Abstract
The aerial part of Wedelia calendulacea have been used in Ayurveda, Unani, Tibetan, Siddha and other folk medicine systems to protect the liver and renal tissue. Liver is considered as primary metabolizing site of body, which is prone to damage by endogenous and exogenous toxicants. A reason for liver toxicity, and major causes of the hepatocellular carcinoma (HCC). 19-α-Hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG), a triterpenoids found in the higher plants, has been known to possess protective effect against various toxicants. The aim of the current study was to scrutinize the hepatoprotective mechanism of HEG against DEN-induced oxidative stress, hyperproliferation, inflammation and apoptosis tissue injury in Wistar rats. Invitro cell lines study of HEG scrutinized against the Hep-G2 and HuH-7 cells. A single dose of DEN (200 mg/kg) and double dose of phenobarbitol were administered to induce the liver damage in rats; the dose treatment of HEG was terminated at the end of 22 weeks. Macroscopical study was performed for the confirmation of hepatic nodules. The serum and hepatic samples were collected for further biochemical and histopathological analysis. Hepatic; non-hepatic; Phase I and II antioxidant enzymes were also examined. Additionally, we also scrutinized the inflammatory cytokines viz., tumor necrosis factor-α, interlukin-6, interlukin-1β, and Nuclear factor kappa beta (NF-kB), respectively. Histopathological study was also performed for analyzing the changes during the HCC. HEG confirmed the reduction of growth and deoxyribonucleic acid synthesis of both cell lines. DEN successfully induced the HCC in all group, which was significantly (p < 0.001) altered by the HEG in a dose-dependent manner. The decreased level of pro-inflammatory cytokines and altered membrane-bound enzyme activity were also observed. HEG inhibits the phase I, II and antioxidant enzymes at the effective dose-dependent manner, which were considered as the precursor of the HCC. The alteration of phase I, II and antioxidant enzymes confirmed the inhibition of inflammatory reaction and oxidative stress, which directly or indirectly inhibited the NF-kB expression. Collectively, we can conclude that the HEG inhibited the growth of Hepatocellular carcinoma via attenuating the NF-kB pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepato-cellular carcinoma isconsidered as the major malignancy Worldwide: the 5th most common malignant tumor among the other types of cancer, especially high incidence in Asian countries. It is believed as the 3rd leading cancer-related death incidence globally (Yin and Evason 2013). The expansion of the liver tumor occurs during the liver cirrhosis setting the stage for chronic liver disease. In most of the cases, the diagnosis of the cancer is not easy and limited options are left for treatment for hepatic cancer viz., liver transplantation. The recurrence rate of this particular cancer is high and the survival rate is poor (Lawrence et al. 1995; Yin and Evason 2013). Other treatment options for the HCC are radiotherapy and chemotherapy; both are ineffective and have minimum effect. Chemoprevention has been considered as the suitable treatment for treatment of HCC with altering the incidence of the disease and also effective in the prognosis of HCC (Kwak et al. 2001).
Diethylnitrosamine (nitrosamine) is the member of the N-nitroso compound and is considered as the most important hepato-toxin and carcinogens in the environment. Nitrosamine-containing compounds are considered to be more effectual hazards to human health as they are widely used in the industry (Marnewick et al. 2009). N-Nitroso is commonly found in alcoholic beverages, agriculture chemical, tobacco smoke, occupational settings, ground water, cosmetics, food like cheese, soybean, dried and salted fish. Alcohol, viral infection, fungal toxin, especially aflatoxin, toxic industrial chemicals, water and air pollutants are considered as the major risk factor for the expansion of HCC. Various drugs are metabolized into the body and start the generation of DEN in a single form or repeated form (Ip et al. 2013). In liver, cytochrome P450 (CYP2E1) stimulates the N-nitrosodiethylamine to form reactive oxygen species and electrophilic, which induce the carcinogenicity, cytotoxicity, and mutagenicity via oxidative damage. Another mechanism of nitrosamine to induce the HCC, CYP2E1 excite the Kupffer cells, which start the generation of reactive oxygen species (superoxide, peroxyl, hydroxyl and hydrogen peroxide) and damage the hepatic cells. ROS also generates the cell death via activation the DNA fragmentation, lipid peroxidation and carbonylation (Pradeep et al. 2007; Bishayee et al. 2010).
Oxygen derived radicals such as hydroxyl (OH), peroxyl (RO2) and superoxide (O2 −) as well as non-radicals viz., peroxynitrite (ONOO−) and hydrogen peroxide (H2O2) are from reactive oxygen species (ROS) family. Various investigations confirm that the generation of oxygen-derived radicals involves in exogenous and endogenous stimulus. ROS is also considered as highly toxic substance of cellular metabolism which directly shows the effect on expansion of cells and confirms the survival on the expansion of cancer (Bishayee et al. 2011; Bingül et al. 2013). During the metabolic biotransformation of DEN, liver is the primary target site to induce the HCC, which enhances the content of oxidative stress and cellular damage due to continuous production of ROS along with the production of the oxygen-derived ROS that results either in damaging the cellular component or activation of signal-specific transduction pathways and can also deactivate the maladaptive molecular response. Enhancing the ROS content in the liver increases the content of free radical, which in turn boosts the consumption of endogenous antioxidant enzymes (Pradeep et al. 2010). Consequently, excessive generation of ROS stimulates the oncogenesis via modulation of redox signal pathways. The previously discussed mechanism confirms that the ROS plays a significant role in the expansion of apoptosis, cellular proliferation, signal transduction and differentiation. In the current experimental study, we made efforts to explore the novel chemo-protective agents which reduce the initiation of malignant transformation.
