Summary
Medicinal plants are a promising source for identification of lead molecules for cancer therapy. In our continuous search to discover bioactive compounds from natural products, we isolated (5R, 10R)-4R, 8R-dihydroxy-2S, 3R:15, 16-diepoxycleroda-13(16), 17, 12S:18,1S-dilactone (ECD), a diterpenoid from Tinospora cordifolia and studied its chemopreventive potential in diethylnitrosamine (DEN) induced hepatocellular carcinoma (HCC) rats. Fifty male Wistar rats were divided into five groups. Group I served as normal control. Group II–IV were given DEN (0.01% in drinking water) for twenty weeks. In addition, Group III (preventive treatment) received ECD (10 mg/kg body weight) throughout the study. Group IV (curative treatment) received ECD (10 mg/kg body weight) for the last 8 weeks. Group V received ECD alone (10 mg/kg body weight) throughout the experimental period. At the end of the experimental period all the animals were sacrificed and analyzed for biochemical end points to assess the effect of ECD treatment in DEN induced HCC. The animals treated with DEN showed a decrease in the activities of antioxidant (SOD, CAT) and detoxification enzymes (GSH, GPx) with increase in the activities of the hepatic markers (SGOT, SGPT, LDH). Treatment of ECD in both preventive and curative DEN induced animals increased the level of antioxidants and detoxification enzymes, and decreased serum transaminase level and hepatic marker enzymes to near normal. Histopathological and nodular incidence also confirmed that ECD remarkably reduced tumor incidence and reversed damaged hepatocytes to normal. Our findings confirm that ECD exhibits preventive effect against chemically induced HCC in rats. ECD can be a potent chemopreventive drug for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequent cancers among humans, with 0.25–1 million newly diagnosed cases each year [1]. The highest frequencies are found in sub-Saharan Africa and far eastern Asia, where hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are endemic and in regions where food contaminated with Aflatoxin B1 is consumed [2]. HCC incidence appears to be rising, even in countries with relatively low incidence, especially in Southern Western Europe and Asia [3].
Carcinogenesis may arise as a result of chemical or biological damage to normal cells in a multistep process that involves changes at the initiation level followed by promotion and progression which lead to malignancy. The promotional stage of cancer is reversible stage and appears to be most appropriate target stage for chemopreventive intervention [4]. Chemoprevention is one of the strategies by which we can revert or delay the response of carcinogen. Cancer chemopreventive agents are able to reduce the incidence of tumorigenesis by intervening in one or more stages of carcinogenesis initiation, promotion or prolongation [5].
Animal experimental models are particularly useful for the study of neoplasm in humans. As experimental model of human HCC, we used rats treated with DEN which induces poor, moderate and well differentiated forms of HCCs with histological features similar to those of the human tumors [6].
In developing countries about 35% of prescribed drugs are derived from natural products. Many investigations are being carried out worldwide to discover naturally occurring compounds which can suppress or prevent the progress of carcinogenesis [7]. It is well known that many anticancer compounds derived from plants include Taxol from Pacific Yew tree, Vinblastine and Vincristine from Catharanthus roseus, Rohitukine from Dysoxylum binectariferum, derivatives of Podophyllin from Podophyllum peltatum and Campothecin from Camptotheca acuminata [8]. It is important to continue efforts aimed at discovering anticancer agents based on natural products [9].
Tinospora cordifolia Miers (Guduchi) is distributed throughout the plains of India. It has been used for several centuries in Ayurvedic medicine for the treatment of liver and intestinal disorders [10]. Plant stem possesses immuno-modulatory [11] antipyretic [12], antiulcer [13] and anticancer [14] activities. Formulation of T. cordifolia has been clinically used to treat jaundice, rheumatoid arthritis and diabetes [15]. It has also been used to treat gout, viral hepatitis, general weakness, and throat cancer in man [16].
