Abstract
The mating system of cirrhitids is generally classified as harem polygyny. However, detailed information on the spatial distribution patterns and mating relationships is lacking in large hawkfish species. We investigated the spatial distribution patterns and mating relationships among individuals of the large hawkfish, Paracirrhites forsteri, on a Kuchierabu-jima Island reef in southern Japan. Large males maintained territories and attacked other large males near the boundaries of their territories. Females usually stayed outside of male territories during the day and moved to prominent coral heads within male territories during the late afternoon for mating. Large males spawned with several females around sunset, and females seldom changed their mating partners. In addition, we confirmed the occurrence of small males with a body size similar to that of females. These small males placed their territories against other small males within/around the territories of large males and pair-spawned with small females. The female distribution pattern and occurrence of small males have never been reported in haremic hawkfish, but their stable mating partnership is very similar to that of haremic hawkfish. Our results reveal considerable variation in mating systems within Cirrhitidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A range of mating systems has been reported by field studies of several reef fish species (Thresher 1984; Turner 1993; Kuwamura 1997; Wong and Buston 2013). Empirical data from field studies suggest that mating systems can be categorized broadly into five types: monogamy, harem polygyny, male-territory-visiting (MTV) polygamy, nonterritorial polygamy, and polyandry (Kuwamura 1996, 1997). Harem polygyny is common among strongly site-attached small reef fish (Thresher 1984; Moyer 1991), e.g., Pomacanthidae (Moyer and Nakazono 1978; Sakai and Kohda 1997), relatively small Labridae (Kuwamura 1984; Moyer 1991), and Pinguipedidae (Nakazono et al. 1985). This mating system is characterized by cohabitation of multiple females within a male’s territory and stable monopolization of mating opportunities with the females by the male (Thresher 1984; Kuwamura 1996, 1997). MTV polygamy is also found widely in reef fish, particularly in relatively large species with good free-swimming ability (e.g., labrids and scarids) (Moyer 1991; Suzuki et al. 2008; Kuwamura et al. 2009). This mating system is different from harem polygyny, as females are usually located outside the male mating territories and choose mating partners (Kuwamura 1996, 1997). MTV polygamy include lek-like mating systems (Kuwamura 1996, 1997).
The Cirrhitidae includes about 33 species in 12 genera, and most species are distributed on rocky and coral reefs in tropical and subtropical waters of the Indo-West Pacific Ocean (Nelson 2006). The freckled hawkfish, Paracirrhites forsteri, is a relatively large cirrhitid (20 cm standard length) that inhabits shallow reefs in the Indo-West Pacific (Randall 1963; Nakabo 2002) and preys on small fish and benthic animals (Hiatt and Strasburg 1960; Hobson 1974). Some hawkfish species have been confirmed protogynous hermaphrodites based on a gonadal histological evaluation (Sadovy and Donaldson 1995; Sadovy de Mitcheson and Liu 2008) or field observations (Kadota et al. 2012). The mating system of cirrhitids, including P. forsteri, is generally classified as harem polygyny (Thresher 1984; Donaldson 1986, 1987, 1989, 1990; Kadota et al. 2011). However, detailed information on the spatial distribution patterns and mating relationships is lacking in large (15 cm standard length or more) hawkfish, e.g., Paracirrhites hemistictus, P. forsteri and Cirrhitus pinnulatus. Mating systems are influenced by a variety of factors, including phylogeny, resource distribution and dispersal of females (Moyer 1991; Shapiro 1991; Kuwamura 1997). Body size is another important factor affecting the mating system (Thresher 1984; Moyer 1991). For instance, among comparative large fishes, enhanced mobility, due to perhaps lower risks of predation, often leads to migrations to favored spawning sites, promoting MTV polygamy or nonterritorial polygamy. Thus, it is predicted that the mating system of large hawkfish is different from those of small hawkfish. We investigated the home range distribution, intraspecific interactions, and spawning events of P. forsteri on Kuchierabu-jima Island reefs, southern Japan to clarify their mating system. We discuss factors affecting the hawkfish mating system from an ecological perspective in terms of feeding and mating tactics based on empirical data from field observations and a removal experiment.
