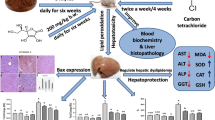
Abstract
This study evaluated the hepatoprotective effect of oral administration of aqueous fraction of methanolic extract of Costus afer leaves (CALAF) during induction of hepatocellular carcinoma (HCC) with diethylnitrosamine (DEN) in rats. The methanolic leaf extract was fractionated into hexane, ethyl acetate, butanol, and aqueous fractions. The in vitro antioxidant potential of the fractions were estimated by the assays of 2,2-diphenyl-1-picrylhydrazine and nitric oxide radical scavenging activity, ferric-reducing antioxidant potential, and total antioxidant capacity. CALAF had the most antioxidant effect. Rats were orally pretreated daily with CALAF at 100, 200, and 400 mg/kg or silymarin (hepatoprotective drug) at 50 mg/kg from 2 weeks prior to HCC induction and through 6 weeks of HCC induction. The HCC induction was by a single intraperitoneal injection of DEN at 200 mg/kg as an initiator, followed 2 weeks later by daily oral administration of 2-acetylaminofluorene at 30 mg/kg as promoter. At the end of HCC induction, levels of alpha-fetoprotein (AFP), liver function and antioxidants, gamma histone 2A family member X (γH2AX), and O6-methylguanine-DNA methyltransferase (MGMT) expressions were determined. HCC rats treated with CALAF at all doses had significantly (p < 0.05) reduced levels of AFP, aspartate aminotransferase, alanine aminotransferase, superoxide dismutase, glutathione peroxidase, reduced glutathione, and γH2AX protein expression, whereas MGMT protein expression was elevated when compared with untreated HCC rats. Thus, CALAF could be effective in protecting against DEN-induced HCC in rats by ameliorating hepatic injury and genotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a primary malignant tumor that originates from the parenchymal cells of the liver (Ahmad et al. 2019). It is the fifth and seventh most common cancer in men and women, respectively (Xu et al. 2018) and the third leading cause of cancer-related deaths globally (Llovet et al. 2016; Bray et al. 2018). Higher HCC incidence was reported in Africa and Asia than in other parts of the world (Mittal and El-Serag 2013). Major risk factors for HCC include hepatitis B and C viral infection, alcohol abuse, primary biliary cirrhosis, diabetes, nonalcoholic steatohepatitis, and exposure to aflatoxin and nitrosamines (Chedid et al. 2017).
Medicinal plant extracts play important role in the prevention of HCC development (Fujiwara et al. 2017; Veeraraghavan et al. 2015). Hence, there is a need to investigate the medicinal properties of Costus species plant extract which have been reported to possess antioxidant, hepatoprotective, and hepatocurative properties (Anyakeme et al. 2014; Tcheghebe et al. 2018; Boison et al. 2019) and exhibit anticancer and apoptotic activities in vitro (Nair et al. 2014; Selim and Al-Jaouni 2015; El-far et al. 2016).
Costus afer is commonly known as bush cane or ginger lily (Anaga et al. 2004), a moderately tall herbaceous monocot rhizome found around the river banks and rain forest regions of tropical West Africa (Aweke 2007) and widely consumed as an ethno-medicinal herb in rural communities (Umoh et al. 2019). Several bioactive compounds found in C. afer leaves include diosgenin, dioscin, flavonoid glycoside kaempferol, saponin aferosides, paryphyllin C, 3-O-α-L-rhamnopyranoside, naphthalene 2,3-dimethyl, naphthalene 1,6-dimethyl, phenol-2,4-bis(1,1-dimethylethyl)-, phytol, 2(4H)-benzo-furanone,5,6,7,7a-tetrahydro-4,4,7a-trimethyl (Lin et al. 1996, 1997; Anyasor et al. 2015). These bioactive components of the extract, including a number of antioxidants, could counteract the induction of HCC (Hassan et al. 2016; Su et al. 2019). Therefore, this present study evaluated the hepatoprotective effect of aqueous fraction of methanolic extract of C. afer leaves during induction of HCC with diethylnitrosamine in rats.
Materials and methods
Chemicals
The following chemicals were used: diethylnitrosamine (DEN) (Sigma-Aldrich, USA), 2-acetylaminofluorene (2-AAF) (Sigma-Aldrich, USA), O6-methylguanine-DNA-methyltransferase (MGMT) kit (Cohension Biosciences, Germany), gamma histone 2A, family member X (γH2AFX) kit (Cusabio Technology, China), silymarin (a reference hepatoprotective drug; Sigma-Aldrich, St. Louis, MO), alanine aminotransferase kit (Randox, United Kingdom), and aspartate aminotransferase kit (Randox, United Kingdom).
