Abstract
We investigated the mating system of three sex changing gobiid fishes: Priolepis akihitoi, Trimma emeryi, and Trimma hayashii by underwater observation or specimen collection. P. akihitoi were found in pairs or were solitary. As the pair bond persisted for at least two months, and pair individuals seemed to mate with each other repeatedly, a monogamous mating system was suggested in P. akihitoi. In contrast, the harem-like group (living in a group composed of one largest male and some smaller females and/or immature individuals) of T. hayashii and T. emeryi suggested that these species have harem polygyny.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex change, also called sequential hermaphroditism, has been well documented in various lineages of teleost fishes (Sadovy de Mitcheson and Liu 2008; Avise and Mank 2009). Recently, functional sex change (e.g., protogyny, protandry, bidirectional sex change) has been confirmed in at least 27 families, and the ability to sex change has been only predicted in at least 22 families (Sadovy de Mitcheson and Liu 2008; Shitamitsu and Sunobe 2017). The size-advantage model (SA model) proposed by Ghiselin (1969) and Warner (1975; 1984) predicts that the mating system act as a selective pressure on the evolution of sex change: the protogynous sex change would evolve in polygynous species because large males obtain greater benefit than small males through male–male competition for mating opportunities or mate choice by females, whereas the evolution of protandry is favored when males reproduce successfully regardless of their body size. A lot of empirical studies have supported this prediction (e.g., Kuwamura and Nakashima 1998; Munday et al. 2006; Erisman et al. 2013). Therefore, the clarification of the mating system in sex-changing fish must provide important cues for us to understand the evolution of sex change. The establishment of the mating system is generally influenced by environmental conditions and/or ecological constraints, such as the spatial dispersion pattern of resources and the temporal availability of receptive mates (Emlen and Oring 1977; Shuster and Wade 2003). Thus, the phylogenetic relationship is not always a principal factor that influences the establishment of a mating system. Indeed, many fish lineages contain species that show various patterns of the mating system. (e.g., McCafferty et al. 2002; Erisman et al. 2009; Erisman et al. 2013).
The genera Priolepis and Trimma, closely related to each other phylogenetically (Thacker and Roje 2011; Agorreta et al. 2013; Sunobe et al. 2017), are colorful tiny gobiid fishes mainly distributed on Indo-Pacific coral reefs, and currently contain 37 (e.g., Allen et al. 2018) and 105 (e.g., Winterbottom et al. 2019) validly described species, respectively. Several studies have investigated the sexuality of nine Priolepis (Cole 1990; Sunobe and Nakazono 1999; Cole 2010; Manabe et al. 2013) and 32 Trimma species (Cole 1990; Sunobe and Nakazono 1993; Shiobara 2000; Manabe et al. 2008; Sakurai et al. 2009; Cole 2010; Sunobe et al. 2017). Most of them have been suggested or confirmed to have the ability to change sex bidirectionally; however, some Trimma species have been reported to be gonochoristic (Fukuda et al. 2017a; Sunobe et al. 2017). Recently, various patterns of the mating systems have been found in this lineage, such as monogamy (Sunobe and Nakazono 1999; Fukuda et al. 2017a), harem polygyny (Sunobe and Nakazono 1990; Fukuda et al. 2017b) and polygynous mating within the multi-male group (Tomatsu et al. 2018). Because of this diversification in sexuality and mating systems, this lineage is expected to serve as a valuable model for understanding the relationship between the evolution of sex change and mating system (Sunobe et al. 2017). However, there is less information on the mating systems of Priolepis and Trimma species compared with that of sexuality. Therefore, it is difficult to conduct a comparative phylogenetic study focusing on the evolution of sex change in this lineage. This is because sexuality has been investigated in 41 species (Cole 1990, 2010; Sunobe et al. 2017), but thus far the mating system of only seven species has been studied (Sunobe and Nakazono 1990, 1999; Manabe et al. 2013; Fukuda et al. 2017a, b; Tomatsu et al. 2018).
In this study, we investigated the mating system of three hermaphroditic species: Priolepis akihitoi, Trimma emeryi, and Trimma hayashii. Bidirectional sex change in P. akihitoi has been confirmed by rearing experiments (Manabe et al. 2013). Also, histological studies suggest that T. emeryi and T. hayashii are bidirectional sex changers (Sunobe et al. 2017). By contrast, little is known about their mating system. Manabe et al. (2013) inferred that the mating system of P. akihitoi is monogamy. However, this inference is based on little evidence given that only one pair was found among eight individuals that were captured. Thus, we conducted observations of reproductive behavior in P. akihitoi under natural conditions to confirm their mating system. Meanwhile, the mating systems of T. emeryi and T. hayashii have never been studied. Therefore, we inferred their mating systems based upon their group structures.