Materials and methods
This study was conducted on reefs in Nishiura Bay of Kuchierabu-jima Island (30°28′N, 130°10′E), Kagoshima, southern Japan, which is the same locality where the timing of P. forsteri spawning was investigated by Kadota et al. (2010). The island is located in a subtropical region close to the Kuroshio Current and hosts over 200 tropical fish species on it reefs (Gushima and Murakami 1976). We set up an observational area of approximately 100 m × 120 m on the reef at a depth of 1–6 m. Where the density of male and female P. forsteri was relatively high around sunset, the observational area (ca. 5000 m2) was divided into 3 m or 10 m grids and a map was drawn (Supplementary material, Fig. S1a). The main observational area was determined by a preliminary study conducted in 2002.
We observed the spatial distribution, intraspecific interactions, spawning events, and feeding activity of P. forsteri using SCUBA or by snorkeling between May and October 2003. Each P. forsteri individual was photographed as it rested on the substrate (Fig. S1), and individual animals were distinguished based on natural variations in the number and appearance of black dots on the head. Total length (TL) was estimated by observing the body length of an individual as it rested on the substrate and then measuring that length with a ruler once the fish had moved. The fish was encouraged to move by the slow approach of the observer. We identified the functional sex of each individual based on spawning behavior and differences in ventral shape (expanded belly signified egg maturation in females just before spawning). We defined the sex of bachelor individuals whose sex could not be identified as male based on agonistic interactions with similar sized males because no mating behavior was observed. Seven males and 12 females were present in the observational area.
Courtship and spawning generally occurs around sunset in P. forsteri (Donaldson 1990; Kadota et al. 2010). We followed each individual fish for 4–124 min (median 49 min) between 3 h before sunset and 1 h after sunset (termed the “late afternoon”) and recorded spawning events, intraspecific interactions, swimming routes, meeting locations with other individuals, and feeding behaviors on the map. If observed individuals moved outside the main observational area, we dropped sinkers attached to small floats as markers to record swimming routes of these individuals whilst following the fish. When a risk of losing the focal animal was increased due to wave condition, we recorded either mating relationships or swimming routes. Each individual was observed 1 to 10 times (median 5 times, 95 observations in total), with the exception of a female whose home range was located outside of the observational area. A total of 189–606 min and 73–238 min of observations were conducted for individual males (n = 7) and females (n = 11), respectively. During these behavioral observations, we usually positioned ourselves about 2.5 m from the focal animal. The presence of observers appeared to have little effect on the behavior of the hawkfish because the hawkfish had been confirmed to naturally spawn despite the presence of observers in a preliminary study. We defined the home range for each individual as an area encompassing the most peripheral swimming route and all meeting locations recorded in observations of other individuals. The overlap ratio (%) of the home range was calculated as follows: total area overlapping another individual’s home range × 100/area of home range of the target individual. If individuals attacked other individuals near the boundaries of their home ranges, we treated these home ranges as territories in this study. Many hawkfish use coral as feeding sites, refuges, or courtship sites (Donaldson 1990; DeMartini 1996; Kane et al. 2009; Kadota et al. 2011). Thus, we set 2 × 10 m lines (n = 40) perpendicularly to shore and calculated the percentage of area that was covered by branching or tabular corals within each line (%). In addition, corals were photographed to identify species.
We also conducted the focal individual observations for 8–163 min (median 60 min) during the morning and early afternoon (between 07:00 and 13:00) to examine diurnal changes in the spatial distribution, frequencies of intraspecific interactions, and feeding behaviors. Each individual was observed 1 to 3 times (median 2 times, 27 observations in total). A total of 60–123 min and 10–172 min of observations were conducted for individual males (n = 6) and females (n = 8), respectively.
We conducted a male removal experiment in the study area to examine social relationships and territoriality among males in detail. We removed the largest male on 24 August and examined the changes in the spatial and mating relationships during the late afternoon for the following 62 days. We conducted the additional observations between May and June 2004 because mating relationships of all individuals were not confirmed in the 62 days.
We used nonparametric tests because of the small sample sizes. All analyses were performed using R V. 3.1.2 (R Development Core Team 2014). A P-value <0.05 was considered significant.
Results
Male spatial distribution and territoriality
Of the seven males present at the start of the study, five were large in size (median TL, 165 mm; range, 158–183 mm; n = 5), and will be called “large males” hereafter. They were significantly larger than females (median TL, 130 mm; range 95–159 mm; n = 11; Mann–Whitney U-test, U = 2.0, P < 0.05). The remaining two males were small in size (TL, 118 and 139 mm) (called “small males” hereafter). Their sizes were not different from those of the females (Mann–Whitney U-test, U = 10.5, P = 1.0). Of the two small males, one was not observed to spawn through the study period. Thus, the sex of the bachelor individual was identified as male based on agonistic interactions with the other small male.