Clerodane derived diterpinoids was rich in T. cordifolia species [17]. The clerodane diterpenoids comprise a large class of natural products which have been studied more in recent years for their wide biological activities [18, 19]. Esculentin A and Esculentin B isolated from Casearia esculenta belonging to clerodane diterpene group showed anticancer activity [19]. ECD derivatives isolated from Croton lechleri showed anti-tumor and apoptotic activity in prostate cancer [20]. ECD based derivatives from shoots of C. hieronymi showed strong activity against lung A-549 carcinoma cells, mouse lymphoma and human colon carcinoma [21].
Generally the toxicity of indigenous drugs has largely been neglected as it is argued that these drugs are used in traditional clinical practices. But it has been suggested that all natural products and active principles must be subjected to toxicity studies [22]. In our present study the ECD crystal from T. cordifolia was subjected to acute toxicity studies in mice. Enzymes are the best markers of tissue damage because of their tissue specific activity and an obvious sign of hepatic injury is leakage of cellular enzymes into serum. Determination of activity of hepatic enzymes such as asparatate transaminase (AST), alanine transaminase (ALT) and lactate dehydrogenase (LDH) released into the blood by the damaged liver is one of the important tools in the study of HCC.
Drugs with multiple mechanisms of protective and preventive action, including anti-oxidant properties, may be one way forward in minimizing tissue injury in human disease [23]. Cells are also equipped with enzymatic antioxidant mechanisms that play an important role in the elimination of free radicals. Superoxide dismutase (SOD) and catalase (CAT) are primary antioxidant enzymes involved in the inactivation of carcinogen and direct elimination of toxin-free radicals and electrophiles, which might otherwise result in amelioration of oxidative damage and lead to cancer progression [24]. Reduced glutathione (GSH), glutathione peroxidase (Gpx) and glutathione S-transferase (GST) have been assumed as significant markers of chemoprevention owing to their antioxidant and detoxification properties [25].
The present study was carried out to investigate the antihepatocarcinogenic potential of ECD isolated from T. cordifolia induced by DEN in male Wistar Albino rats.
Material and methods
Chemicals
DEN, gamma glutamyl paranitroanalide, hematoxylin and eosin were purchased from Sigma Chemical Company, Bangalore, India. Serum glutamyl oxalate transaminase (SGOT), Serum glutamyl pyruvate transaminase (SGPT) and LDH kits were purchased from Futura systems (Italy). All other chemicals, including solvents were of high purity and analytical grade marketed by Himedia Chemicals, Mumbai, India.
Extraction, isolation and identification of ECD from T. cordifolia
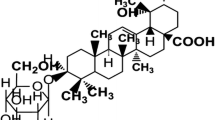
Stems of T. cordifolia were shade dried, powdered (1 kg) and extracted with alcohol by cold percolation method. The extract was evaporated to dryness in vacuum which was (25 gm) chromatographed using silica gel (100–200 mesh). The column was eluted by increasing solvent polarity (hexane to methanol). When increasing the solvent polarity at chloroform: methanol (19:1), a white color compound was eluted which was confirmed as terpinoid [26]. The compound upon repeated crystallization from methanol yielded transparent crystals. Crystal of dimensions 0.22 × 0.20 × 0.16 mm was selected for data collection (Indian Institute of Technology, Chennai) using graphite radiation monochromator (Bruker 1999). The isolated crystal was confirmed as (5R,10R)-4R,8R-dihydroxy-2S,3R:15,16-diepoxycleroda-13(16),17,12S,18,1S-dilactone which has been reported previously [27] (Fig. 1).
Animals
Male Wistar Albino strain rats of body weight 120–150 g were procured from Tamil Nadu University of Animal and Veterinary Science, Chennai. The animal experiments were performed in accordance with legislation on animal welfare of Indian National law (reg. no. 833/a/2004/CPCSEA). Animals were kept in polypropylene cage with hygienic bed of husk under controlled conditions of a 12 h light/12 h dark cycle at 22 ± 2°C till the end of the experimental period. They were fed with normal rat chow marketed by Kings Institute, Chennai and were provided with clean drinking water ad libitum.
Behavioral and toxicological effects
Three groups (ten in each group) of Wistar Albino mice were treated with graded doses of the ECD (10, 20 and 50 mg/kg body weight) given by intragastric intubations orally and observed continuously for 1 h for any gross behavioral changes and death, if any, and then, intermittently for the next 6 h, and then again at 24 h.