Plant material
Costus afer leaves were collected from a farmland at 6° 53′ N and 33° 44′ E in Irolu, Ikenne Local Government, Ogun State. It was identified, authenticated, and assigned a voucher specimen number at the Forestry Herbarium Ibadan (FHI) as FHI-108001.
Extraction and partitioning procedures
Costus afer leaves were thoroughly washed and oven dried at 40 °C. The dried leaves were pulverized using a mechanical grinder. Six hundred and twenty grams of pulverized leaf samples was extracted using 4960 mL 70% methanol with intermittent shaking for 72 h. The extract obtained was filtered using Whatman No. 1 filter paper, and the filtrates were subsequently concentrated using a rotary evaporator (Buchi Rotavapor RE-3; Buchi Labortecknic AG, Switzerland) at 30 °C. The concentrates were reconstituted with distilled water in 1:2 ratio and partitioned using successive solvent method to obtain hexane (CALHF), ethyl acetate (CALEF), butanol (CALBF), and aqueous (CALAF) fractions. The fractions were concentrated again using the rotary evaporator at 30 °C and kept at 4 °C as stock until further use.
Determination of antioxidant activity in vitro
2,2-Diphenyl-1-picrylhydrazyl assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of C. afer leaf fractions was determined by following the method described by McCune and Johns (2002). The reaction mixture consisted of 1.0 mL DPPH (0.3 mM) and 2.5 mL of 50, 100, 250, 500, and 1000 μg/mL C. afer fractions or gallic acid (served as standard) prepared in 10% dimethyl sulfoxide (DMSO). The reaction mixture was mixed and incubated in the dark for 30 min, and afterward, the absorbance was measured at 517 nm using a double-beam UV–visible spectrophotometer (Shimadzu Double Beam UV-2600, Nakagyo-Ku, Kyoto, Japan).
The percentage inhibition (I%) was calculated using the following formula:
where, A0 is absorbance of control and A1 is absorbance of test.
The fifty percent inhibitory concentration (IC50) was extrapolated from a linear regression plot of the percentage inhibition against concentration of the test fraction/standard. The assay was performed in triplicates.
Nitric oxide scavenging activity assay
The nitric oxide radical scavenging activity of C. afer leaf fractions was determined following the method described by Ebrahimzadeh et al. (2010). Two milliliters of 10 mM sodium nitroprusside was dissolved in 0.5 mL phosphate-buffered saline (pH 7.4) mixed with 0.5 mL of 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL of C. afer fractions, 70% methanol as control and gallic acid as standard. The mixture was incubated at 25 °C for 150 min after which 0.5 mL of the incubated solution was withdrawn and mixed with 0.5 mL Griess reagent. The Griess reagent mixture was incubated at 25 °C for 30 min. Subsequently, the absorbance was measured at 546 nm using a double-beam UV–visible spectrophotometer (Shimadzu Double Beam UV-2600, Nakagyo-Ku, Kyoto, Japan). The amount of nitric oxide radical inhibition was calculated using the following equation:
where Ao is the absorbance before reaction with Griess reagent and A1 is the absorbance after reaction has taken place with Griess reagent.
The fifty percent inhibitory concentration (IC50) was extrapolated from a linear regression plot of the percentage inhibition against concentration of the test fraction/standard. The assay was performed in triplicates.
Ferric-reducing antioxidant potential assay
Ferric-reducing antioxidant potential (FRAP) of C. afer leaf fractions was determined using the method described by Benzie and Strain (1996). Seventy-five microliters of C. afer leaf fractions was added to 2 mL of ferric reducing antioxidant potential reagent to form an intense blue Fe2+-2,4,6-tripyridyl-S-triazine complex, and the optical density was read at 593 nm after 2 min using a double-beam UV–visible spectrophotometer (Shimadzu Double Beam UV-2600, Nakagyo-Ku, Kyoto, Japan). The assay was performed in triplicate, and a standard linear curve for gallic acid was plotted. Total phenolic content was extrapolated from the curve, and values were expressed as mg gallic acid equivalent (GAE)/g.