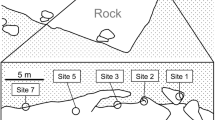
The large males maintained territories against other large males based on late afternoon observations (median, 290 m2; range, 102–504 m2; n = 5; Fig. 1a). The territories of the large males rarely overlapped (overlap ratio: median, 1.4 %; range, 0.2–9.9 %; n = 5). Coral coverage in their territories (median, 9.6 %; range 4.4–45.9 %; n = 15) was significantly higher than that in the other areas (median, 2.8 %; range, 0.2–40.0 %; n = 25; Mann–Whitney U-test, U = 89.5, P < 0.01). The males maintained territories in the same place during the morning and early afternoon as in the late afternoon (median, 106 m2; range, 70–166 m2; n = 4; Fig. 1b), except for one male (M-d). That male eventually moved outside the observational area.
Spatial distribution of male territories and female home ranges during the late afternoon a and morning and early afternoon b. Shaded areas and thick lines indicate large and small males, respectively. Medium lines indicate the female home ranges. Thin broken lines illustrate outlines of rocks or cracks. Triangles and circles indicate the prominent coral heads where females appeared during the late afternoon and spawning sites, respectively. Some females spawned on the prominent coral heads. The mating relationships are shown by common letters; e.g., M-a (Male-a) had mating opportunities with F-a1 (Female-a1) and F-a2 (Female-a2). The two females that were not observed spawning before the experiment are indicated by “?”. The territories of males and home ranges of females during the late afternoon were drawn from 270 – 591 min (median, 471 min) and 73–238 min (median, 110 min) of observations, respectively. The territories of males and home ranges of females during the morning and early afternoon were based on 108–123 min (median, 121 min) and 58–172 min (median, 121 min) of observations, respectively. The home ranges of two individuals (M-d and F-?1) were not drawn on the map in the morning and early afternoon. These individuals were observed in the observational area for a short time but M-d eventually moved outside the observational area and F-?1 eventually disappeared in the observational area. Data were taken from 2 May 2003 to 23 August 2003
Intraspecific interactions among the large males sometimes occurred on the boundaries of their territories during the morning and afternoon observations (morning and early afternoon: median, 0.5 times/h; range, 0–1.0 times/h; n = 5; late afternoon: median, 0.3 times/h; range, 0–0.6 times/h; n = 5). Aligning spots (Supplementary material, Fig. S1b) appeared on the lateral sides of the bodies of two large males when they encountered each other, and they alternately circled around each other with their pectoral fins moving intensely (called “struggling”). In some instances, we observed one individual biting the other during these displays. Besides struggling, the males swam away from other approaching males.
The territories of small males (M-f and M-g) were placed around the territories of large males. Their territories seemed to be relatively smaller in size (39 m2 and 110 m2), compared to those of the large males (Fig. 1a) and were not significantly different from female home ranges (Mann–Whitney U-test, U = 4.0, P = 0.23). The small males overlapped their territories considerably with those of the large males (overlap ratios, 18.8 % and 59.7 %) and the small males (overlap ratios, 13.6 % and 38.0 %). Their territories included none, or only one, prominent coral head where females appeared in the late afternoon (see below), whereas the territories of the large males included one to three coral heads (n = 5). One small male (M-g) maintained a territory during the morning and early afternoon in the same location as that in the late afternoon, but another male (M-f) was not found within the observational area (Fig. 1b).
Large male-small male interactions were observed only during the late afternoon (1.5 times/h and 2.3 times/h, n = 2). Small males either swam away from approaching large males or hid in the coral interstices. Interactions between small males also occurred only during the late afternoon (0.1 and 1.0 times/h, n = 2). We observed struggling between small males on the boundaries of their territories.
Female spatial distribution and diurnal movements
All 12 females were found within the territories of the large males during the late afternoon where they appeared on the same prominent coral heads (Acropora intermedia, A. robusta, A. valenciennesi or Pocillopora eydouxi) (Fig. 1a). They hid in shelters within male territories just after spawning around sunset and were not observed to re-emerge from their shelter on the day.