Experimental design for hepatocarcinogenesis
Fifty rats (120 to 150 kg/body weight) were divided into five groups (ten animals per group). Liver tumors were induced in group II, III and IV by supplementation of DEN (0.01%) via drinking water [28]. Group I served as normal control and Group II–IV were given DEN till the end of the experimental period of twenty weeks. Group III received ECD (10 mg/kg body weight) throughout the experimental period (preventive treatment). ECD was given by intragastric intubations orally by dissolving in poly ethylene glycol (PEG) due to low solubility in water. Group IV received ECD (10 mg/kg body weight) from the 12th week (curative treatment). Group V received only ECD 10 mg/kg body weight) till the end of the experiment. All the experimental animals were sacrificed at the end of 20th week by administering ketamine intramuscularly (30 mg/kg body weight).
Morphology and morphometry of liver
Soon after the sacrifice, livers were promptly excised from all the treated and control rats, weighed and examined on the surface for subcapsular micro and macroscopic lesions (hypo and hyperplasic nodules). The nodules with approximate spheres were measured in to three categories via, ≥3 (hyperplasic), ≤3–≥1 (Hypoplasic) and <1 mm.
Biochemical estimations
All the biochemical estimations were completed within 24 h of animal sacrifice. SGOT, SGPT and LDH were estimated according to the manufacturer’s kit. Gamma glutamyl transpeptidase (GGT) activity was estimated according to the method of Jacobs [29]. GST and GSH were determined by the method of Moron et al. [30]. GPx activity was estimated by Rotruck et al. method [31]. Protein content in all samples was estimated by the Lowry et al. method using bovine serum albumin as standard [32].
Histopathological study
For histopathological examination, livers were removed, washed in saline and kept in 10% formalin and the fixed paraffin embedded sections were stained with hematoxylin and eosin (H&E).
Statistical analysis
Statistical analysis was performed using ANOVA with SPSS software program while the Duncan multiple range test (DMRT) was used for significance differences between the means.
Results
Toxicity studies
ECD administered mice did not produce any abnormalities such as atoxia, circling, lacrimation, labored breathing, etc. during acute toxicity study up to 50 mg/kg bodyweight.
Effect of ECD on number and size of hepatocellular nodules in DEN induced rats
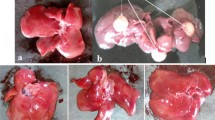
Table 1 shows the number and size of nodules in experimental animals. No macroscopic and microscopic liver nodules were present in Group I and V. DEN alone rats showed 100% nodule incidence in group II (Fig. 2). The most prominent tumor, with a maximum diameter of 8 mm, was seen in group II, which was neoplastic nodule when examined histopathologically. The ECD preventive treatment (3.66 ± 0.88) and curative (Group IV) treatment (8.00 ± 1.05) groups showed a noteworthy decrease in the number of nodules when compared to DEN alone animals (16.80 ± 1.33).
Assessment of body weight and liver weight
Table 2 shows the body weight, liver weight and relative liver weight of control and experimental group of animals. Body weight of DEN treated animals declined significantly by the end of the 20th week when compared with normal control animals (P < 0.05). Treatment with ECD improved the decline of animal body weight remarkably in preventive (Group III) and curative (Group IV) treatment groups significantly (P < 0.05). Liver weight and relative liver weight were increased in DEN alone treated animals when compared with normal control animals (P < 0.05). There was significant decrease in liver weight and relative liver weight of ECD treated preventive treatment (Group III) and curative treatment (Group IV) animals when compared to DEN alone treated animals (P < 0.05). ECD supplied rats showed better resistance against HCC.
Effects on serum transaminase levels
Figure 3 shows SGOT, SGPT and LDH activities in the serum of DEN alone treated rats. The values of SGOT, SGPT and LDH were elevated when compared to normal rats (P < 0.05). ECD supplemented rats in both preventive (Group III) and curative (Group IV) treatments showed remarkable decrease in SGOT, SGPT and LDH values (P < 0.05); ECD reversed the toxic effect of DEN. In the case of ECD alone group there was no significant difference between serum transaminase values when compared to normal control animals (Group I).