Total antioxidant capacity assay
Total antioxidant capacity (TAC) of C. afer leaf fraction was determined using the method described by Prieto et al. (1999). An aliquot of 0.1 mL 1 mg/mL varying concentrations of C. afer leaf fractions was combined with 1 mL of molybdate reagent solution in a test tube. The test tubes were incubated in a water bath at 95 °C for 90 min. Subsequently, the samples were cooled to room temperature, and the absorbance of the reaction mixture was measured at 695 nm using a double-beam UV–visible spectrophotometer (Shimadzu Double Beam UV-2600, Nakagyo-Ku, Kyoto, Japan). The assay was performed in triplicate, and a standard linear curve for gallic acid was plotted. Total phenolic content was extrapolated from the curve, and values were expressed as mg gallic acid equivalent (GAE)/g.
Experimental animals
Forty-two adult male Albino rats (Wistar strain) weighing 100 to 200 g were housed in properly ventilated polypropylene cages lined with dried wood shavings as bedding under room temperature. Twelve hours photoperiod of light and dark cycles was maintained. Rats were fed with a commercial pellet diet (Ladokun Pelleted Feeds, Ibadan) and water ad libitum. The animals were allowed to acclimatize for 14 days at the Babcock University Animal Facility. The animals were properly handled following ethical standards and protocols established for the care and use of laboratory animals by the National Institutes of Health (2011) and Babcock University Health, Research and Ethics Committee (BUHREC) with certificate number BUHREC 715/18.
Acute toxicity study
Acute toxicity study to assess the safe dose for the administration of C. afer aqueous leaf fraction to experimental rats was performed in accordance to the guideline set by the Organization for Economic Cooperation Development (2001). A rat was orally administered with 5000 mg/kg body weight (b.w.) CALAF after fasting overnight. The animal was observed for 24 h for any clinical sign of toxicity, such as a change in fur color, accelerated breathing, and mortality. The animal survived without noticeable sign of toxicity. Subsequently, five male rats were selected randomly and subjected to the same protocol for 72 h and no mortality was recorded. The 5000 mg/kg b.w. CALAF was considered safe, and doses of 100, 200, and 400 mg/kg b.w. were adopted for the animal study.
Experimental hepatocellular carcinoma model
Diethylnitrosamine (DEN) was used to chemically induce the formation of hepatocellular carcinoma (HCC) in rats following the method described by Amereh et al. (2017). The rats were fasted (without feed alone, while water was given ad libitum) for 96 h as a stimulant for the development of hepatocarcinogenesis. Subsequently, each rat was injected intraperitoneally with a single dose of DEN at 200 mg/kg b.w. to initiate hepatocarcinogenesis, and re-feeding commenced for the remaining duration of the study. Two weeks later, 30 mg/kg b.w. of 2-AAF was orally administered once daily for 2 weeks to promote the development of liver cancer. HCC was investigated by measurement of HCC markers (alpha-fetoprotein (AFP), λH2AX, and MGMT proteins) and liver function markers (alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities) and by histological evaluation.
Experimental design
Experimental rats were randomly divided into six groups of six rats each. Group 1: control rats (no HCC induction; negative control); group 2: untreated HCC rats (positive control); group 3: HCC rats treated with silymarin (reference hepatoprotective drug) at 50 mg/kg (Vargas-Mendoza et al. 2014). Groups 4 to 6: HCC rats treated with CALAF at 100, 200, and 400 mg/kg b.w., respectively. The treated groups (3 to 6) were daily administered with silymarin or CALAF orally by gavage for 2 weeks before HCC induction and 6 weeks during HCC induction, within 8 weeks of treatment after which the animals were anesthetized using diethyl ether and sacrificed to obtain blood and liver samples for analysis.
Blood and liver sample preparations
Immediately after animal sacrifice, whole blood samples were collected through cardiac puncture using hypodermal syringes into lithium heparinized bottles and plain bottles. The anticoagulated and clotted blood samples were centrifuged at 4200 rpm for 5 min to separately obtain plasma and serum for biochemical analysis, such as alpha-fetoprotein (AFP) and liver function enzymes. Liver samples were dissected out, washed immediately in ice-cold saline buffer, blotted with a filter paper, and weighed afterward. The liver samples were divided into two portions, one portion for immunohistochemical study and the other portion was homogenized in 0.1 M Tris-HCl buffer (pH 7.4) with a Teflon homogenizer. The liver homogenates were used to assay for antioxidant and membrane-bound enzyme activities.