Materials and methods
Field observations of Priolepis akihitoi. We conducted underwater observations using SCUBA at Ito Beach, Tateyama, Japan from June to August 2015. The study area was a rocky reef approximately 35 m in length (Fig. 1) that was situated approximately 400 m off the shore at depths of 18-24 m. The water temperature ranged from 14.0 to 25.5 °C during the study period. At the beginning of the observation period, we collected 5 individuals on 15 June 2015, and also collected additional 5 individuals on 27 June 2015 occurring in seven rocky caves or crevices in the study area using a hand net (Fig. 1). All individuals were transported to the laboratory, anesthetized with quinaldine, measured for total length (TL) to the nearest 0.5 mm, and sexed by examination of the shape of the urogenital papilla that were either long and tapered posteriorly in the male, or bulbous with several processes at the papillae opening in the female (Sunobe and Nakazono 1999; Manabe et al. 2013). An individual with urogenital papilla not matching either shape was regarded as being immature. We identified all individuals using subcutaneous injection of a visible implant Elastomer Tag (Northwest Marine Technology Inc., Shaw Island, WA, USA), and released them at collection sites on the next day. Then, we observed each site twice a day between 0900 and 1200 hours. We observed individuals collected on 15 and 27 June 2015 for 23 days out of 46 days and 16 days out of 34 days study period, respectively.
Specimen collection of Trimma emeryi and Trimma hayashii. To estimate the mating system, we collected T. emeryi and T. hayashii using SCUBA and hand nets offshore of Atetsu, Amami Island, Japan, at depths of 6-12 m on 30 July 2014 and 24 April 2015. Previous studies show that Trimma species usually live in a social group in natural habitats, and the group members reproduce within their group; the social group is also the mating group (Sunobe and Nakazono 1990; Fukuda et al. 2017b). Therefore, if multiple individuals were collected from the same cave or crevice, we considered them as a group and analyzed each group structure separately. After collection, all individuals were euthanized in iced seawater, frozen in seawater at approximately − 18 °C, and transported to the laboratory for use in future molecular experiments. In the laboratory. Specimens were defrosted and measured for TL to the nearest 0.5 mm, then sexed using the above methods.
Statistical analyses. Values are presented as means ± standard error. All statistical analyses were conducted on R version 3.5.0. For all tests, the significance level used was α = 0.05. We assessed the normality of datasets based on the Shapiro–Wilk test before each statistical test. We also assessed the homogeneity between two and three statistically compared datasets using the F test and Levene’s test, respectively. The sexual size (TL) dimorphism in P. akihitoi and T. emeryi was examined using Welch’s two-sample t test, followed by the calculation of the effect size index, Cohen’s d (Cohen 1988). An immature P. akihitoi individual was not included in the analysis (see results). The difference in TL between paired and solitary individuals in P. akihitoi was examined with the Wilcoxon Rank Sum test, followed by the calculation of the effect size index r (Cohen 1988) because homoscedasticity was assumed but normality was not expected. A one-way ANOVA followed by the post hoc Tukey–Kramer test was used to compare the TL among males, females, and immature individuals of T. hayashii. We reported the effect size index η2 (Cohen 1988) for the one-way ANOVA and Cohen’s d for the Tukey–Kramer test.
Results
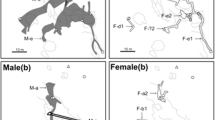
Mating system of Priolepis akihitoi. We collected 10 individuals (42.3 ± 4.0 mm TL, n = 10) from seven sites (Table 1). There was not any significant difference in TL between males and females (t = 0.43, P = 0.68, d = 0.29, n = 9). Three pairs and four solitary individuals were found (Table 1; Fig. 2). There was not any significant difference in TL between paired individuals (45.7 ± 5.0 mm TL, n = 6) and mature solitary individuals (41.7 ± 6.9 mm TL, n = 3) (W = 13.5, P = 0.29, r = 0.34, n = 9). All reproductive pairs consisted of similar size individuals, and males were always larger than females within each pair (Fig. 2). Individuals of each pair were observed close to one another on all observation days. Interactive behavior, such as aggression and courtship, was not observed. Although spawning was not observed, we found egg masses on sites 1 (n = 3), 4 (n = 2) and 5 (n = 2). Eggs were laid on the surface of the bottom, roof, or wall of caves or crevices, and were cared for by the males. Because we did not observe other conspecifics at these sites, we assumed that the eggs found were spawned by the pairs we observed in each. All pairs and two solitary individuals in site 2 and 3 were present throughout the study period. The other two solitary individuals in site 6 and 7 had disappeared on 28 and 5 July, respectively.