Effects on antioxidant and hepatic marker enzymes
As shown in Table 3, SOD and CAT levels in the livers of group II rats significantly decreased (P < 0.05) when compared with the normal group. Administration of ECD increased the levels of SOD when compared to that of the DEN alone treated rats. The CAT level in group II significantly decreased in comparison with the normal group. Preventive and curative treatment rats showed significant (P < 0.05) increase in CAT levels when compared to that of the DEN alone treated rats. GGT and GST values in DEN alone treated rats were increased significantly when compared to normal group (P < 0.05). In the case of preventive treatment (Group III) the levels became near normal. In curative treatment (Group IV) the levels were diminished compared to group II (P < 0.05).
Effects on detoxification enzymes
DEN alone treated rats showed decreased level of detoxifying enzymes GSH and GPx (significant at P < 0.05) when compared to normal control animals (Fig. 4). There was a remarkable increase in the levels GSH and GPx on preventive and curative ECD treatment rats (significant at P < 0.05). There was no significant difference between the normal control animals and ECD alone treated animals.
Histopathological results
Histological examination of the liver showed normal architecture in both normal control group (Fig. 5a) and ECD supplemented rats (Fig. 5e). However, cellular damage with malignancy was obvious in the DEN treated liver. The liver showed loss of architecture, neoplastic hepatocytes with large cells, vesicular nuclei and prominent nucleoli. It showed nodular arrangement (Pseudolobule formation) surrounded by lymphocytic infiltrate (Fig. 5b). In contrast, DEN with ECD preventive treatment showed near normal hepatocytes with lymphocytic infiltration formed around the central vein without disruption of the liver architecture (Fig. 5c). In DEN with ECD curative treatment, liver showed pseudolobule formation with less nuclear changes when compared to DEN alone treated rats. Lymphocytes in the fibrous (pseudolobule) bands were formed. The hepatocytes were near normal (Fig. 5d).
Discussion
Tumorigenesis is a multistep process that begins with cellular transformation and progresses to hyper proliferation leading to metastatic lesions [33]. This progress can be activated by the carcinogenotoxic substances which are widely employed to develop cancers in specific organs of experimental animals. DEN is a potent carcinogen for HCC [30]. DEN is well known to cause perturbations in the nuclear enzymes involved in DNA repair and is normally used as a carcinogen to induce liver cancer in animal models and these compounds are considered to be effective health hazards in causing HCC.
Liver plays a pivotal role in regulation of physiological processes such as metabolism, secretion and storage. Unfortunately it is a common target for a number of toxicants [34]. The multitude of pathological changes caused by the progression of tumor as well as its inhibition through chemotherapy is expected to be reflected in the biochemical and histological parameters of the host system, particularly pertaining to the liver which is known to be the major organ affected in carcinogenesis.
Plant materials are a promising source for the identification of lead molecules against cancer. Certainly, to move from a crude plant extract to a prescription drug with proven effectiveness as a cancer chemopreventive agent is a long and arduous process. In the present study ECD, a diterpenoid, was isolated from the stems of T. cordifolia which was found to have chemopreventive potential in DEN induced HCC.
In short-term toxicity studies, the administration of ECD did not exhibit any adverse effect. The acute and subacute toxicity studies of T. cordifolia had no toxic effects on heart, liver and kidney [34]. In earlier studies the methanolic crude extract of T. cordifolia was found to be nontoxic and no sign of death of mice up to 10 g/kg body weight was noticed indicating the safety of the treatment [35]. In our present study the compound ECD did not exhibit any toxicity signs up to 50 mg/kg body weight. Histopathological examinations suggested that no gross abnormalities or pathological lesions developed in the ECD alone treated animals.