Evaluation of biochemical and immunohistochemical assays
Alpha-fetoprotein (AFP) assay was carried out in accordance with the steps described in serum AFP enzyme-linked immunosorbent assay (ELISA) kit. Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were determined following the description in Randox diagnostic kits. Assessment of λH2AX and O6-methylguanine-DNA methyltransferase (MGMT) protein levels were determined in the liver samples following the standard immunohistochemical staining kit procedure. Immunohistochemistry of paraffin-embedded liver samples was done post-fixation of liver samples with 10% buffered formalin as described by Oyagbemi et al. (2015). Paraffin sections were melted at 60 °C in the hot air-oven. Dewaxing of the liver samples in xylene was followed by passage through graded ethanol. Peroxidase quenching with 1% H2O2/methanol was followed by antigen retrieval performed by microwave heating in 0.01 mol/L citrate buffer (pH 6.0) to boil. The liver sections were blocked in goat serum and probed with anti-λH2AX and MGMT antibodies in 1:200 ratio overnight at room temperature. Detection of bound antibody was carried out using biotinylated (goat anti-rabbit, 2.0 μg/mL) secondary antibody and, subsequently, streptavidin antibody peroxidase (horseradish peroxidase-streptavidin) according to protocol. The reaction was enhanced with diaminobenzidine (DAB, Amresco, USA) for 2 to 3 min and counterstained with high definition hematoxylin (Enzo, New York, USA) with dehydration in ethanol. The slides were covered with coverslips and sealed with resinous solution. The immune-reactive positive expressions of λH2AX and MGMT intensive regions were viewed starting from low magnification using photomicroscope (Olympus) and a digital camera (Toupcam; Touptek Photonics, Zhejiang, China). The assessment of immune-reactive positive expression of λH2AX and MGMT was carried out digitally using quantification software (ImageJ 1.48 v; National Institutes of Health, Bethesda, MD, USA). Five photomicrographs were analyzed per group for λH2AX and MGMT.
Determination of hepatic antioxidants
The following antioxidant activities were estimated using liver homogenates: superoxide dismutase (SOD) activity was determined in accordance with the method described by McCord and Fridovich (1969). Reduced glutathione (GSH) level was assayed using the method described by Buetler et al. (1963). Glutathione peroxidase (GPx) activity was determined using the method described by Flohe and Gunzler (1984).
Statistical analysis
Data were expressed as means ± standard deviations. Linear regression of the percentage inhibition on the concentration of extract fractions or standard was carried out to determine the 50% inhibitory concentration (IC50) values. The means were compared using one-way analysis of variance (ANOVA), followed by Tukey’s test with statistical significance set at p < 0.05 using GraphPad Prism® version 6.0.
Results
Data in Figs. 1 and 2 show that varying concentrations of the leaf fractions and standard gallic acid scavenged DPPH and NO• radicals. DPPH 50% inhibitory concentration (IC50) values showed that CALAF had high scavenging activity when compared with CALHF, CALEF, and CALBF. In addition, NO• IC50 values showed that CALAF had high scavenging activity when compared with CALHF, CALBF, and CALEF. The NO• scavenging activity of CALAF was comparable to standard gallic acid (Table 1). Further studies showed that CALAF had high FRAP and TAC values when compared with CALHF, CALBF, and CALEF (Table 1).
Table 2 shows that animals in group 2 had serum AFP level that was significantly (p < 0.05) higher than group 1. Animals in group 3 to 6 had significantly reduced (p < 0.05) serum AFP when compared with group 2. Groups 4 to 6 had reduced serum AFP levels. Furthermore, animals in groups 3 to 6 had significantly (p < 0.05) reduced plasma ALT and AST, respectively, when compared with ALT and AST activities in group 2. Group 6 had significantly (p < 0.05) reduced plasma ALT and AST activities when compared with group 3.
Figure 3 shows the immunohistochemical sections of liver γH2AX protein expressions. Group 1 suggests a normal hepatic architecture, whereas group 2 indicates disordered arrangement of dysplastic hepatocytes, loss of lobular architecture, trabecular growth pattern, lack of normal parenchymal portal tracts, and high λH2AX protein expression identified by pronounced brown patches. Group 4 to 6 liver sections suggested restoration of hepatic architecture with reduced γH2AX protein expression. In addition, data in Fig. 4 show the quantitative analysis of γH2AX protein expression. Group 1 (38.02 ± 3.84%), group 3 (31.26 ± 8.90%), group 4 (35.37 ± 2.97%), group 5 (33.62 ± 4.07%), and group 6 (31.48 ± 2.33%) had significantly reduced (p < 0.05) γH2AX protein levels when compared with group 2 (47.28 ± 1.10%). Figure 5 shows the immunohistochemical sections of liver MGMT protein expressions. Group 1 suggests normal histoarchitecture whereas group 2 indicates an altered histoarchitecture with reduced MGMT protein expression. Furthermore, data in Fig. 6 show that groups 3 to 6 had restored liver histoarchitecture with elevated MGMT protein expression. Furthermore, group 1 (93.83 ± 2.55%), group 3 (92.80 ± 3.15%), group 4 (92.85 ± 3.63%), group 5 (93.75 ± 3.04%), and group 6 (95.59 ± 1.25%) had significantly (p < 0.05) elevated MGMT protein expression when compared with group 2 (76.87 ± 2.48%).