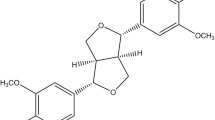
Various chemo-protective and epidemiologic investigation already proved that the regular intake of fruit and vegetable has been reducing the risk factor of cancer in animal and in human. Wedelia calendulacea. (Compositae), is the only species of the genus Wedelia native to tropical and sub-tropical region including India, Ceylon, Burma, Japan and China. In India, Wedelia propagated in Assam, West Bangel, Jammu and Kashmir, Himachal Pradesh and Tamilnadu, etc. The root, rhizomes, bark, young stalks and leaves of the plants are edible, and traditionally it is used as renal protective tonic. In the remote areas, the plant is commonly used in the treatment of renal diseases, jaundice, cholagogue, hepatic enlargement and deobstruent. It is also used in treatment of swelling, distended stomach, hepatitis, headaches, baldness and various types of skin diseases and disorder. The bioactive chemical constituents viz., Norwedelic acid, bisdesmosidic oleanolic acid, ginsenoside, bisdesmosidic oleanolic acid glycoside and Kauren diterpene are isolated from the plant. Several shreds of evidence have accentuated that biologically active products present in functional food may be exploited as a complementary treatment for hepatic cancer (Jang et al. 1997; Wagner et al. 2001; Butler 2008). Various forms of the foods and drinks confirm the presence of Triterpenoids and have been scrutinized as the potential treatment of various inflammatory and cancer diseases (Setzer and Setzer 2003; Neto 2007; James and Dubery 2009). Triterpenoids, metabolites of isopentenyl pyrophosphate oligomers, are widely distributed in different species of higher plants in the form of phytosterol, triterpene glycosides (saponin), free triterpenoids and their precursors with a protective effect against various diseases (Mahato et al. 1992; Neto 2007; James and Dubery 2009; Hill and Connolly 2012). The anti-inflammatory, antidiabetic, anticancer and anti-arthritic effects of Triterpenoids have already been proved by several researchers. Numerous studies confirmed the antioxidant effect of triterpenoids, which confirm the antioxidant potential and their capacity to scavenge the free radicals generated via oxidative stress(Mahato et al. 1992; Neto 2007; James and Dubery 2009; Hill and Connolly 2012). Triterpenoids get more attention due to their free radical scavenging effect and antioxidant action. The current experimental study was designed as a follow-up to our group research interest in the discovery of novel hepatoprotective phytoconstitutents from traditional Indian medicine . We made an attempt to scrutinize the chemoprotective effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-α-d-glucopyranoside (HEG) (Fig. 1) isolated by Verma et al. (2017) against the diethylnitrosamine (DEN)-induced hepatocellular carcinoma in Wistar rats and tried to decipher the possible mechanism.
Materials and methods
Chemicals
Diethylnitrosamine and other reagents were purchased from the Sigma-Aldrich (USA). Other chemicals and solvents used in the current study were of analytical grade and purchased from the local vendor.
Effect 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) (Fig. 1) on the growth inhibition in HCC cell lines
HCC cell line viz., HuH-7 and Hep G2 was used for the current experimental study. The cells were cultured according to the reported method of Anwar et al. (2015b) with minor modification. Both cell lines were grown in Dulbecco’s Eagle medium (DMEM). The medium was supplemented with penicillin–streptomycin, l-glutamine, fetal bovine serum and was subjected to incubation.
The drug was dissolved in solvent with different dilution to get the stock of the drug prepared. For getting the desired concentration of the drug, the drug was further diluted with the solution and then applied to the growing adherent cells. Different doses of HEG (0.1–0 mM) were used for the in vitro study.
Determination of growth inhibition
Direct cell counting method was used for scrutinizing the effect of HEG on the cell growth. Cell growth was determined via using the Haemocytometer. Different concentrations of HEG (10–50 mM) were added in the 5 × 105 cells, and the cells seeded into the 60 mm plates for 24 h. After 48 h, both adherent and floated cells were harvested, and viable cells were dyed with the trypan blue dye exclusion. The cell growth was counted in presence and absence of the HEG.
DNA synthesis assay
Both cell lines were seeded in the 24-well plates. After 24 h, the different dilution of the HEG dissolved was added in the growing cells. After 24-h treatment of HEG to cells, 24-well plates were treated with the 1 µCi [methyl-3H]-thymidine and incubated for the 6 h. Both cell lines were trypsinated and each well was again harvested onto a glass fiber filter mat via cell harvester; the plates were dried for 1 h and the DNA incorporation was estimated by using the 1450 Microbeta scintillation counter.
Experimental study
Animals
Pathogen-free Swiss albino Wistar rats were used for the current investigation. Wistar rats (150–200 g, sex = male) were collected from the Faculty Animal House. The rats were kept in the single cage and maintained as per the committee for the purpose of control and supervision of experiments on animals (CPCSEA) guidelines. The rats were kept in relative humidity (30–70%) and temperature (25 ± 2 °C) with a 12/12 h light and dark cycle of light in the Faculty Animal House. The rats were treated with the standard diet pallet and water ad libitum. The study was approved by the Laboratory Animal Facility (LAF), Sam Higginbottom University of Agriculture, Technology and Sciences, Allahabad, Uttar Pradesh, India 211007 (IAEC/SHIATS/PA151X/FVK01).
Induction of HCC
Diethylnitrosamine (200 mg/kg) was used for the induction of HCC in the rats (Anwar et al. 2015b). Single intraperitoneal injection of DEN (prepared via dissolved in the phosphate buffer, pH = 4.5) was used for induction of HCC and after 1 week Phenobarbital was administered to promote the HCC. After the above discussed treatment, alpha feto protein (AFP) level was estimated for confirmation of HCC in the rats.
Experimental treatment
Each group contains six rats and groups were divided in the following manner: Group I: normal control (treated with saline only); Group II: normal control + HEG treated; Group III: DEN control; Group IV: DEN + HEG (5 mg/kg); Group V: DEN + HEG (10 mg/kg); Group VI and DEN + HEG (20 mg/kg).
At the end of the experimental study, all group rats were subjected to mild anesthesia, and blood samples collected from the retro-orbital plexus; the collected blood samples were subjected to centrifuge for separate the serum. The serum samples were further used for the estimation of different biochemical, hepatic, nonhepatic and antioxidant parameters. All groups of rats were killed via cervical decapitation and the liver samples were immediately removed, washed with the ice cold saline and blotted to dryness. 5 mm sample of the liver tissue was further used for the histopathology and small tissue sample was homogenate in Tris–HCl buffer and centrifuged at 2000 rpm for 15 min at 4 °C to obtain the clear supernatant (remove the cell debris).
Biochemical estimation
The hepatic parameters viz., ALP and AST were estimated via standard kits (Nicholas India) using instruction of the manufacturer. Non-hepatic parameters viz., albumin, BUN, total bilirubin and total protein were also estimated via using the standard kits (Nicholas India Pvt. Ltd., India). AFP was determined according to the reported method of Khan et al., with minor modification (Khan et al. 2015). The membrane-bound enzymes including Mg2+ ATPase, Ca2+ ATPase and Na+/K+ ATPase were determined via using the reported method of Pradeep et al. (2007) with minor modification.