Three individual females were always observed within male territories during the morning and early afternoon. Four other females maintained their home ranges outside the male territories (Fig. 1b). The other five females (F-a1, F-b4, F-d1, F-e1, and F?-1) were not seen within the observational area during the morning and early afternoon and had probably moved far away from the observational area. The median female home range size was 24 m2 during the morning and early afternoon (range, 5–53 m2, n = 7 females within the observational area) and was not significantly different from that during the late afternoon (median, 23 m2; range 1–96 m2; n = 11; Wilcoxon signed-ranks test: T = 10, P = 0.58). Females rarely overlapped their home range within the territory of a large male during the morning and early afternoon (median, 10.3 % of female home ranges; range, 0.0–74.3 %; n = 7 females within the observational area). The overlap ratio between large males and females increased significantly during the late afternoon (median, 99.7 % of the female home range; range, 63.8–100 %, n = 11; Wilcoxon signed-ranks test: T = 0, P < 0.05). Similarly, the female home ranges did not overlap with each other during the morning and early afternoon (median, 0 %; n = 7). The overlap ratio among females tended to increase during the late afternoon (median, 7.6 %; range, 0–100 %; n = 11; Wilcoxon signed-ranks test: T = 0, P = 0.06). The density of males and females was lower during the morning and early afternoon (0.16 individuals/100 m2 in a minimum oblong area that encompassed all home ranges and territories of observed individuals) than during the late afternoon (0.25 individuals/100 m2).
Intraspecific interactions were rare among females during the late afternoon (median, 0 times/h; range, 0–0.9; n = 11). Females hid in the coral interstices if approached by other females sharing the same prominent coral. However, the females were not evicted from the prominent corals by other females. In contrast, females either struggled with or chased away other females that did not share the prominent coral. Encounters between females during the morning and early afternoon were not observed.
Feeding activity
Each individual usually rested still on a coral head or on the bottom of the reef when feeding, and then lunged suddenly at prey. In the majority of feeding events (92 % of the 72 feeding events), hawkfish were observed lunging at small benthic animals, such as crustaceans or small fish. In contrast, pelagic animals, such as the blue sprat, Spratelloides gracilis, and zooplankton were occasionally (7 %) preyed upon. The feeding frequency of the hawkfish was significantly higher during the morning and early afternoon (median, 1.5 times/60 min; range, 0–4.0 times/60 min; n = 13) than that during the late afternoon (median, 0 times/60 min; range 0–3.3 times/60 min; n = 13; Wilcoxon signed-ranks test, T = 3. P < 0.01).
Courtship and spawning
Male–female interactions occurred infrequently during the morning and early afternoon (median, 0 times/60 min; range 0–2.0 times/60 min; n = 5). Females either swam away or hid in the coral interstices if approached by males.
Large males moved frequently between prominent corals during the late afternoon. Males tended to move greater distances during the late afternoon (median, 142 m/h; range, 67–212 m/h; n = 5) than during the morning and early afternoon (median, 68 m/h; range, 31–82 m/h; n = 4). Females appeared on prominent corals approximately 2 h before sunset and waited for males. Male–female interactions increased during the late afternoon (median, 3.1 times/60 min; range, 1.3–3.9 times/60 min; n = 5). If a large male met a female on a prominent coral head, the female either swam away or hid in the coral interstices at first. However, the female did not swim away from an approaching large male as spawning approaches and moved from the prominent coral head to a spawning site (median, 0.7 m; range, 0–26.4 m; n = 9) with the large male. Just prior to spawning, the large male positioned himself beside and parallel to the female (Supplementary material, Fig. S1c), and the pair executed a spawning ascent into the water column around sunset. Once spawning was finished, the large male moved immediately to another prominent coral to search for and mate with other females.
Mating relationship stability between sexes
Each large male had mating opportunities with 1–4 females (median, 2; n = 4), whereas a small male had mating opportunities with one female. One large male and one small male did not mate with females. The number of females that males mated with tended to increase with male body size, although there was no significant correlation between the number and the body size probably because of the small sample sizes (Spearman’s rank correlation coefficient r s = 0.66, n = 7, P = 0.10). Of the nine females observed to spawn with large males, none mated with more than one male (Table 1). Seven females always spawned with large males dominating the prominent coral heads, on which the females always appeared during late afternoon. As an exceptional case, one female (F-e1) appeared on two prominent coral heads dominated by two different large males (M-b and M-e), but spawned with only one of the males (M-e). The other exception was a female (F-b4) that appeared at a particular prominent coral head within the territory of a large male (M-c) but moved to a neighboring territory of another large male (M-b) to spawn.