Photomicrograph sections showing the effects of 100, 200, and 400 mg/kg b.w. Costus afer leaf aqueous fraction (CALAF) on immunohistochemical λH2AX expression in rats induced with HCC (×400). Thin sections of liver tissue stained with a polyclonal antibody against λH2AX, detected by a biotinylated immunoglobulin G (IgG) secondary antibody and streptavidin-horseradish peroxidase, followed by 3,3′-diaminobenzidine (DAB) for color detection and counterstained with hematoxylin. Group 1: normal group shows a normal hepatic architecture; group 2: HCC group shows disordered arrangement of dysplastic hepatocytes, loss of lobular architecture, trabecular growth pattern, lack of normal parenchymal portal tracts (orange arrow), and high λH2AX activity identified by pronounced brown patches (blue arrow); group 3: silymarin group indicated reduced distortion of hepatocytes; groups 4, 5, and 6: show restoration of hepatic architecture. Blue arrow represents λH2AX activity; orange arrow represents disordered arrangement of hepatocytes
Effects of varying doses of Costus afer leaf aqueous fraction on immunohistochemical γH2AX expression in the liver during chemical induction of hepatocellular carcinoma in rats. Different symbols indicate significantly different at p < 0.05; CALAF, Costus afer leaf aqueous fraction; DEN, diethylnitrosamine; 2-AAF, 2-acetylaminoflourene; γH2AX, gamma histone 2A, family member X; group 1: normal; group 2: untreated control; group 3: 50 mg/kg b.w. silymarin; group 4: 100 mg/kg b.w. CALAF; group 5: 200 mg/kg b.w. CALAF; and group 6: 400 mg/kg b.w. CALAF
Photomicrograph sections showing the effects of 100, 200, and 400 mg/kg b.w. Costus afer leaf aqueous fraction (CALAF) on immunohistochemical MGMT expression in rats during chemical induction of hepatocellular carcinoma (×400). Black arrow represents MGMT protein. Thin sections of liver tissue stained with a polyclonal antibody against MGMT, detected by a biotinylated IgG secondary antibody and streptavidin-horseradish peroxidase, followed by 3,3′-diaminobenzidine (DAB) for color detection and counterstained with hematoxylin. Group 1: had normal liver histoarchitecture and MGMT protein expression; group 2: untreated HCC induced group indicates an altered histoarchitecture with reduced MGMT protein expression level; groups 3 to 6: indicates restored histoarchitecture with elevated MGMT expression levels
Effects of varying doses of Costus afer leaf aqueous fraction (CALAF) on MGMT expression in in the liver of rats during chemical induction of hepatocellular carcinoma. Different symbols indicate significantly different at p < 0.05; CALAF, Costus afer leaf aqueous fraction; DEN, diethylnitrosamine; 2-AAF, 2-acetylaminoflourene; MGMT, O6-methylguanine-DNA-methyltransferase (MGMT); group 1: normal; group 2: untreated control; group 3: 50 mg/kg b.w. silymarin; group 4: 100 mg/kg b.w. CALAF; group 5: 200 mg/kg b.w. CALAF; and group 6: 400 mg/kg b.w. CALAF
Data in Table 3 show that GSH level and SOD and GPx activities were significantly (p < 0.05) elevated in group 2 when compared with the values in group 1. However, animals in groups 4, 5, and 6 had significantly (p < 0.05) reduced GSH levels and SOD and GPx activities, respectively, when compared with group 2. Group 3 also had significantly (p < 0.05) reduced GSH, SOD, and GPx activities when compared with group 2.