Group structure of Trimma emeryi and Trimma hayashii. We collected nine T. emeryi individuals (21.5 ± 0.4 mm TL, n = 9) from three groups (Table 2). Each group consisted of three individuals (one largest male and two smaller females) (Fig. 3a). There was significant difference in TL between males and females (t = 2.71, P = 0.035, d = 1.65, n = 9).
Twenty-nine T. hayashii individuals (20.9 ± 1.0 mm TL, n = 29) were collected from eight groups (Table 2). Those groups contained one male (n = 8), 0-3 females (1.8 ± 0.4 individuals, n = 8) and 0-3 immature individuals (0.9 ± 0.4 individuals, n = 8) (Fig. 3b). The largest individual in the group was always male. There were significant differences in TL between males, females and immature individuals (one-way ANOVA, F (2, 26) = 212.5, P < 0.001, η2 = 0.94; Tukey-Kramer test, male vs female: P = 0.02, d = 1.34, n = 22; male vs immature individual: P < 0.001, d = 8.36, n = 15; female vs immature individual: P < 0.001, d = 9.42, n = 21).
Discussion
The mating system of three species of Priolepis has been suggested to be or confirmed as being monogamous (Sunobe and Nakazono 1999; Manabe et al. 2013). However, field observation has been conducted in only one species, Priolepis cincta (Sunobe and Nakazono 1999). The present study suggests that the mating system of Priolepis akihitoi is monogamy according to the definition proposed by Barlow (1988), in that the male and female confine most of their spawning to the same partner, or they remain partners after fertilization until the young no longer requires care. Whiteman and Côté (2004) divided the evolutionary factors leading to monogamy in marine fishes into six hypotheses: biparental care, resource limitation, low mate availability, increased reproductive efficiency, territorial defense, and a net benefit of single mate sequestration. The monogamous mating system of P. akihitoi probably results from the low mate availability based on their low population density and low mobility. The present study showed that only 10 individuals were found within the study area (approximately 27.4 m × 17.0 m; the lower part of Fig. 1). This population density seems to be relatively low, because we previously found at least 67 individuals of Trimma grammistes, a phylogenetically related species to Priolepis, within the same study area (Fukuda et al. 2017b). Low population density should increase costs when searching for additional mates (Whiteman and Côté 2004). Furthermore, small fishes are generally exposed to greater predation risk (Munday and Jones 1998; Goatley and Bellwood 2016), and the same should apply to P. akihitoi owing to their small body size. Searching for the additional mates would have an enormous cost because potential mortality may be increased in this situation. These costs favor monogamy in P. akihitoi.
Functional bidirectional sex change has been confirmed in P. akihitoi (Manabe et al. 2013). The coral-dwelling monogamous gobies Paragobiodon echinocephalus (Nakashima et al. 1995) and Gobiodon histrio (Munday et al. 1998) also change sex bidirectionally, and reproductive ecology is similar to that of P. akihitoi. Both species spawn in crevices, have paternal care, and males are larger than their female partners even though the differences in body sizes between them are small. Kuwamura et al. (1994), Nakashima et al. (1995), and Munday (2002) explain the adaptive significance of the bidirectional sex change in these species based on the two hypotheses discussed below. Our results and the demonstration of Manabe et al. (2013) indicate that there is a possibility that these hypotheses also explain the evolution of hermaphroditism in P. akihitoi. The first hypothesis is a risk-of-movement model (Nakashima et al. 1995; Munday et al. 1998; Munday 2002). Bidirectional sex change is favored in P. echinocephalus and G. histrio because individuals can avoid predation risk by mating with the closest occurring individual regardless of their sex when the individuals have lost or lack a partner (Nakashima et al. 1995; Munday et al. 1998; Munday 2002). Because P. akihitoi are also small fish and live secluded in rocky caves and crevices, they should be exposed to similar costs when searching for mates. Thus, bidirectional sex change may decrease predation risk and maximize the lifetime reproductive success of P. akihitoi individuals. The second hypothesis is a growth-rate advantage model (Iwasa 1991). In P. echinocephalus, individuals maximize their lifetime reproductive success by reproducing as a female first, because the female grows faster than the male (Kuwamura et al. 1994; Nakashima et al. 1995). Although it is unclear whether the growth rate is different between sexes or not, the fact that females are always smaller than male partners, even if the size difference is relatively small, suggests that the smaller individual in a newly formed pair remains as or changes to female. This has been demonstrated in aquaria (Manabe et al. 2013). Therefore, as with P. echinocephalus, there is a possibility that P. akihitoi conforms to the growth-rate advantage model.