A small male (M-g, 118 mm TL) had a mating opportunity with the smallest female (F-g1, 110 mm TL) within the territory of a large male (M-b, 183 mm TL). The smallest female used a prominent coral head, which was commonly used by a mating pair between a large male (M-b) and a female (F-b1, 148 mm TL), but mated only with the small male (M-g). Struggling between the small male (M-g) and the larger female (F-b1) were observed at the coral head. Besides struggling, the small male either swam away or hid in the coral interstices if approached by the large female. The small male was never observed to spawn with the large female.
Large male removal experiment
Two large males (M-a, 183 mm TL and M-c, 158 mm TL) expanded their territories to take over a prominent coral head occupied previously by a large male (M-b, 183 mm TL) one day after that male was removed from the observational area (Fig. 2). Although a small male (M-g) appeared on one of the prominent coral heads, it was chased away by a large male (M-a). As a result, the small male lost a mating opportunity with the smallest female (F-g1), and the female subsequently mated with male M-a. Of the four females that had spawned with M-b, two females (F-b4 and F-b1) started to spawn with M-a on days 16 and 46 after the male was removed. The other two females (F-b3 and F-b2) spawned with M-c three days after the male was removed and during the next mating season.
Changes in the spatial distribution of male territories and female home ranges before a and after removing a large male (M-b) b. Shaded areas and thick lines indicate the large male and small male, respectively. Medium lines indicate the female home ranges. Thin broken lines illustrate outlines of rocks or cracks. Triangles indicate the prominent coral heads where females appeared
Discussion
Each female appeared on a prominent coral head located within a male territory during the late afternoon, and large males monopolized mating opportunities with the females. This stable mating relationship appears to be similar to that of haremic fish, such as small hawkfish (Kadota et al. 2011). The majority of females however left male home territories during the morning and early afternoon, unlike haremic fish. This distribution pattern is an important characteristic of MTV polygamy (Thresher 1984; Kuwamura 1996, 1997). Why did female P. forsteri leave male territories during the morning and early afternoon? Hawkfish more frequently ambushed prey during the morning and early afternoon than during the late afternoon, suggesting that the female movement patterns are considerably related to their feeding activities. Females may be attempting to maximize feeding opportunities over a larger area that could have a relatively greater abundance of prey.
Hawkfish spawned within a short time around sunset (Kadota et al. 2010). In addition, females were dispersed widely and interacted rarely with their mating partners during the morning and early afternoon. Thus, interaction between mating partners would be limited to a relative narrow time frame commencing late in the afternoon and continuing past sunset. Hawkfish females always appeared and remained on the same coral heads during the late afternoon, whereas males moved between the coral heads searching for mates. This result suggests the specific corals are used as rendezvous sites (sensu Moyer and Zaiser 1981; Thresher 1982) to reduce the risk of losing a mating opportunity. Thus, rendezvous sites may be important resources for territorial males to defend.
Another important P. forsteri characteristic is the coexistence of small males with large territorial males. One of these small males was observed pair-spawning with a small female within a large male’s territory. Another case of pair-spawning by a small male was confirmed in a preliminary study conducted in 2002 (T. Kadota, unpubl. Data). In the large male removal experiment, the small male lost mating opportunities because of intrusion by adjacent large males. This result suggests that small males are subordinate competitors among territorial males and acquire mating opportunities differently than large males, although the large male removal experiment were conducted only once. Alternative reproductive tactics of males have been reported in several species of fish (Gross 1996; Taborsky 2001). A similar example of the coexistence of two types of males was reported in the left eye flounder, Engyprosopon grandisquama (Manabe et al. 2000), although small males have rarely been reported in haremic species in general (Kuwamura 1984; Sakai and Kohda 1997; Kadota et al. 2011). Small E. grandisquama males have opportunities to pair-spawn with small females because only a few females mate with large males, which prefer large females, within the narrow mating time window around sunset (Manabe et al. 2000). P. forsteri spawned around sunset, and small males spawned with the smallest females within large male’s territories. Thus, small hawkfish males may be restricted to mating with only small remnant females, as is seen in small left eye flounder males. This tactic used by small males could make the best of a bad situation because the number of females that small males mated with was lower than the number of females that large males mated with. Further support for this comes from the fact that a small male observed in the preliminary study became a large territorial male in the present study (T. Kadota, unpubl. Data).