Discussion
In this present study, the results showed that CALAF exhibited the strongest DPPH scavenging activity when compared with the other test fractions. This indicates that CALAF could possess high antioxidant activity with the capacity to donate hydrogen ion, a key event involved in the quenching of the deleterious reactive oxygen species. This finding also suggests that CALAF could contain polar antioxidant compound(s). This seems to corroborate with the previous reports that most antioxidant compounds possess polar functional groups (Prashant et al. 2011; Lourenço et al. 2019). Previous findings have shown that aqueous extract of plant leaves exhibits high antioxidant activity, partly due to the presence of polyphenolic compounds (Castro-López et al. 2019). Findings from our laboratory had shown that polyphenolic compounds in C. afer leaf aqueous fraction are largely responsible for its pharmacological activities (Anyasor et al. 2014). Other researchers have also shown that plant polyphenolic compounds play an important role in cancer prevention and treatment through their antioxidant mechanism of action (Ezejiofor and Orisakwe 2017).
CALAF had high NO• scavenging activity and FRAP when compared with the other test fractions. These findings suggest that CALAF perhaps could mitigate the NO-induced nitrosylation of DNA bases. The nitrosylation of DNA bases has been demonstrated to impair DNA repair mechanism causing genotoxicity (Salvatore and Giuseppe 2018). An elevated generation of NO• has been linked to hyperdynamic circulation and cytotoxic effect observed in liver cirrhosis and could lead to hepatic dysfunction and severe hepatic injury (Vairappan 2015). FRAP data suggest that CALAF could inhibit the generation of hydroxyl and hydroxide ions which are by-products of Fenton reaction (Sudan et al. 2014). More so, CALAF had high TAC when compared with the other test fractions. CALAF antioxidant property could perhaps explain the ethnomedical use of C. afer leaves as therapy in the management of hepatic disorder as reported by Ezejiofor et al. (2013). Hence, the selection of CALAF as the test agent is to be investigated in the animal experiment.
The animal study revealed that CALAF-treated groups had reduced serum AFP level compared with the untreated control group. This suggests that CALAF could possess anticancer property. Previous reports have shown that 60% to 80% of HCC patients presented with high AFP levels (Biselli et al. 2015; Chou et al. 2018). It has also been demonstrated that high AFP level inhibits caspase 3 apoptotic activity by altering caspase 8 activity through colocalization at its active domain (Park et al. 2017). Hence, the reduced serum AFP levels in CALAF-treated rats could be suggestive of CALAF promotion of caspase 3 apoptotic activity as a mechanism to mitigate DEN-induced HCC. Furthermore, the reduction in serum AFP level in CALAF-treated rats might be attributable to the presence of cytoprotective bioactive compounds including polyphenols, such as phenol 2,4-bis(1,1-dimethylethyl) (Anyasor et al. 2015), and sapogenins, such as diosgenin (Shiraishi et al. 2000). Moreover, the anticancer property of CALAF could be attributed to the presence of diosgenin. Diosgenin, a steroidal sapogenin isolated from C. afer (Lin et al. 1996) and Costus speciosus had been reported to exhibit anticancer and apoptotic effects on cell proliferation (Selim and Al-Jaouni 2015). In addition, diosgenin has been shown to inactivate the STAT3 signaling pathway in HCC by inhibiting c-SRC, JAK1, and JAK2 (Li et al. 2010; Sethi et al. 2018).
Plasma AST and ALT are sensitive markers employed in the detection of hepatic damage due to their native cytoplasmic location, and, hence, release into the blood circulation is an indicator of tissue damage. Investigation of these markers reflects mechanisms of cellular damage, subsequent release of proteins, their extracellular turnover, and mechanisms of HCC process (Jahan et al. 2011). The assessment of the hepatic function showed that animals in group 2 had significantly elevated plasma ALT and AST activities than group 1 whereas silymarin- and CALAF-treated groups had reduced plasma ALT and AST activities. This indicates that DEN-induced HCC had a toxic effect on hepatic cells, probably through increased membrane permeability and necrosis of hepatocytes causing enhanced leakages of ALT and AST into the blood. Conversely, data showed that CALAF exhibited hepatoprotective effect on DEN-induced HCC rats. Previous work had shown that hepatic injury is a key factor that instigates tumor formation in DEN-induced carcinogenesis (Tolba et al. 2015). Hence, it seems that the hepatoprotective capacity of CALAF might have contributed to its anticancer activity.
