedings of the International Large River Symposium. Canadian Special Publication of Fisheries and Aquatic Science, vol. 106, pp. 110–127.
Junk, W. J., M. G. Soares & U. Saint-Paul, 1997. The fish. In Junk, W. J. (ed.), The central amazon floodplain: Ecology of a pulsing system. Ecological Studies. Springer, Berlin: 385–408.
Lowe-McConnell, R., 1999. Estudos ecológicos em comunidades de peixes tropicais. EDUSP, São Paulo, SP, 524 pp.
Marengo, J. A., C. A. Nobre, J. Tomasella, M. D. Oyama, G. S. R. de Oliveira, R. de Oliveira, H. Camargo, L. M. Alves & I. F. Brown, 2008. The drought of Amazonia in 2005. Journal of Climatology 21: 495–516.
Martelo, J., K. Lorenzen, M. Crossa & D. McGrath, 2008. Habitat associations of exploited fish species in the Lower Amazon river-floodplain system. Freshwater Biology 53: 2455–2464.
Mendonça, F., V. Pazin, H. Espírito-Santo, J. A. Zuanon & W. E. Magnusson, 2008. Peixes. In M. L. Oliveira, F. B. Baccaro, R. Braga-Neto, W. E. (eds) Magnusson (Org.) Reserva Ducke—A biodiversidade amazônica através de uma grade, pp. 63–75.
Miyazono, S., J. N. Aycock, L. E. Miranda & T. E. Tietjen, 2010. Assemblage patterns of fish functional groups relative to habitat connectivity and conditions in floodplain lakes. Ecology of Freshwater Fish 19(578): 585.
Mojica, J. L., C. Castellanos & J. Lobón-Cerviá, 2009. High temporal species turnover enhances the complexity of fish assemblages in Amazonian terra firme streams. Ecology of Freshwater Fish 18: 520–526.
Oksanen, J., F. Guillaume Blanchet, R. Kindt, P. Legendre, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Vagner, 2011. Vegan: Community Ecology Package version 1.17-9. http://CRAN.R-project.org/package=vegan
Petry, A. C., A. A. Agostinho & L. C. Gomes, 2003a. Fish assemblages of tropical floodplain lagoons: exploring the role of connectivity in a dry year. Neotropical Ichthyology 1: 111–119.
Petry, P., P. B. Bayley & D. F. Markle, 2003b. Relationships between fish assemblages, macrophytes and environmental gradients in the Amazon River floodplain. Journal of Fish Biology 63: 547–579.
Petry, A. C., L. C. Gomes, P. Piana & A. A. Agostinho, 2010. The role of the predatory trahira (Pisces: Erythrinidae) in structuring fish assemblages in lakes of a Neotroplical floodplain. Hydrobiologia 651: 115–126.
Pittock, J., L. J. Hansen & R. Abell, 2008. Running dry: freshwater biodiversity, protected areas and climate change. Biodiversity 9: 30–39.
Qian, H., R. E. Ricklefs & P. White, 2005. Beta diversity of angiosperms in temperate floras of eastern Asia and eastern North America. Ecology Letters 8: 15–22.
R 2.14.2. R Development Core Team, 2012. R: A language and environmental for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0. http://CRAN.Rproject.org.
Reis, R. E., S. O. Kullander & C. J. Ferraris Jr, 2003. Check list of the freshwater fishes of South and Central America. Edipucrs, Porto Alegre.
Rodríguez, M. A. & W. M. Lewis Jr, 1997. Structure of fish assemblages along environmental gradients in floodplain lakes of the Orinoco River. Ecological Monographs 67: 109–128.
Saint-Paul, U., J. A. Zuanon, M. A. V. Correa, M. Garcia, N. N. Fabré, U. Berger & W. J. Junk, 2000. Fish communities in central Amazonian white and blackwater floodplains. Environmental Biology of Fishes 57: 235–250.
Sánchez-Botero, J. I. & C. A. R. M. Araújo-Lima, 2001. As macrófitas aquáticas como berçário para a ictiofauna da várzea do rio Amazonas. Acta Amazonica 3: 437–448.
Scarabotti, P. A., J. A. López & M. Pouilly, 2011. Flood pulse and the dynamics of fish assemblage structure from neotropical floodplain lakes. Ecology of Freshwater Fish 20: 605–618.
Siqueira-Souza, F. K. & C. E. C. Freitas, 2004. Fish diversity of floodplain lakes on the lower stretch of the Solimões river. Brazilian Journal of Biology 64: 501–510.
Soares, L. C., 1977. Hidrografia. Geografia do Brasil. Região Norte. IBGE, Rio de Janeiro: 95–166.
Soares, M. G. M., E. L. Costa, F. K. Siqueira-Souza, H. D. B. Anjos, K. C. Yamamoto & C. E. C. Freitas, 2007. Peixes de lagos do médio rio Solimões. EDUA, Manaus.
Soares, M.G.M., F. R. Silva, H. D. B. Anjos, L. Prestes, D. R. Bevilacqua & C. Campos, 2009. Ambientes de pesca e a ictiofauna do complexo lacustre do lago Grande de Manacapuru, AM: composição taxonômica e parâmetros populacionais. In Fraxe, T. J. P. & A. C. Witkoski (org), A pesca na Amazônia Central: Ecologia, conhecimento tradicional e formas de manejo. EDUA, Manaus, pp. 77–178.
Súarez, Y. R., M. Petrere-Jr & A. C. Catella, 2004. Factors regulating diversity and abundance of fish communities in Pantanal lagoons, Brazil. Fisheries Management and Ecology 11: 45–50.
Taylor, C. M. & M. L. Warren Jr, 2001. Dynamics in species composition of stream fish assemblages: Environmental variability and nested subsets. Ecology 82: 2320–2330.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
van de Wolfshaar, K. E., H. Middelkoop, E. Addink, H. V. Winter & L. A. J. Nagelkerke, 2011. Linking flow regime, floodplain lake connectivity and fish catch in a large river-floodplain system, the Volga-Akhtuba floodplain (Russian Federation). Ecosystems 14: 920–934.
Acknowledgements
We thank I. Santos, W. Dias, J. Pena, and L. Santos for help in field work and J. Zuanon (INPA) and K. Yamamoto (UFAM) for contribution in identification of fish. The first author was supported by a CAPES PhD scholarship: Process BEX: 9706/11-9. Additional support came from Project CNPq No. 563073/2010-1, Piatam Project (FINEP) and from a Brazilian research fellowship from CAPES and Lenfest grants from Washington and Lee University to LEH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luiz Carlos Gomes
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Siqueira-Souza, F.K., Freitas, C.E.C., Hurd, L.E. et al. Amazon floodplain fish diversity at different scales: do time and place really matter?. Hydrobiologia 776, 99–110 (2016). https://doi.org/10.1007/s10750-016-2738-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2738-2