The P. forsteri mating system has previously been classified as harem polygyny by Donaldson (1990). In this study, we confirmed a stable mating relationship in this species. However, the spatial distribution of the fish did not fit the definition of harem polygyny at our study site. The spatial distributions of some reef fish change according to habitat conditions (e.g., Fricke 1980; Shapiro 1991; Karino et al. 2000). Thus, habitat conditions, such as proximity between suitable feeding and spawning sites and abundance of predators, may have differed between sites in the previous and the present study. In addition, body size (TL) was considerably smaller in the previous study (male: mean, 141 mm; female: 98 mm) than that in the present study (male: mean, 158 mm; female: 128 mm). This size difference may also have affected spatial distribution because body size can influence mobility (Thresher 1984; Moyer 1991). Further study on the P. forsteri mating system in other habitats is expected to reveal phenotypic plasticity in the mating system of this species.
The P. forsteri mating system observed in this study has not been reported in other small hawkfish species, although small hawkfish are relatively well-studied (Thresher 1984; Donaldson 1986, 1987, 1989, 1990; Kadota et al. 2011, 2012). This observation indicates that spatial distribution may vary more in large hawkfish than in small hawkfish. Thus, it is plausible that a mating system similar to that of P. forsteri occurs in other large hawkfish.
References
DeMartini EE (1996) Sheltering and foraging substrate uses of the arc-eye hawkfish Paracirrhites arcatus (Pisces: Cirrhitidae). Bull Mar Sci 58:826–837
Development Core Team R (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Donaldson TJ (1986) Courtship and spawning of the hawkfish Cirrhitichthys falco at Miyake-jima, Japan. Jpn J Ichthyol 33:329–333
Donaldson TJ (1987) Social organization and reproductive behavior of the hawkfish Cirrhitichthys falco (Cirrhitidae). Bull Mar Sci 41:531–540
Donaldson TJ (1989) Facultative monogamy in obligate coral-dwelling hawkfishes (Cirrhitidae). Environ Biol Fish 26:295–302
Donaldson TJ (1990) Reproductive behavior and social organization of some Pacific hawkfishes (Cirrhitidae). Jpn J Ichthyol 36:439–458
Fricke HW (1980) Control of different mating systems in a coral reef fish by one environmental factor. Anim Behav 28:561–569
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–98
Gushima K, Murakami Y (1976) The reef fish fauna of Kuchierabu, offshore Island of southern Japan. J Fac Anim Husb, Hiroshima Univ 15:47–56
Hiatt RW, Strasburg DW (1960) Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecol Monogr 30:65–127
Hobson ES (1974) Feeding relationships of teleostean fishes on coral reefs in Kona, Hawaii. Fish Bull 72:915–1031
Kadota T, Sakai Y, Hashimoto H, Gushima K (2010) Diel and lunar spawning periodicity of the hawkfish Paracirrhites forsteri (Cirrhitidae) on the reefs of Kuchierabu-jima Island, southern Japan. Ichthyol Res 57:102–106
Kadota T, Osato J, Hashimoto H, Sakai Y (2011) Harem structure and female territoriality in the dwarf hawkfish Cirrhitichthys falco (Cirrhitidae). Environ Biol Fish 92:79–88
Kadota T, Osato J, Nagata K, Sakai Y (2012) Reversed sex change in the haremic protogynous hawkfish Cirrhitichthys falco in natural conditions. Ethology 118:226–234
Kane CN, Brooks AJ, Holbrook SJ, Schmitt RJ (2009) The role of microhabitat preference and social organization in determining the spatial distribution of a coral reef fish. Environ Biol Fish 84:1–10
Karino K, Kuwamura T, Nakashima Y, Sakai Y (2000) Predation risk and the opportunity for female mate choice in a coral reef fish. J Ethol 18:109–114
Kuwamura T (1984) Social structure of the protogynous fish Labroides dimidiatus. Publ Seto Mar Biol Lab 29:117–177
Kuwamura T (1996) An introduction to reproductive strategies of fishes. In: Kuwamura T, Nakashima Y (eds) Reproductive strategies in fishes, vol 1. Kaiyusha, Tokyo, pp. 1–41