The mating systems of four Trimma species had been investigated in the field (Sunobe and Nakazono 1990; Fukuda et al. 2017b) and captivity (Fukuda et al. 2017a; Tomatsu et al. 2018), and three types of mating systems have been reported: monogamy, harem polygyny, and polygynous mating within a multi-male group. Our results showed that the group structure and size distribution of Trimma emeryi and Trimma hayashii is typically seen in harem polygynous species, such as Trimma okinawae (Sunobe and Nakazono 1990) and T. grammistes (Fukuda et al. 2017b). Therefore, it is strongly inferred that the mating systems of T. emeryi and T. hayashii are harem polygyny. In the genus Trimma, the mating system diversification has been found within closely related species (Sunobe et al. 2017). Emlen and Oring (1977) predicted that the temporal and spatial clumping distribution of fertilizable females increases the probability of polygyny. According to this prediction, the pattern of distribution of group structure for almost all Trimma species studied suggests a high potential for polygyny because they live in groups (clumping distribution) and have a female-biased sex ratio (Sunobe and Nakazono 1990; Sakurai et al. 2009; Fukuda et al. 2017a, b; Tomatsu et al. 2018). Indeed, the polygynous mating system dominates in this lineage (Sunobe et al. 2017). However, Trimma marinae establishes a monogamous mating system by female–female competition for males even though they live in groups and the female-biased sex ratio is the same as in polygynous species (Fukuda et al. 2017a). Therefore, the diversification of the mating system in this lineage should result from not only the diversification of the environmental conditions, but also the differentiation of social behavior in each species. In other species, some social factors have a significant influence on the establishment of the mating system (e.g., Whiteman and Côté 2004). Future large-scale data collection on the mating system, habitat condition, and proximate mechanisms underlying social behavior will make this lineage an excellent model for clarifying how environmental and proximate factors are related to the diversification of mating systems.
Sunobe et al. (2017) suggested that T. emeryi and T. hayashii are bidirectional sex changers based upon their intersexual gonadal structure that consists of both ovarian and testicular tissues simultaneously. The adaptive significance of bidirectional sex change in harem polygynous species has been explained based on the size-advantage (SA) model (Nakashima et al. 1995; Munday et al. 2010). When a male of harem polygynous and protogynous species migrates to another harem with a larger male or a male migrates to another harem with a smaller male, the reproductive success of the smaller males should become lower than that of females of the same size. In these situations, the smaller males change their sex to female (reverse sex change) and reproduce as a female to avoid the loss of reproductive opportunities and cost of inter-harem movement (Munday et al. 2010). Reverse sex change in the above situation has been observed in two Trimma species, T. okinawae (Manabe et al. 2007) and T. grammistes (Fukuda et al. 2017b). Both species show similar group structure to that of T. emeryi and T. hayashii under the natural conditions. Therefore, it is presumed that the pattern of sex change and its adaptive significance in T. emeryi and T. hayashii are the same as that of common harem polygynous species, such as T. okinawae and T. grammistes.
In some fish lineages, the evolution of hermaphroditism and gonochorism is related to the diversification of mating systems as expected by the SA model (e.g., Erisman et al. 2009; Kazancıoğlu and Alonzo 2010). Is there the same relationship between the mating system and sexuality in Priolepis and Trimma lineage? The SA model successfully explains the adaptive significance of sex change in the polygynous Trimma species, as seen in T. okinawae (Sunobe and Nakazono 1993; Manabe et al. 2007), T. grammistes (Fukuda et al. 2017b), and Trimma caudomaculatum (Tomatsu et al. 2018). Also, it is suggested that the same is true in some hermaphroditic Trimma species that are expected to have a polygynous mating system, such as T. emeryi and T. hayashii (Sunobe et al. 2017). However, T. marinae does not have the ability to change sex even though their monogamous mating system with size-unrelated pairing should favor the evolution of protandrous sex change (Fukuda et al. 2017a). This suggests that the evolution of gonochorism results from selective pressures other than their mating system. In P. akihitoi, some features of its life history and pattern of distribution may be responsible for (rather than its mating system) the evolution of sex change in this species. Therefore, the mating system does not necessarily explain the diversification of sexuality in this lineage. Charnov (1982, 1986) has pointed out that sex change should not always be favored even if the reproductive ecology of a species fits the SA model. For example, a sexual difference in mortality and growth rate would contribute to the evolution of sex change (Iwasa 1991). Future research on not only the mating system, but also the entire life history of species is needed to clarify the evolutionary background of hermaphroditism and gonochorism in this lineage.