One major symptom of HCC is weight loss [36]. A significant (P > 0.05) weight loss was observed in rats exposed to DEN. Treatment with ECD improved the body weight which indicated the reversal of chemically induced hepatocarcinogenesis. This suggested that ECD had no adverse effect on the growth response of rats. ECD was found to significantly reduce the number of macroscopic nodules; however more number and large size of nodules were found in DEN alone treated animals. In curative treatment the nodular incidence was decreased when compared to DEN alone treated animals. Not all nodules become cancerous during their life span, but several observations suggest that the nodules are precursors of hepatic cancer. A large body of data in experimental and human disease has found a correlation between the size of hyperplasic nodules and the likelihood of HCC [37]. The inhibitory effect of nodule development may be due to ECD.
Hepatospecific enzymes were activated when hepatocellular damage gave rise to abnormalities of liver function [38]. SGOT and SGPT activities in blood serum are generally accepted as an index of liver damage and this tendency is also known to be distinct in rodents. SGPT is accepted to be a highly liver specific enzyme. SGOT might be a non specific-index because it is distributed not only in the liver but also in the heart, skeletal muscle, brain and kidney. Significantly elevated levels of SGOT and SGPT were observed which indicated the liver damage and loss of functional integrity of cell membranes, thus proving to be an excellent ‘marker’ enzyme for this tumor model. [39]. Hepatocellular necrosis leads to high levels of SGOT and SGPT which are released from the liver into the blood. On the other hand, LDH activity is related to functional hepatocytes. A high rate of glycolysis is manifested in malignant conditions because dividing neoplastic cells consume more energy than required in the normal status, in that way leading to an abnormal increase in LDH activity. Treatment with ECD in DEN treated animals significantly reduced the level of these enzymes indicating preventive effect by stabilization of plasma membrane as well as repair of hepatic tissue damage caused by tumor induction.
GGT and GST are important markers for HCC. GGT is the only known enzyme to break the γ-glutamyl bond of glutathione and, as such, is important for recycling of glutathione and the metabolism of glutathione xenobiotic conjugates [40]. This enzyme is induced mainly in the periportal areas of the liver by a wide range of chemopreventive agents. An increase in the activity of GGT may lead to the accumulation of glycine and cysteine, thereby reducing ferric nitrilotriacetate (Fe-NTA) to its ferrous complex and enhancing peroxidative damage to the tissue or membrane [41]. GST is prominent phase-II enzyme involved in detoxification, conjugation and elimination of carcinogens in the form of glucuronides, sulfates and glutathione conjugates. GST, used as a marker for evaluating anticarcinogenic potential, catalyzes electrophilic conjugation with reduced glutathione thus counteracting a variety of carcinogens. ECD administrated animals showed markedly decreased levels of GGT and GST indicating that ECD has the ability to prevent HCC.
A possible mechanism is that ECD might either inhibit methionine aminopeptidase which is involved in the removal of N-terminal methionine from peptides and proteins and thus affect the key regulator of proliferation of cancer [42] or ECD might inhibit the production of interleukin-8 (IL-8) by preventing DNA binding of amino peptidase (AP-1) since ECD derivatives of sesquiterpene lactones are known to do this [43].
The ability of plant-derived compounds to function as antioxidants has been well established, and their role in chemoprevention is supported by numerous epidemiological studies [44]. The antioxidant properties of crude extract of T. cordifolia were earlier investigated and found to possess free radical scavenging property [45]. The endogenous antioxidant system may frustrate the reactive oxygen species (ROS) there by reduce the oxidative stress with the enzymic antioxidants such as SOD and CAT. SOD initiates the conversion of superoxide radical (O−2) to hydrogen peroxide while CAT converts H2O2 to H2O. Depletion in the activity of these enzymes can be due to an enhanced radical production during DEN alone treated rats [46]. ECD has been able to enhance the antioxidant enzymes SOD and CAT in tumor-bearing rats to near normal levels. ECD exhibits preventive effect in DEN induced HCC by reducing the levels of oxidative injuries on DNA and other components of hepatocytes during the early stage of hepatocarcinogenesis.