Phase I enzymes
The phase I enzymes such as cytochrome b5 reductase, cytochrome P450 reductase and NADH-cytochrome b5 reductase were determined via using the reported method with minor modification (Dhanasekaran et al. 2009; Ghosh et al. 2012).
Antioxidant parameters
Antioxidant parameters such as malondialdehyde (MDA), glutathione reductase (GR), catalase (CAT), glutathione peroxidase (GPx), glutathione (GSH), superoxide dismutase (SOD), reduced glutathione, glutathione-S-transferase (GST), p. carbonyl, myeloperoxidase (MPO) were estimated using the reported method with minor modification (Kumar et al. 2013, 2014, 2015, 2016, Anwar et al. 2015a, b; Khan et al. 2015; V. et al. 2015; Verma et al. 2016; Yuan et al. 2016).
Pro-inflammatory cytokines and inflammatory mediator
Pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α and inflammatory mediators such as NF-kB were determined according to the the manufacturer’s instruction.
Statistical analysis
The data obtained were subjected to one-way ANOVA and Dennett’s multiple comparison tests were performed using GraphPad Prism statistical package. Values are expressed as mean ± SEM. p value <0.05 was considered as significant.
Results
HEG reduced DNA synthesis
3H-thymidine incorporation was used for the estimation of inhibitory effect of HEG on both the cell lines, We used various concentrations of HEG for confirming the inhibitory effect against the 3H-thymidine incorporation; it was found that the all does of HEG significantly reduced the 3H-thymidine incorporation in both cell lines (Hep G2 and HhH-7). From the Fig. 2a, HEG dose the reduction in the 3H-thymidine incorporation, 96% at dose 50 mM.
a Expresses the DNA synthesis inhibition in HCC cell lines. Cells were treated with ligands in 0.1–50 µM concentration and after 24 h, a standard 3H-thymidine incorporation assay was performed. b The effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on proliferation of HCC cell lines. All the values are given as mean ± SEM
HEG induced growth inhibition
After 48 h of HEG treatment, both visible cells (Trypan blue stained) and cells without stain were counted. Different concentrations of HEG such as 10, 25 and 50 mM considerably confirmed the reduction in the growth of cells (Fig. 2b).
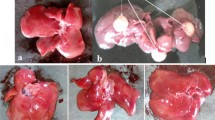
Macroscopical evaluation
Supplementary Figure 1 illustrates the microscopical evaluation of the different group of rats. NC and NC treated with HEG (20 mg/kg) confirmed the unchanged hepatic morphological character (figure not shown). On the other hand, DEN control group rats confirmed the growth of HCC via expansion of hepatic nodules; generate the patches on the surface of the liver. The liver of this group of rats also showed rough surface, which confirms the expansion of disease. DEN group rats treated with HEG (5 and 10 mg/kg) displayed hepatic nodules, which were less in number as compared to DEN control. None or less hepatic nodules were found in DEN-treated group with the HEG (20 mg/kg); this group showed white patches only on the outer surface of the liver and on touch, we feel the smooth surface as compared to the DEN control group rats.
Table 1 demonstrates the development of the hepatic nodules in the different group of rats except NC and NC treated with HEG (20 mg/kg). DEN control group rats confirmed the expansion of hepatic nodules in all rats with 100% incidence rate, and the hepatic nodule incidence was considerably decreased by the HEG in a dose-dependent manner.
Table 1 illustrates the hepatic nodules, the average size of hepatic nodules and relative size of nodules. DEN control group rats showed the expansion of the hepatic nodules with maximum size more than 1 mm and the HEG treatment group rats showed the less hepatic nodules with less in size (less than 1 mm).
Effect of HEG on body weight and relative liver weight
The results of the current investigation prove that the rats of all group have increased the body weight till the end of the experimental study. NC group rats showed the initial body weight of 158.4 ± 4.65–332.8 ± 8.94 with growth gain rate 1.13 per day and NC group rats treated with HEG (20 mg/kg) demonstrated the original body weight of 163.4 ± 5.43–343.2 ± 10.34 with growth gain rate of 1.17 per day. DEN control group also confirmed the enhanced body weight with growth gain rate of 0.83 per day, as compared to the NC and HEG control group rats. DEN group rat treated with HEG also confirmed the improved body weight at end of the experimental study with growth gain rate 0.90, 1.0 and 1.12 at a dose of 5, 10 and 20 mg/kg, respectively (Fig. 3a).
The effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on the body weight. a Shows the initial body weight and final body weight of the different group rat. b explains the liver weight and relative body weight of the different group rats. Relative body weight of the different group rats was estimated as gm liver/100 g body weight. The comparisons were made by ANOVA followed by Dunnett’s test. *p < 0.05 is considered as significant, **p < 0.01 is considered as very significant, ***p < 0.001 is considered as extremely significant
The liver weight and relative liver weight were also estimated for all groups at the end of the experimental study. We found that the liver weight and relative weight of NC and NC treated with the HEG (20 mg/kg) were almost similar. But the DEN control group confirmed the increased liver weight (16.2 ± 0.73) and relative liver weight (5.4 ± 0.23), which were higher than the control group. HEG-treated group proved the down-regulation pattern for liver weight (15 ± 0.65, 13.8 ± 0.34 and 11.4 ± 0.13) and relative liver weight (4.8 ± 0.11, 4.2 ± 0.09 and 3.4 ± 0.04) as compared to DEN control (Fig. 3b).
Effect of HEG on hepatic parameters
Significant variation was observed in the hepatic parameter profile when compared to all group rats (Fig. 5). In fact, unsubstantial enhancement of AFP concentration was observed after the DEN treatment, which remained higher at the end of the experimental study. A down-regulated AFP level was available on increasing the dose of HEG as well. On the other hand, HEG dose (5 mg/kg) (235.2 ± 6.03) was associated with decreased level of AFP as compared to DEN control and the higher dose HEG (20 mg/kg) confirmed the down-regulation of the level of AFP (50.8 ± 3.92) (Fig. 4).
The effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on the hepatic parameters of DEN-induced HCC rats. Hepatic parameters were scrutinized in terms of altered level of a AFP, b ALT, c AST, d NO. AFP Alpha feto protein, ALT Alanine transaminase, AST aspartate aminotransferase, NO. The comparisons were made by ANOVA followed by Dunnett’s test. *p < 0.05 is considered as significant, **p < 0.01 is considered as very significant, ***p < 0.001 is considered as extremely significant
A similar trend of hepatic parameters including viz., ALT and ASTwas observed in DEN-induced group rats and dose-dependent treatment of UFD significantly (p < 0.001) altered the hepatic parameter content.