Kuwamura T (1997) The evolution of parental care and mating systems among Tanganyikan cichlids. In: Kawanabe H, Hori M, Nagoshi M (eds) Fish communities in lake Tanganyika. Kyoto University press, Kyoto, pp. 57–86
Kuwamura T, Sagawa T, Suzuki S (2009) Interspecific variation in spawning time and male mating tactics of the parrotfishes on a fringing coral reef at Iriomote Island, Okinawa. Icthyol Res 56:354–362
Manabe H, Ide M, Shinomiya A (2000) Mating system of the lefteye flounder, Engyprosopon grandisquama. Icthyol Res 47:69–74
Moyer JT (1991) Comparative mating strategies of labrid fishes. Watanabe Ichthyol Inst Monogr 1:1–90
Moyer JT, Nakazono A (1978) Population structure, reproductive behavior and protogynous hermaphroditism in the angelfish Centropyge interruptus at Miyake-jima, Japan. Jpn J Ichthyol 25:25–39
Moyer JT, Zaiser MJ (1981) Social organization and spawning behavior of the pteroine fish Dendrochirus zebra at Miyake-jima, Japan. Jpn J Ichthyol 28:52–69
Nakabo T (2002) Fishes of Japan with pictorial keys to the species, English edn. Tokai University Press, Tokyo
Nakazono A, Nakatani H, Tsukahara H (1985) Reproductive ecology of the Japanese reef fish, Parapercis snyderi. Proc 5th Int Coral Reef Congr 5:355–360
Nelson JS (2006) Fishes of the world, 4th edn. Wiley, New York
Randall JE (1963) Review of the hawkfishes (family Cirrhitidae). Proc US Natn Mus 114:389–451
Sadovy Y, Donaldson TJ (1995) Sexual pattern of Neocirrhites armatus (Cirrhitidae) with notes on other hawkfish species. Environ Biol Fish 42:143–150
Sadovy de Mitcheson Y, Liu M (2008) Functional hermaphroditism in teleosts. Fish Fish 9:1–43
Sakai Y, Kohda M (1997) Harem structure of the protogynous angelfish, Centropyge ferrugatus (Pomacanthidae). Environ Biol Fish 49:333–339
Shapiro DY (1991) Intraspecific variability in social systems of coral reef fishes. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic Press, San Diego, pp. 331–355
Suzuki S, Toguchi K, Makino Y, Kuwamura T, Nakashima Y, Karino K (2008) Group spawning results from the streaking of small males into a sneaking pair: male alternative reproductive tactics in the threespot wrasse Halichoeres trimaculatus. J Ethol 26:397–404
Taborsky M (2001) The evolution of bourgeois, parasitic, and cooperative reproductive behaviors in fishes. J Hered 92:100–110
Thresher RE (1982) Courtship and spawning in the emperor angelfish Pomacanthus imperator, with comments on reproduction by other pomachanthid fishes. Mar Biol 70:149–156
Thresher RE (1984) Reproduction in reef fishes. T.F.H. Publications, Neptune City
Turner GF (1993) Teleost mating behaviour. In: Picher TJ (ed) Behaviour of teleost fishes, 2nd edn. Chapman and Hall, London, pp. 307–331
Wong MYL, Buston PM (2013) Social systems in habitat-specialist reef fishes: key concepts in evolutionary ecology. Bioscience 63:453–463
Acknowledgments
We are grateful to the people of Kuchierabu-jima Island for allowing us to conduct the field work. We also thank N. Shimizu, O. Fujita, S. Takayanagi, and K. Tsukamura for their support during the fieldwork and H. Hashimoto, the late Y. Yogo, Y. Masui, Y. Yamane, and colleagues at the Laboratory of the Biology of Aquatic Resources, Hiroshima University, for their advice. Thanks are also due to G. Suzuki for providing advice on identifying the corals. Finally, we thank T. Kuwamura for comments on an earlier draft of the manuscript. This work was partially supported by JSPS KAKENHI Grant Number 15 K07222 and Inamori Grants to Y. Sakai. This study complies with the current laws of Japan and the guidelines of the Japan Ethological Society. We dedicate this study to the memory of the late K. Gushima, who provided considerable support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 613 kb)
Rights and permissions
About this article
Cite this article
Kadota, T., Sakai, Y. Mating system of the freckled hawkfish, Paracirrhites forsteri (Cirrhitidae) on Kuchierabu-jima Island reefs, southern Japan. Environ Biol Fish 99, 761–769 (2016). https://doi.org/10.1007/s10641-016-0520-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-016-0520-y