GSH and GPx are the most potent enzymes playing an important role in detoxification mechanisms or in the protection of cellular constituents against oxidating and alkylating agents [47]. The levels of GSH and GPx were used to monitor the balance of oxidative stress and chemopreventive ability. A decrease in the GSH level could therefore expose the mitochondria to their own endogenously generated free radicals, leading to irreversible damages [48]. The reactivity of ECD against GSH could be useful in anti-multidrug resistance therapy because elevated intracellular GSH level can augment cell resistance to irradiation and chemotherapy and the use of alkylating agents to deplete intracellular GSH content can increase cell response to chemotherapy.
In conclusion, ECD from T. cordifolia was able to prevent malignancy. It was effective in inhibiting tumor growth in solid tumor model. The present investigation highlights the chemopreventive potential of ECD in DEN induced hepatocarcinogenesis which might be due to the antioxidant and detoxification mechanisms. Decrease in serum transaminase enzymes maintained the functional integrity of membrane because of protective effect of ECD. The biochemical and histological studies also supported the chemopreventive properties of ECD. Our study confirms that ECD plays duel role by blocking carcinogen metabolic activation and enhancing carcinogen detoxification.
Abbreviations
- HCC:
-
hepatocellular carcinoma
- DEN:
-
diethyl nitrosamine
- ECD:
-
epoxy clerodane diterpene
- SGOT:
-
serum glutamyl oxalate transaminase
- SGPT:
-
serum glutamyl pyruvate transaminase
- LDH:
-
lactate dehydrogenase
- GGT:
-
gamma glutamyl transpeptidase
- GST:
-
glutathione-S-transferase
- GSH:
-
glutathione reductase
- GPx:
-
glutathione peroxidase
- SOD:
-
superoxide dismutase
- CAT:
-
catalase
- PEG:
-
poly ethylene glycol
- ROS:
-
reactive oxygen species
- Fe-NTA:
-
ferric nitrilotriacetate
References
Feo FM, Miglio RD, Simile MM, Muroni MR, Calvisi DF, Frau M, Pascale RM (2006) Hepatocellular carcinoma as a complex polygenic disease. Interpretive analysis of recent developments on genetic predisposition. Biochim Biophys Acta 1765:126–147
Feitelson MA, Sun B, Tufan NLS, Liu J, Pan J, Lian Z (2002) Genetic mechanisms of hepatocarcinogenesis. Oncogene 21:2593–2604, doi:10.1038/sj.onc.1205434
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917, doi:10.1016/S0140-6736(03)14964-1
Hong WK, Sporn MB (1997) Recent advances in chemoprevention of cancer. Science 278:1073–1077, doi:10.1126/science.278.5340.1073
Digiovanni J (1994) Multistage carcinogenesis in mouse skin. Pharmacol Ther 54:63–128, doi:10.1016/0163-7258(92)90051-Z
Di Stefano G, Fiume L, Bolondi L, Lanza M, Pariali M, Chieco P (2005) Enhanced uptake of lactosaminated human albumin by rat hepatocarcinomas: implications for an improved chemotherapy of primary liver tumours. Liver Int 25:854–860, doi:10.1111/j.1478-3231.2005.1118.x
Cheng YL, Change WL, Lee SC, Liu YG, Chen CJ, Harn HJ (2004) Acetone extract of Angelica sinensis inhibits proliferation of human cancer cells via including cell cycle arrest and apoptosis. Life Sci 75:1579–1594, doi:10.1016/j.lfs.2004.03.009
Mukherjee AK, Basu S, Sarkar N, Ghosh AC (2001) Advances in cancer therapy with plant based natural products. Curr Med Chem 8:1467–1486
Pezzuto JM (1997) Plant derived anticancer agents. Biochem Pharmacol 53:121–133, doi:10.1016/S0006-2952(96)00654-5
Chadha YR (1976) Publication and Information Directorate. CSIR, NewDelhi, The wealth of India, 10:251