Effect of HEG on non-hepatic enzymes
Non-hepatic parameters such as albumin, total protein, BUN and total bilirubin were estimated in rats of all groups. DEN-induced group confirmed the down-regulation of albumin and total protein and boosted the level of BUN and bilirubin as compared to the NC- and HEG-treated rats. HEG rats improved the level of albumin and total protein and reduced the BUN and bilirubin level in a dose-dependent manner (Fig. 5).
The effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on the hepatic parameters of DEN induced HCC rats. Hepatic parameters were scrutinized in terms of altered level of Albumin, BUN, total bilirubin and total protein. BUN blood urea nitrogen. The comparisons were made by ANOVA followed by Dunnett’s test. *p < 0.05 is considered as significant, **p < 0.01 is considered as very significant, ***p < 0.001 is considered as extremely significant
Effect of HEG on antioxidant markers
Phase I antioxidant enzymes including NADPH-cytochrome P450 reductase, NADH-cytochrome b5 reductase, cytochrome b5 and cytochrome P450 considerably altered during the DEN-induced HCC. An effective treatment of HEG significantly (p < 0.001) modulated the phase I antioxidant enzymes in a dose-dependent manner (Fig. 6).
Effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on the phase I enzymes in liver a NADPH cytochrome P450 reductase b cytochrome P420 c NADH cytochorme b5 and d cytochrome b5 content. The comparisons were made by ANOVA followed by Dunnett’s test. *p < 0.05 is considered as significant, **p < 0.01 is considered as very significant, ***p < 0.001 is considered as extremely significant
When LPO concentration was estimated , the DEN control showed the up-regulation of MDA level (an indicator of LPO). A dose-dependent reduction of MDA was observed in HEG treatment (Fig. 7).
The effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on the DEN-induced hepatocarcinogenesis rat. Antioxidant parameters were scrutinized in terms of altered level of a MDA, b P. Carbonyl, c CAT, d SOD, e MPO, f GSH, g GST, h GPx as described in “Materials and Methods”. ns non-significant, DEN diethylinitrosamine, CAT catalase, SOD superoxide dismutase, GPx glutathione peroxidase, GST glutathione transferase. The comparisons were made by ANOVA followed by Dunnett’s test. *p < 0.05 is considered as significant, **p < 0.01 is considered as very significant, ***p < 0.001 is considered as extremely significant
A similar pattern of up-regulation was found in the MPO/GSH/P. Carbonyl/GST level, which was significantly (p < 0.001) down-regulated in a dose-dependent pattern.
On the contrary, endogenous antioxidants viz., SOD, CAT and GPx were significantly (p < 0.001) reduced in DEN group. Administration of DEN down-regulation the concentration of endogenous antioxidant marker viz., SOD, CAT and GPx, a dose-dependent treatment of HEG significantly (p < 0.001) up-regulated the level of endogenous antioxidant (Fig. 7).
Effect of HEG on membrane-bound enzymes
The Ca2+ ATPase level decrease was observe in the DEN control group as compared to NC control group rats. Pre-treatment of HEG showed the dose-dependent up-regulation of Ca2+ ATPase level.
An almost similar pattern of reduction was available for the Na+/K+ and Mg2+ ATPase activity in the hepatic tissue. HEG showedsignificantly (p < 0.001) increased activity of Mg2+ ATPase level in a dose-dependent manner (Fig. 8).
Effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on the membrane-bound enzyme level of hepatic tissue in all group rats. The comparisons were made by ANOVA followed by Dunnett’s test. *p < 0.05 is considered as significant, **p < 0.01 is considered as very significant, ***p < 0.001 is considered as extremely significant
Effect of HEG on pro-inflammatory and inflammatory mediator
When scrutinizing the pro-inflammatory cytokines in the DEN-induced group rats, a similar pattern of up-regulation was observed at end of the study and dose-dependent treatment of HEG significantly down-regulated the pro-inflammatory cytokines (Fig. 9). DEN afforded up regulation of NF-kB; the use of HEG was evident with a dose-dependently reduced the NF-kB activity (Fig. 9).
Effect of 19-α-hydroxyurs-12(13)-ene-28 oic acid-3-O-β-d-glucopyranoside (HEG) on the pro-inflammatory and inflammatory mediator in DEN-induced HCC rats. Pro-inflammatory cytokines and inflammatory mediator were scrutinized in terms of altered level of a IL-6, b IL-1β, c TNF-α, d NF-kB as described in “Materials and methods”. The comparisons were made by ANOVA followed by Dunnett’s test. *p < 0.05 is considered as significant, **p < 0.01 is considered as very significant, ***p < 0.001 is considered as extremely significant
Effect of HEG on histopathology
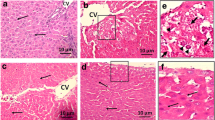
Table 2 represents the histopathological changes of all group rats. DEN group rats confirmed the expansion of HCC via the visible changes viz., generated the cell necrosis, thick cords of unsystematic hepatic parenchyma, inflamed blood vessels, irregular cells with rich sinusoids, cell swelling, basophilic, cytoplasm having irregular shapes and pseudoacini. It also showed the hepatocytes, which were enclosed with macro lipid droplets and contain masses of eosinophilic in vaculation covered by cytoplasm. The histopathological observation confirmed that enlargement of hyperplasia and nuclei (karyomegaly) in the bile duct confirm the carcinogenic effect on the tissue. The tissue also confirmed the proliferation in the portal area of hepatic stellate cells (HSCs), which further confirm the focal proliferation. DEN rat treated with HEG (5 and 10 mg/kg) proved its protective effect by displaying the less inflammatory cells, necrotic cell, enlarge karyomegaly and expansion of proliferation in the HSCs area and less HSCs focal proliferation as compared to DEN group rat. Another dose of HEG (20 mg/kg) confirms the chemo-protective effect against the DEN group via showing the less microdroplet, HSCs and focal proliferation (Supplementary Figure 2).