References
Allen GR, Erdmann MV, Brooks WM (2018) A new species of Priolepis (Pisces: Gobiidae) from Papua New Guinea. J Ocean Sci Found 31:32–37
Agorreta A, San Mauro D, Schliewen U, Tassell JL, Van Kovačić M, Zardoya R, Rüber L (2013) Molecular phylogenetics of Gobioidei and phylogenetic placement of European gobies. Mol Phylogenet Evol 69:619–633
Avise JC, Mank JE (2009) Evolutionary perspectives on hermaphroditism in fishes. Sex Dev 3:152–163
Barlow GW (1988) Monogamy in relation to resource. In: Slobodchikoff CN (ed) The ecology of social behavior. Academic Press, New York, pp 55–79
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton, New Jersey
Charnov EL (1986) Size advantage may not always favor sex change. J Theor Biol 119:283–285
Cohen J (1988) Statistical power analysis for the behavioral sciences. second edition. Lawrence Erlbaum Associates, New Jersey
Cole KS (1990) Patterns of gonad structure in hermaphroditic gobies (Teleostei: Gobiidae). Environ Biol Fish 28:125–142
Cole KS (2010) Gonad morphology in hermaphroditic gobies. In: Cole KS (ed) Reproduction and sexuality in marine fishes: patterns and processes. University of California Press, California, pp 117–162
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating system. Science 197:215–223
Erisman BE, Craig MT, Hastings PA (2009) A phylogenetic test of the size-advantage model: evolutionary changes in mating behavior influence the loss of sex change in a fish lineage. Am Nat 174:83–99
Erisman BE, Petersen CW, Hasting PA, Warner RR (2013) Phylogenetic perspectives on the evolution of functional hermaphroditism in teleost fishes. Integr Comp Biol 53:736–754
Fukuda K, Manabe H, Sakurai M, Dewa S, Shinomiya A, Sunobe T (2017a) Monogamous mating system and sexuality in the gobiid fish, Trimma marinae (Actinopterygii: Gobiidae). J Ethol 35:121–130
Fukuda K, Tanazawa T, Sunobe T (2017b) Polygynous mating system and field evidence for bidirectional sex change in the gobiid fish Trimma grammistes. Int J Pure Appl Zool 5:92–99
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Goatley CHR, Bellwood DR (2016) Body size and mortality rates in coral reef fish: a three-phase relationship. Proc R Soc Lond B Biol Sci 283:20161858
Iwasa Y (1991) Sex change evolution and cost of reproduction. Behav Ecol 2:56–68
Kazancıoğlu E, Alonzo SH (2010) A comparative analysis of sex change in Labridae supports the size advantage hypothesis. Evolution 64:2254-2264
Kuwamura T, Nakashima Y (1998) New aspects of sex change among reef fishes: recent studies in Japan. Environ Biol Fish 52:125–135
Kuwamura T, Nakashima Y, Yogo Y (1994) Sex change in either direction by growth rate advantage in a monogamous coral goby Paragobiodon echinocephalus. Behav Ecol 5:434–438
Manabe H, Ishimura M, Shinomiya A, Sunobe T (2007) Field evidence for bi-directional sex change in the polygynous gobiid fish Trimma okinawae. J Fish Biol 70:600–609
Manabe H, Matsuoka M, Goto K, Dewa S, Shinomiya A, Sakurai M, Sunobe T (2008) Bi-directional sex change in the gobiid fish Trimma sp.: does size-advantage exist? Behaviour 145:99–113
Manabe H, Toyoda K, Nagamoto K, Dewa S, Sakurai M, Hagiwara K, Shinomiya A, Sunobe T (2013) Bidirectional sex change in seven species of Priolepis (Actinopterygii: Gobiidae). Bull Mar Sci 89:635–642
McCafferty S, Bermingham E, Quenouille B, Planes S, Hoelzer G, Asoh K (2002) Historical biogeography and molecular systematic of the Indo-Pacific genus Dascyllus (Teleostei: Pomacentridae). Mol Ecol 11:1377–1392
Munday PL (2002) Bi-directional sex change: testing the growth-rate advantage model. Behav Ecol Sociobiol 52:247–254
Munday PL, Jones GP (1998) The ecological implications of small body size among coral-reef fishes. Oceanogr Mar Biol Annu Rev 36:373–411
Munday PL, Buston PM, Warner RR (2006) Diversity and flexibility of sex-change strategies in animals. Trends Ecol Evol 21:89–95
Munday PL, Caley MJ, Jones GP (1998) Bi-directional sex change in a coral-dwelling goby. Behav Ecol Sociobiol 43:371–377.