Atal CK, Sharma ML, Kaul A, Khajuria A (1986) Immunomodulating agents of plant origin. Preliminary screening. J Ethnopharmacol 18(2):133–141, doi:10.1016/0378-8741(86)90025-5
Vedavathy S, Rao KN (1991) Antipyretic activity of six indigenous medicinal plants of Tirumala Hills Andhra Pradesh, India. J Ethnopharmacol 33:1–2, doi:10.1016/0378-8741(91)90153-5
Sarma DNK, Khosa RL, Chansouria JPN, Sahai M (1995) Antiulcer activity of Tinospora cordifolia Miers and Centella asiatica Linn extracts. Phytother Res 9(8):589–590, doi:10.1002/ptr.2650090811
Jagetia GC, Nayak V, Vidyasagar MS (1998) Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in cultured HeLa cells. Cancer Lett 127:71–82, doi:10.1016/S0304-3835(98)00047-0
Kirtikar KR, Basu DD (1980) Indian medicinal plants. Lalit Mohan Basu Allahabad 1(2):75–81
Chauhan K (1995) Successful treatment of throat cancer with ayurvedic drugs. Suchitra Ayurved 47:840–842
Maurya R, Wazir V, Tyagi A, Kapil SR (1995) Clerodane diterpenoids from Tinospora cordifolia. Phytochemistry 38:659–661, doi:10.1016/0031-9422(94)00686-N
Oberlies NM, Burgess JP, Navarro HA, Pinos RE, Fairchild CR, Peterson RW, Soejatto DD, Famaworth NR, Kingdom AD, Wani MC, Wall ME (2002) Novel bioactive clerodane diterpenoids from the leaves and twings of Casearia sylvestris. J Nat Prod 65:95–99, doi:10.1021/np010459m
Prakash SCV, Hoch JM, Kingston DG (2002) Structure and stereochemistry of new cytotoxic clerodane diterpenoids from the bark of Casearia lucida from the Madagascar rainforest. J Nat Prod 65:100–107, doi:10.1021/np010405c
Morita H (1991) Structures and cytotoxic activity relationship of Casearins, New Clerodane Diterpenes from Casearia sylvestris Sw. Chem Pharm Bull (Tokyo) 39(2):693–697
Huang DM, Shen YC, Kung LF, Teng CM, Guh JH (2004) Investigation of extrinsic and intrinsic apoptosis pathways of new clerodane diterpenoids in human prostate cancer PC-3 cells. Eur J Pharm 503:17–24, doi:10.1016/j.ejphar.2004.09.040
Sadzuka Y, Sugiyama T, Shimoi K, Kinae N, Hirota S (1997) Protective effect of flavonoids on duxorubicin-induced cardiotoxicity. Toxicol Lett 92:1–7, doi:10.1016/S0378-4274(97)00028-3
Barry H (1991) Antioxidant effects a basis for drug selection. Drugs 42:569, doi:10.2165/00003495-199142040-00003
Prestera T, Zhang Y, Spencer SR, Wilczak C, Talalay P (1993) The electrophilic counter attack responses: protection against neoplasia and toxicity. Adv Enzyme Regul 33:281–296, doi:10.1016/0065-2571(93)90024-8
Saydam N, Kirb A, Demir O, Hazan E, Oto O, Saydam O (1997) Determination of glutathione reductase, glutathione peroxidase and glutathione-S transferase levels in human lung cancer tissues. Cancer Lett 119:13–19, doi:10.1016/S0304-3835(97)00245-0
Malairajan P, Narashiman S, Jessikalaveni K, Kavimani S (2007) Antiulcer activity of crude alcoholic extract of Toona ciliate Romer (heart wood). J Ethnopharmacol 110:348–351, doi:10.1016/j.jep.2006.10.018
Swaminathan K, Bhatt RK, Sabata BK, Sinha UC (1988) Crystal structure of a new diterpenoid furanolactone from Tinospora cordifolia Meirs. Acta Crystallogr C 44:1421–1424, doi:10.1107/S0108270188003798
Woo-song HA, Klm Ck, Song SE, Kang CB (2001) Study on mechanism of multistep hepatotumorigenesis in rat: development of hepatotumorigenesis. J Vet Sci 2(1):53–58
Jacobs WL (1971) A colorimetric assay for gamma-glutamyl transpeptidase. Clin Chim Acta 31(1):175–179, doi:10.1016/0009-8981(71)90375-5