Discussion
DEN model is widely used for the estimation of the anticancer effect of the compound on various stage viz., initiation, expansion and neoplastic transformation of hepatic cancer. Several investigations confirmed that the DEN expands the preneoplastic and neoplastic nodules in rodents, which is closely similar to the human HCC (Sivalokanathan et al. 2004; Agren et al. 2014). Recently, few studies confirmed that the DEN-induced hepatic cancer via gene expression patterns in rodent closely resembles the subclass of human HCC. DEN-induced control group rats showed the development of the hepatic nodules (3 mm in size), which were considered as the detection of the pre-neoplastic nodules via elevated the hyperplasia. Pre-neoplastic nodules are recognized as the precursor of HCC. The current model is mostly used for scrutinizing the potential effect of chemo-preventive effect in the clinical setting. Various researchers proved the strong correlation between the hepato-carcinogenesis and number of nodular hyperplasia in both human disease and experimental rodent model (Jagan et al. 2008; Jayakumar et al. 2012).
Alpha feto protein (AFP) is considered as the gold parameter, which is commonly elevated during the HCC (Dhanasekaran et al. 2009). The size and shape of AFP are similar to another serum protein, but the concentration of the AFP was found in minute quantity in the normal adult. The altered level of AFP confirmed the expansion of HCC. The current experimental study showed the elevated level of AFP in DEN-induced rats and confirmed the development of hepatic nodules, which was further proved by the macroscopical and histopathological studies (Shizuma et al. 2011). DEN-induced rats treated with HEG showed the reduced concentration of AFP and claim the chemo-protective effect; the current statement is supported by the macroscopical and histopathological study (supplementary Figures 1, 2).
Most tissue in human body is flooded with transminase enzymes like ALT and AST catalyzing the formation of amino groups from 2-oxacids and amino acids. Hepatic parameter viz., ALT and AST are considered as the determinants of the normal and altered function of the liver. Several clinical studies showed that the content of both enzymes increase 20–40 times during the hepatocyte damage and also boost the level more than 100 times during the serve injury of hepatic cells. The activities of both enzymes viz., AST and ALT are mostly found in the cytoplasm cells of liver, muscle and heart (Jayakumar et al. 2012). During the hepatic cell damage, the liver starts the leakage of these specific liver enzymes into the plasma. Increased content of AST and ALT into the plasma initiates the effect on the functional integrity of liver cells and provokes the cellular injury in liver. DEN-induced group rats showed increased content of hepatic parameters (AST and ALT), which was significantly altered by the HEG in a dose-dependent manner.
DEN-induced group rats demonstrated augmented content of bilirubin and ALP in serum; both are considered as the hepatic toxicity marker. The high concentration of bilirubin in the serum confirmed the increased degradation rate of red blood cells, while modulation of ALP content directly showed the pathological changes in biliary flow (Zhao et al. 2015). DEN-induced rats treated with the HEG proved the chemo-protective effect via reducing the leakage of ALP and bilirubin in the serum.
In the entire experimental study, all group rats showed almost similar water and food intake. DEN-induced group rats demonstrated significantly (p < 0.001) reduced body weight as compared to the NC group rats, which was significantly (p < 0.001) improved by HEG at dose-dependent manner. Various researchers confirmed that the attenuation of tumor expansion via natural product is well known that has increased renewed interest (Pradeep et al. 2007; Jagan et al. 2008). Few of researchers observed that the decreased content of nutrition inducing the loss of body weight may be connected with reduced tumor volume.
Several incidences confirmed the possible mechanism for damage of the corpuscle in LPO, which is generally induced by the free radicals. LPO is considered as the classical marker of oxidative stress. During the oxidative stress, free radicals absorb the electron from the cell membrane and starts damaging the cells with lipid degradation. During the oxidative stress, constant generation of oxygen reacts with the unstable fatty acid and forms the delicious product such as peroxy fatty acid radical and the produced radical continues to react with the another free radical and forms the unpredictable chain of lipid peroxide (Jagan et al. 2008; Ghosh et al. 2012; Thangavel and Vaiyapuri 2013). During the DEN-induced carcinogenesis, LPO such as 4-hydroxy nominal and MDA react with the various free radicals and initiate the oxidative stress, which causes the carcinogenic effect. The enhanced content of LPO during the DEN treatment provoked the generation of free radicals, which was confirmed by reduce content of endogenous antioxidant or initiate the production of ROS. In the current study, we observed the increased level of LPO in the DEN-induced HCC rats, which was significantly (p < 0.005) reduced by the HEG dose dependently. The current results confirmed the anti-LPO effect of HEG, which is attributed to their free radical scavenging effect.
GSH and its oxidized product are considered as the primary defense system of cells. Various studies confirmed the possible mechanism action of GSH; it may behave like non-enzymatic antioxidant via direct contact with –SH group or it may be involved in the detoxification reaction of ROS. On the other hand, we observed the enhanced content of thiol group (SH), which is sensitive to inducing the oxidative damage (Ghosh et al. 2012; Jayakumar et al. 2012). In the current study, DEN-induced group of rats treated with HEG altered the level of GSH and thiol group in a dose-dependent manner. The possible mechanism of action of HEG may be directly improving the endogenous antioxidant parameters by increasing the biosynthesis of GSH.
Various clinical studies proved that there is a low content of ROS, during the normal cell physiological progression. Several factors viz., directly affect the macromolecules (nucleic acid, protein, and lipids), environmental factors, stressful condition and carcinogenesis; all of these factors are responsible for increasing the ROS production (Dhanasekaran et al. 2009; Ghosh et al. 2012). Various investigations showed that the expansion of neoplastic and pre-neoplastic directly or indirectly affect the hepatic cells, which results in increase of ROS production and inhibit the capacity of endogenous antioxidant parameters. It is the hypothesis that increasing the overload of abnormal cells may affect the antioxidant parameter via down-regulating and detoxifying the endogenous antioxidant defense mechanism. SOD and CAT both are considered as the primary defense antioxidant system. SOD converts the superoxide anion into O2 and H2O2; on the other hand, CAT converts the H2O2 to H2O and confirms the reduction in generation of delicious free radicals. During the DEN-induced carcinogenesis, the content of SOD and CAT significantly decreases due to biomolecule changes, which is involved in the neoplastic and pre-neoplastic transformation (Jayakumar et al. 2012). SOD is considered as the first line antioxidant; it plays an important role to protect the cell from the superoxide injury via inhibiting the reaction of superoxide. The antioxidant content was estimated during the various pathological conditions of rodent during the different conditions of diseases. Scientific reports are sufficient to confirm the deaths of experimental rats from increase oxidative stress in them (Pradeep et al. 2007; Shizuma et al. 2011; Jayakumar et al. 2012). It can be concluded that the HEG significantly improved the antioxidant content and established the chemo-protective effect via their anti-free radical scavenging nature.