Immunohistochemical analysis showed an increased λH2AX expression in group 2 than CALAF-treated groups 4 to 6. This indicates that CALAF might have protected the animals against alkylation of DNA structure thereby preventing DNA strand break, which is one of the early events that initiate the process of hepatocellular carcinogenesis. Previous report had shown λH2AX as a sensitive metabolite for targeting DNA strand breaks and a pronounced expression of this marker serves as an indicator of mutagenic activity (Ivashkevich et al. 2012). Furthermore, groups 3 to 6 had elevated MGMT protein expression when compared with group 2. This indicates that silymarin and CALAF might have mitigated DEN-generated toxic-reactive metabolites that interfere with the DNA repair mechanism. Previous investigations have shown that methylating agents, like DEN, generate reactive electrophilic species as a by-product of metabolism that alkylate and form adducts at the N- and O-atoms in DNA bases, generating O6-methylguanine (Kaina et al. 2007; Kaina and Fahrer 2013). However, DNA base alkylation can be removed by the DNA repair gene MGMT (Jacinto and Esteller 2007; Soll et al. 2016). Hence, the elevation in MGMT protein expression suggests reduced carcinogenesis in the CALAF-treated animals.
Investigation of the status of endogenous antioxidant in vivo showed that animals in the untreated group had significantly elevated GSH level and SOD and GPx activities, when compared with normal, silymarin-, and CALAF-treated groups. This suggests that the endogenous antioxidant defense system might have been triggered to counteract the heightened oxidative stress elicited by the highly reactive by-products of DEN, such as methyl diazonium and carbonium ions, in group 2 animals. In addition, 2-nitrosofluorene, a metabolite of 2-AAF induces redox cycling leading to superoxide anion production capable of causing damage to DNA. It has also been reported that oxidative stress plays a crucial role during the initiation and progression of HCC (Hussain et al. 2000; Sasaki et al. 2006). The oxidative stress in livers of HCC rats might be attributed to DEN metabolized in the livers by cytochrome p450 enzymes, and the reactive metabolites are mainly responsible for the hepatotoxic effects. These reactive metabolites induce oxidative stress and cytotoxicity by damaging biomolecules, such as DNA, lipids, and proteins (Verna et al. 1996).
During carcinogenesis, tumor cells protect themselves against increased oxidative stress through the sequestration of intracellular GSH concentration (Chen et al. 2017). Similar findings were also observed in rat hepatoma tissues and human HCC tissue (Huang et al. 2010; Marengo et al. 2010). In addition, overproduction of GSH has been reported in breast and ovarian tumor tissue (Snezhkina et al. 2019). Glutathione peroxidase (GPx) is known to detoxify hydrogen peroxide and organic hydroperoxides using GSH as cosubstrate (He et al. 2017; Sarikaya and Dogan 2020). Previous work had shown an elevated GSH-dependent GPx status in hepatocellular carcinoma (Chen et al. 2017). Hence, reduction in GSH concentration and SOD and GPx activities in CALAF-treated groups could be attributed to the antioxidant effect of CALAF thereby conferring further protection in DEN-induced HCC animals.
Conclusion
The plant extract, CALAF, possesses hepatoprotective effect on DEN-induced HCC in rats. The anticancer mechanism of action of CALAF might possibly be through its antioxidants and effects on γH2AX and MGMT protein expressions.
References
Ahmad MS, Suardi N, Shukri A, Mohammad H, Oglat AA, Abunahel BM, Mohamed ABM, Makhamrah O (2019) Current status regarding tumour progression, surveillance, diagnosis, staging, and treatment of hcc: a literature review. J Gastroenterol Hepatol Res 8:2841–2852
Amereh Z, Hatami N, Shirazi F, Gholami S, Hosseini HS, Noubarani M, Kamalinejad M, Andalib S, Keyhanfar F, Eskandari RM (2017) Cancer chemoprevention by oleaster (Elaeagnus angustifoli L.) fruit extract in a model of hepatocellular carcinoma induced by diethylnitrosamine in rats. ExCLI J Sci 16:1046–1056
Anaga AO, Njoku CJ, Ekejuiba ES, Esiaka MN, Asuzu IU (2004) Investigations of the methanolic leaf extract of Costus afer Ker Gawl. for pharmacological activities in vitro and in vivo. Phytomed 11:242–248
Anyakeme T, Essien ES, Akinnawor JO (2014) Hepatoprotective effect of methanolic stem extract of bush cane (Costus afer) on immunologic response generated reactive oxygen species (ROS) in alcohol-induced liver cirrhosis in rats. Asian J Biochem Pharm Res 4:215–223
Anyasor GN, Onajobi F, Osilesi O, Adebawo O (2014) Proximate composition, mineral content and in vitro antioxidant activity of leaf and stem of Costus afer (ginger lily). J Intercult Ethnopharmacol 3:128–134
Anyasor GN, Onajobi F, Osilesi O, Adebawo O, Oboutor E (2015) Evaluation of Costus afer Ker Gawl. in vitro anti-inflammatory activity and its chemical constituents identified using gas chromatography mass spectrometry analysis. J Coast Life Med 3:132–138
Aweke G (2007) Costus afer Ker Gawl. record from PROTA4U. Schmelzer GH & Gurib-Fakim A (Editors). PROTA (Plant Resources of Tropical Africa/Ressourcesvégétales de l’Afrique Tropicale), Wageningen, Netherlands. Retrieved December 16, 2019, from http://www.prota4u.org/search.asp.