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochim Biophys Acta 582(1):67–78
Rotruck JT, Pope AL, Ganther HE (1973) Selenium: biochemical role as a component of glutathione peroxidase, purification and assay. Science 179:588–590, doi:10.1126/science.179.4073.588
Lowry OH, Rosenrough NJ, Farr AI, Randall RJ (1951) Protein determination using Folin Ciocalteau reagent. J Biol Chem 193:265–275
Hahn WC, Weinberg RA (2002) Rules for making human tumor cells. N Engl J Med 347:1593–1603, doi:10.1056/NEJMra021902
Mayer SA, Kulkarni AP (2001) Hepatotoxicity. In: Hodgson E, Smart RC (eds) Introduction to biochemical toxicology. Wiley, New York, pp 599–628
Mary NK, Babu BH, Padikkala J (2003) Antiatherogenic effect of Caps HT2, a herbal Ayurvedic medicine formulation. Phytomedicine 10:474–482, doi:10.1078/094471103322331412
Kwe MC (1996) Tumors of the liver. In: Zakim D, Boyer TD (eds) Hepatology—a text book of liver disease. Saunders, Philadelphia, pp 1513–1548
Bishayee A, Chatterjee M (1995) Inhibitory effect of vanadium on rat liver carcinogenesis initiated with diethylnitrosamine and promoted by phenobarbitol. Br J Cancer 71:1214–1220
Ansari RA, Tripathi SC, Patnaik GK, Dhawan BN (1991) Antihepatotoxic properties of picroliv, an active fraction from rhizomes of Picrorhiza kurroa. J Ethnopharmacol 34:61, doi:10.1016/0378-8741(91)90189-K
Gupta M, Mazumder UK, Sambath kumar R, Sivashankar T, Vamsi MLM (2004) Antitumor activity and antioxidant status of Caesalpinia bonducella against Ehrlich ascites carcinoma in Swiss albino mice. J Pharmacol Sci 94:177–184, doi:10.1254/jphs.94.177
Rocchi E, Seium Y, Camellini L (1997) Hepatic tocopherol content in primary hepatocellular carcinoma and liver metastatis. Hepatology 26:67–72, doi:10.1002/hep.510260109
Khan N, Sultana S (2005) Anticarcinogenic effect of Nymphaea alba against oxidative damage. Hyperproliferative response and renal carcinogenesis in wistar rats. Mol Cell Biochem 271:1–11, doi:10.1007/s11010-005-2258-2
Klohs WD, Hamby JM (1999) Antiangiogenic agents. Curr Opin Biotechnol 10:544–549, doi:10.1016/S0958-1669(99)00033-6
Lindenmeyer MT, Hrenn A, Kern C, Castro V, Siedle B, Merfort I et al (2008) Sesquiterpenes lactones as inhibitor of IL-8 expression in HeLa cells. Bioorg Med Chem 14(8):2487–2497, doi:10.1016/j.bmc.2005.11.027
Chung FL, Schwartz J, Herzog CR, Yang YM (2003) Tea and cancer prevention: studies in animals and humans. J Nutr 133:3268S–3274S
Prince PS, Menon VP (2001) Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytother Res 5(3):213–218, doi:10.1002/ptr.707
Seifried HE, McDonald SS, Anderson DE, Greenwald P, Milner JA (2003) The antioxidant conundrum in cancer. Cancer Res 63:4295–4298
Masella R, Vari R, D’Archivio M, Benedetto R, Matarrese P, Malorni W, Scazzocchio B, Giovannini C (2004) Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J Nutr 134:785–791
Hatono S, Jimenez A, Wargovich MJ (1996) Chemopreventive effect of S-allylcysteine and its relationship to the detoxication enzyme glutathione S-transferase. Carcinogenesis 17:1041–1044, doi:10.1093/carcin/17.5.1041
Acknowledgments
We are grateful to Dr. C. S. Vijayalakshmi, Professor, Kilpauk Medical College, Chennai for helping in histopathological assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhanasekaran, M., Baskar, AA., Ignacimuthu, S. et al. Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest New Drugs 27, 347–355 (2009). https://doi.org/10.1007/s10637-008-9181-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9181-9