ROS not only damages macromolecules (protein, lipids, and DNA) of cell but are also one of the prime factor for toxicity in cells, this can be attributed to the fact that they creates imbalance between endogenous antioxidant defense system or alters the detoxifying capacity of natural enzymes viz., GSH-Red, CAT, SOD, Glc-6-PD and GSH-Px. The DEN-induced carcinogenesis rats revealed the reduced content of these ROS detoxifying enzymes due to the excessive mobilization of endogenous antioxidant enzymes toward the detoxification of ROS such as (H2O2, O2, ONOO and RO2) (Kweon et al. 2003; Qi et al. 2008; Chen et al. 2012). The imbalance between the endogenous antioxidants and increased oxidative stress on cellular macromolecules start the oxidative injury and cell death. DEN-induced rats demonstrated the reduced content of ROS-detoxifying enzymes, which was significantly (p < 0.001) improved by HEG treatment. The current effect of HEG may be due to free radical scavenging ability of the compound; the hypothesis was confirmed by the in vitro antioxidant effect of HEG.
During the proliferation, cells showed increased content of GSH, GST and glutathione metabolizing enzymes. The elevated content of GST and GSH is due to over-expression of proliferation and quick sequestration of antioxidants via tumor cells (Shizuma et al. 2011; Thangavel and Vaiyapuri 2013). GST is considered as the cyto-protective phase II enzyme, which is involved in the detoxification of hydrophobic, conjugates, xenobiotics, electrophilic, lipid hydro-peroxides and H2O2. The prime role of GST is protecting the cell from the free radical-induced damage. The purpose of the GST is to protect the cell via catalyzing the production of lipid hydro-peroxides, which are regularly generated during the oxidative damage of cellular lipid molecules. Oxidative stress in cells is controlled by phase II enzymes system. This enzyme system work on mechanism of electron transport phenomenon where cytochrome P450 reduces one electron from xenobiotics to give a stable compound with reduce two atoms (Liu et al. 2010; Shaban et al. 2013). DEN-induced group rats confirmed the increased content of phase II enzymes, but the DEN-induced rats treated with HEG altered the activity of phase II enzymes, and probably influenced the hepatic damage. GPx is the antioxidant enzyme, which protects the cell from the oxidative damage. The primary function of GPx is to inhibit the lipid hydro-peroxides to their alcohols free hydrogen peroxidase to water (Shaban et al. 2013).
Researchers claim that the alteration of lipid metabolism provoked the hepatocellular carcinoma. It also affected the development of cells and cellular function. Available literature clearly states that alteration in lipid profile and increase oxidative stress are common features observe during expansion of hepatic nodules in experimental rat (Li et al. 2006). Altered lipid profile in malignant tissue is considered as the critical parameter, due to an effect on the fluidity, membrane integrity and circulation cellular process, which is directly related to the cell survival and growth.
Several experimental evidence confirms that membrane-bound enzymes viz., Mg2+, Ca22+ and Na+/K+ ATPase are responsible for the transport of ions across the cell membrane at the expense of ATP. During the hepatic injury that starts from the lipid membrane peroxidation, resultant generate the alteration of the functional and structural characteristic of a membrane, which further directly affects the membrane-bound ATPase activity. During the DEN-induced HCC, decrease in the activity of membrane-bound ATPase due to specific sensitivity from the superoxide and hydro-peroxides radicals is observed. DEN-induced HCC rats confirmed the reduction of the membrane-bound enzyme, HEG treatment up-regulated the membrane-bound enzyme activity via stabilizing the interruption in the potassium and calcium metabolism. On the other hand, we already confirmed that the HEG is preventing the lipid peroxidation during the DEN-induced HCC. On the basis of above fact, we can conclude that HEG prevents the peroxidation of membrane lipids. We can say that HEG prevented the hepatocytes damage and also maintained the membrane integrity during the healthy state via potent antioxidant nature.
IL-1β, a member of interleukin (IL) family, is considered as the potent pro-inflammatory cytokines, which play a significant role in the initiation and expansion of inflammatory reaction. Several clinical studies confirmed that the low concentration of IL-1β causes the local inflammatory response pursued by extending the potential immune response; on the other hand, high level of IL-1β induces the inflammation-associated tumor invasiveness and tissue injury. Several clinical studies confirmed up-regulated concentration of IL-1β in the HCC patient and the same result was observed in our study. DEN-induced disease control rats confirmed the increased level of IL-1β, which further showed the inflammatory reaction in the disease. On the other hand, HEG significantly (p < 0.001) suppressed the concentration of IL-1β in a dose-dependent manner. On the basis of the current result, we can conclude that HEG inhibits the IL-1β level, which confirmed the anti-inflammatory effect of HEG.
Pro-inflammatory cytokines such as interlukin-6 and TNF-α, both, are considered as the paracrine and autocrine growth factor, which bind to the cell receptors. The main actions of both pro-inflammatory mediators are targeting the abandoned cell proliferation (Naik et al. 2011; Zhang et al. 2012). Tumor necrosis factor-α (TNF-α) is considered as the expansion factor for most tumor cells and also take part in the promotion, initiation, and metastasis of tumors. The various clinical investigations confirmed that the level of NF-kB boosts up during the HCC. The same result was observed in the current study. The various clinical studies showed that the TNF-α activate the up-regulation of NF-kB expression and start the inflammatory reaction. IL-6 regulates the genes expression involved in the apoptosis inhibition and cell cycle progression via alteration of activation of transcription signaling pathway and Janus-activated kinase-signal transducers (Mbimba et al. 2012). The increased level of IL-6 has been observed in the patient suffering from the HCC as compared to the normal volunteer. The same result was observed in the disease control group rats; HEG treatment significantly (p < 0.001) down-regulated the IL-6 concentration in adose-dependent manner. The reduction of IL-6 concentration via HEG may be due to an inflammatory mechanism.
Nuclear factor-kappa beta (NF-kβ) is the member of five closely related proteins, which are commonly found in the various dimeric combinations and bind with DNA to the kB sites. Several clinical studies showed that the NF-kB activation has been recurrently observed in the tumor tissues as well as non-tumor tissues (Majumder et al. 2010; Mbimba et al. 2012). The level of NF-kB was up-regulated via pro-inflammatory cytokines, free radicals, tumor promoters, inflammatory reaction, carcinogens and endotoxins (Cho et al. 2003; Lawrence 2009). In the current study, we observed the increased content of free radical, pro-inflammatory cytokines, which boosted the NF-kB in the all group rats. HEG showed the inhibition of NF-kB at dose-dependent manner and confirm the chemoprotective effect. The possible mechanism of HEG may be down-regulation the oxidative stress and pro-inflammatory cytokines.