Munday PL, Kuwamura T, Kroon FJ (2010) Bidirectional sex change in marine fishes. In: Cole KS (ed) Reproduction and sexuality in marine fishes: patterns and processes. University of California Press, California, pp 241-271.
Nakashima Y, Kuwamura T, Yogo Y (1995) Why be a both-ways sex changer. Ethology 101:301–307
Sadovy de Mitcheson Y, Liu M (2008) Functional hermaphroditism in teleosts. Fish Fish 9:1–43
Sakurai M, Nakakoji S, Manabe H, Dewa S, Shinomiya A, Sunobe T (2009) Bi-directional sex change and gonad structure in the gobiid fish Trimma yanagitai. Ichthyol Res 56:82–86
Shiobara Y (2000) Reproduction and hermaphroditism of the gobiid fish, Trimma grammistes, from Suruga Bay, central Japan. Sci Rep Mus Tokai Univ 2:19–30
Shitamitsu T, Sunobe T (2017) Notes on protandry in the creediid fishes Limnichthys fasciatus and L. nitidus (Teleostei: Creediidae). Ichthyol Res 64:365–367
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Sunobe T, Nakazono A (1990) Polygynous mating system of Trimma okinawae (Pisces: Gobiidae) at Kagoshima, Japan with a note on sex change. Ethology 84:133–143
Sunobe T, Nakazono A (1993) Sex change in both directions by alteration of social dominance in Trimma okinawae (Pisces: Gobiidae). Ethology 94:339–345
Sunobe T, Nakazono A (1999) Mating system and hermaphroditism in the gobiid fish, Priolepis cincta, at Kagoshima, Japan. Ichthyol Res 46:103–105
Sunobe T, Sado T, Hagiwara K, Manabe H, Suzuki T, Kobayashi Y, Sakurai M, Dewa S, Matsuoka M, Shinomiya A, Fukuda K, Miya M (2017) Evolution of bidirectional sex change and gonochorism in fishes of the gobiid genera Trimma, Priolepis, and Trimmatom. Sci Nat. https://doi.org/10.1007/s00114-017-1434-z
Thacker CE, Roje DM (2011) Phylogeny of Gobiidae and identification of gobiid lineages. Syst Biodivers 9:329–347
Tomatsu S, Ogiso K, Fukuda K, Deki M, Dewa S, Manabe H, Sakurai M, Shinomiya A, Sunobe T (2018) Multi-male group and bidirectional sex change in the gobiid fish, Trimma caudomaculatum. Ichthyol Res 65:502–506
Warner RR (1975) The adaptive significance of sequential hermaphroditism in animals. Am Nat 109:61–82
Warner RR (1984) Mating behavior and hermaphroditism in coral reef fishes. Am Sci 72:128–136
Whiteman EA, Côté IM (2004) Monogamy in marine fishes. Biol Rev 79: 351–375
Winterbottom R, Erdmann MV, Mambrasar (2019) A new species of Trimma (Teleostei: Gobiidae) from Indonesia and Timor-Leste. J Ocean Sci Found 32:47–55
Acknowledgments
The Ito diving service BOMMIE provided the facilities for the fieldwork. We are grateful to K. Shiota, N. Yamamoto, R. Tamaki, Y. Kazama and other staff of the above diving service for their encouragement during the study. Thanks are also due to S. Yokoyama for his assistance. Critical reading by two anonymous reviewers led to significant improvement of the manuscript. This work was supported by JSPS KAKENHI Grants to T.S. (No. 19570016, 24370006 and 16K07507) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Fukuda, K., Sunobe, T. Group structure and putative mating system of three hermaphrodite gobiid fish, Priolepis akihitoi, Trimma emeryi, and Trimma hayashii (Actinopterygii: Gobiiformes). Ichthyol Res 67, 552–558 (2020). https://doi.org/10.1007/s10228-020-00750-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-020-00750-w