Conclusion
From the above results, we can conclude our experimental study with two major findings. First, HEG down-regulates the production of free radicals and inhibits the oxidative stress via multiple mechanisms and it also stabilizes the membrane-bound enzyme activity. Second, HEG showed the effects via regulation of diverse transcription factors, inhibition of pro-inflammatory cytokines, protein kinase and activation of NF-kB. Given these promising findings, the current investigation suggests that HEG inhibits the oxidative stress and pro-inflammatory cytokines via reduction of NF-kB. But, to find out the exact mechanism of HEG in DEN-induced HCC, more cellular and molecular investigation is required.
Author contributions
All authors equally contributed to the manuscript.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HEG:
-
19-α-Hydroxyurs-12(13)-ene-28 oic acid-3-O-α-d-glucopyranoside
- DEN:
-
Diethylnitrosamine
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interlukin-6
- IL-1 β:
-
Interlukin-1β
- NF-kB:
-
Nuclear factor kappa beta
- DNA:
-
Deoxyribonucleic acid
- CYP2E1:
-
Cytochrome P450
- OH:
-
Hydroxyl
- RO2 :
-
Peroxyl
- O2 − :
-
Superoxide
- ONOO− :
-
Peroxynitrite
- H2O2 :
-
Hydrogen peroxide
- ROS:
-
Reactive oxygen species
- AFP:
-
Alpha feto protein
- ALP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- LPO:
-
Lipid peroxidation
- GR:
-
Glutathione reductase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- SOD:
-
Superoxide dismutase
- GST:
-
Glutathione-S-transferase
- MPO:
-
Myeloperoxidase
- NC:
-
Normal control
References
Agren R, Mardinoglu A, Asplund A et al (2014) Identification of anticancer drugs for hepatocellular carcinoma through personalized genome-scale metabolic modeling. Mol Syst Biol. doi:10.1002/msb.145122
Anwar F, Al-Abbasi FA, Bhatt PC et al (2015a) Umbelliferone β-d-galactopyranoside inhibits chemically induced renal carcinogenesis via alteration of oxidative stress, hyperproliferation and inflammation: possible role of NF-κB. Toxicol Res (Camb) 4:1308–1323. doi:10.1039/c5tx00146c
Anwar F, Mushtaq G, Kazmi I et al (2015b) Anticancer effect of rosiglitazone in rats treated with N-nitrosodiethylamine via inhibition of DNA synthesis: an implication for hepatocellular carcinoma. RSC Adv 5:68385–68391. doi:10.1039/C5RA07291C
Bingül I, Başaran-Küçükgergin C, Tekkeşin MS et al (2013) Effect of blueberry pretreatment on diethylnitrosamine-induced oxidative stress and liver injury in rats. Environ Toxicol Pharmacol 36:529–538. doi:10.1016/j.etap.2013.05.014
Bishayee A, Barnes KF, Bhatia D et al (2010) Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev Res 3:753–763. doi:10.1158/1940-6207.CAPR-09-0171
Bishayee A, Mbimba T, Thoppil RJ et al (2011) Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J Nutr Biochem 22:1035–1046. doi:10.1016/j.jnutbio.2010.09.001
Butler MS (2008) Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep 25:475–516. doi:10.1039/b514294f
Chen B, Ning M, Yang G (2012) Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 17:4672–4683. doi:10.3390/molecules17044672
Cho SY, Park SJ, Kwon MJ et al (2003) Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem 243:153–160. doi:10.1023/A:1021624520740
Dhanasekaran M, Baskar AA, Ignacimuthu S et al (2009) Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest New Drugs 27:347–355. doi:10.1007/s10637-008-9181-9
Ghosh D, Choudhury ST, Ghosh S et al (2012) Nanocapsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem Biol Interact 195:206–214. doi:10.1016/j.cbi.2011.12.004
Hill RA, Connolly JD (2012) Triterpenoids. Nat Prod Rep 29:780. doi:10.1039/c2np20027a
Ip BC, Hu KQ, Liu C et al (2013) Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res 6:1304–1316. doi:10.1158/1940-6207.CAPR-13-0178
Jagan S, Ramakrishnan G, Anandakumar P et al (2008) Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Mol Cell Biochem 319:51–59. doi:10.1007/s11010-008-9876-4
James JT, Dubery IA (2009) Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 14:3922–3941
Jang M, Cai L, Udeani GO et al (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 80(275):218–220. doi:10.1126/science.275.5297.218
Jayakumar S, Madankumar A, Asokkumar S et al (2012) Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem 360:51–60. doi:10.1007/s11010-011-1043-7
Khan R, Kazmi I, Afzal M et al (2015) Fixed dose combination therapy loperamide and niacin ameliorates diethylnitrosamine-induced liver carcinogenesis in albino Wistar rats. RSC Adv 5:67996–68002. doi:10.1039/c5ra11201j
Kumar V, Verma A, Ahmed D et al (2013) Fostered antiarthritic upshot of Moringa oleifera lam. stem bark extract in diversely induced arthritis in wistar rats with plausible mechanism. Int J Pharm Sci Res 4:3894–3901. doi:10.13040/IJPSR.0975-8232.4(10).3894-01
Kumar V, Anwar F, Verma A, Mujeeb M (2014) Therapeutic effect of umbelliferon-α-d-glucopyranosyl-(2I→1II)-α-d-glucopyranoside on adjuvant-induced arthritic rats. J Food Sci Technol 52:3402–3411. doi:10.1007/s13197-014-1403-x
Kumar V, Al-Abbasi FA, Ahmed D et al (2015) Paederia foetida Linn. inhibits adjuvant induced arthritis by suppression of PGE 2 and COX-2 expression via nuclear factor-κB. Food Funct 6:1652–1666. doi:10.1039/c5fo00178a
Kumar V, Bhatt PC, Rahman M et al (2016) Melastoma malabathricum Linn attenuates complete freund’s adjuvant-induced chronic inflammation in Wistar rats via inflammation response. BMC Complement Altern Med 16:510. doi:10.1186/s12906-016-1470-9
Kwak MK, Itoh K, Yamamoto M et al (2001) Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dimethiole-3-thione. Mol Med 7:135–145
Kweon S, Park KA, Choi H (2003) Chemopreventive effect of garlic powder diet in diethylnitrosamine-induced rat hepatocarcinogenesis. Life Sci 73:2515–2526. doi:10.1016/S0024-3205(03)00660-X
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect, Biol, p 1
Lawrence TS, Robertson JM, Anscher MS et al (1995) Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 31:1237–1248. doi:10.1016/0360-3016(94)00418-K
Li J-J, Tang Q, Li Y et al (2006) Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol Sin 27:1078–1084. doi:10.1111/j.1745-7254.2006.00345.x
Liu X, Dewaele S, Vanhooren V et al (2010) Alteration of N-glycome in diethylnitrosamine-induced hepatocellular carcinoma mice: a non-invasive monitoring tool for liver cancer. Liver Int 30:1221–1228. doi:10.1111/j.1478-3231.2010.02279.x
Mahato SB, Nandy AK, Roy G (1992) Triterpenoids. Phytochemistry 31:2199–2249
Majumder S, Roy S, Kaffenberger T et al (2010) Loss of metallothionein predisposes mice to diethylnitrosamine-induced hepatocarcinogenesis by activating NF-κB target genes. Cancer Res 70:10265–10276. doi:10.1158/0008-5472.CAN-10-2839
Marnewick JL, van der Westhuizen FH, Joubert E et al (2009) Chemoprotective properties of rooibos (Aspalathus linearis), honeybush (Cyclopia intermedia) herbal and green and black (Camellia sinensis) teas against cancer promotion induced by fumonisin B1 in rat liver. Food Chem Toxicol 47:220–229. doi:10.1016/j.fct.2008.11.004
Mbimba T, Awale P, Bhatia D et al (2012) Alteration of hepatic proinflammatory cytokines is involved in the resveratrol-mediated chemoprevention of chemically-induced hepatocarcinogenesis. Curr Pharm Biotechnol 13:229–234. doi:10.2174/138920112798868575
Naik SR, Thakare VN, Patil SR (2011) Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol 63:419–431. doi:10.1016/j.etp.2010.03.001
Neto CC (2007) Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res 51:652–664
Pradeep K, Mohan CVR, Gobianand K, Karthikeyan S (2007) Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol 560:110–116. doi:10.1016/j.ejphar.2006.12.023
Pradeep K, Mohan CVR, Gobianand K, Karthikeyan S (2010) Protective effect of Cassia fistula Linn. on diethylnitrosamine induced hepatocellular damage and oxidative stress in ethanol pretreated rats. Biol Res 43:113–125. doi:10.4067/S0716-97602010000100013
Qi Y, Chen X, Chan CY et al (2008) Two-dimensional differential gel electrophoresis/analysis of diethylnitrosamine induced rat hepatocellular carcinoma. Int J Cancer 122:2682–2688. doi:10.1002/ijc.23464
Setzer WN, Setzer MC (2003) Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev Med Chem 3:540–556. doi:10.2174/1389557033487854
Shaban NZ, El-Kersh MAL, El-Rashidy FH, Habashy NH (2013) Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem 141:1587–1596. doi:10.1016/j.foodchem.2013.04.134
Shizuma T, Ishiwata K, Nagano M et al (2011) Protective effects of fermented rice vinegar sediment (Kurozu moromimatsu) in a diethylnitrosamine-induced hepatocellular carcinoma animal model. J Clin Biochem Nutr 49:31–35. doi:10.3164/jcbn.10-112
Sivalokanathan S, Ilayaraja M, Balasubramanian MP (2004) Anticancer potency of Terminalia arjuna bark on N-nitrosodiethylamine- induced hepatocellular carcinoma in rats. Nat Prod Sci 10:190–195. doi:10.1007/s11010-006-0433-8
Thangavel P, Vaiyapuri M (2013) Antiproliferative and apoptotic effects of naringin on diethylnitrosamine induced hepatocellular carcinoma in rats. Biomed Aging Pathol 3:59–64. doi:10.1016/j.biomag.2013.01.006
V.K, F.A. A-A, A.V, et al (2015) Umbelliferone β-d-galactopyranoside exerts an anti-inflammatory effect by attenuating COX-1 and COX-2. Toxicol Res (Camb) 4:1072–1084. doi:10.1039/c5tx90017d
Verma A, Bhatt PC, Kaithwas G et al (2016) Chemomodulatory effect Melastoma malabathricum Linn against chemically induced renal carcinogenesis rats via attenuation of inflammation, oxidative stress, and early markers of tumor expansion. Inflammopharmacology 24:233–251. doi:10.1007/s10787-016-0276-1
Verma A, Ahmed B, Anwar F et al (2017) Novel glycoside from Wedelia calendulacea inhibits diethyl nitrosamine-induced renal cancer via downregulating the COX-2 and PEG2 through nuclear factor-κB pathway. Inflammopharmacology 25:159–175. doi:10.1007/s10787-017-0310-y
Wagner JS, Adson MA, Van Heerden JA et al (2001) The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg 199:502–508
Yin C, Evason K (2013) Hepatic stellate cells in liver development, regeneration, and cancer. J Cin Investig 123:1902–1910. doi:10.1172/JCI66369.1902
Yuan C, Wang C, Wang J et al (2016) Inhibition on the growth of human MDA-MB-231 breast cancer cells in vitro and tumor growth in a mouse xenograft model by Se-containing polysaccharides from Pyracantha fortuneana. Nutr Res 36:1243–1254. doi:10.1016/j.nutres.2016.09.012
Zhang CL, Zeng T, Zhao XL et al (2012) Protective effects of garlic oil on hepatocarcinoma induced by N-nitrosodiethylamine in rats. Int J Biol Sci 8:363–374. doi:10.7150/ijbs.3796
Zhao X, Chen Q, Li Y et al (2015) Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur J Pharm Biopharm 93:27–36. doi:10.1016/j.ejpb.2015.03.003
Acknowledgements
We gratefully acknowledge the Department of Pharmaceutical Sciences, Faculty of Health Sciences, Sam Higginbottom University of Agriculture, Technology and Sciences (SHUATS) for providing the facility for conducting the experimental study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, A., Singh, D., Anwar, F. et al. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway. Inflammopharmacol 26, 133–146 (2018). https://doi.org/10.1007/s10787-017-0350-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0350-3