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Ann Biochem 239:70–76
Biselli M, Conti F, Gramenzi A, Frigerio M, Cucchetti A, Fatti G, D’Angelo M, Dall’Agata M, Giannini EG, Farinati F, Ciccarese F, Andreone P, Bernardi M, Trevisani F (2015) A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br J Cancer 112:69–76
Boison D, Adinortey CA, Babanyinah GW, Quasie O, Agbeko R, Wiabo-Asabil GK, Adinortey MB (2019) Costus afer: a systematic review of evidence-based data in support of its medicinal relevance. Scientifica (Cairo) 2019:3732687–3732610. https://doi.org/10.1155/2019/3732687
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:1–31. https://doi.org/10.3322/caac.21492
Buetler E, Duran O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Castro-López C, Bautista-Hernández I, González-Hernández MD, Martínez-Ávila GCG, Rojas R, Gutiérrez Díez A, Medina-Herrera N, Aguirre-Arzola VE (2019) Polyphenolic profile and antioxidant activity of leaf purified hydroalcoholic extracts from seven Mexican Persea americana cultivars. Molecules 24(1):173. https://doi.org/10.3390/molecules24010173
Chedid F, Kruel C, Pinto M, Grezzan-filho T, Ian L (2017) Hepatocellular carcinoma: diagnosis and operative management. Arq Bras Cir Dig 30:272–278
Chen S, Liu H, Chen S, Lin P, Lai C et al (2017) Changes of oxidative stress, glutathione and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS One 12:e0170016. https://doi.org/10.1371/journal.pone.0170016
Chou W-C, Lee C-N, Yang T-S, Huang C-Y, Teng W, Tseng Y-T, Chen J-S, Lin Y-C, Hou M-M, Chang HH, Hsieh JC-H (2018) Changes in serum α-fetoprotein level predicts treatment response and survival in hepatocellular carcinoma patients and literature review. J Formos Med Assoc 117(2):153–163
Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR (2010) Antioxidant and free radical scavenging activity of H. officinalis, L. Var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak J Pharm Sci 23:29–34
El-Far AH, Badria FA, Shaheen HM (2016) Possible anticancer mechanisms of some Costus speciosus active ingredients concerning drug discovery. Curr Drug Discov Technol 13(3):123–143
Ezejiofor AN, Orisakwe OE (2017) Evaluation of protective effect of aqueous leave extract of Costus afer on female albino Wistar rats exposed to lead acetate. EC Pharmacol Toxicol 4:75–92
Ezejiofor AN, Orish CN, Orisakwe OE (2013) Effect of aqueous leaves extract of Costus afer Ker Gawl. (Zingiberaceae) on the liver and kidney of male albino Wistar rat. Anc Sci Life 33:4–9
Flohe L, Gunzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105:114–121. https://doi.org/10.1016/s0076-6879(84)05015-1
Fujiwara N, Friedman S, Goossens N, Hoshida Y (2017) Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol 68:526–549
Hassan HA, El-Gharib NE, Azhari AF (2016) Role of natural antioxidants in the therapeutic management of hepatocellular carcinoma. Hepatom 2:216–223. https://doi.org/10.20517/2394-5079.2016.12
Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennet WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC (2000) Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res 60:3333–3337
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidant maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44:532–553. https://doi.org/10.1159/000485089
Huang ZZ, Chen C, Zeng Z, Yang H, Oh H et al (2010) Mechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver generation. FASEB 15:19–21
Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Olga A, Martin OA (2012) Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett 321(1–2):123–133. https://doi.org/10.1016/j.canlet.2011.12.025
Jacinto FV, Esteller M (2007) Mutator pathways unleashed by epigenetic silencing in human cancer. Mutagenesis 22:247–253
Jahan MS, Vani G, Shyamaladevi CS (2011) Anticarcinogenic effect of Solarium trilobatum in diethylnitrosamine induced and phenobarbital promoted hepatocarcinogenesis in rats. Asian J Biochem 6(1):74–81
Kaina B, Fahrer J (2013) O6-methylguanine-DNA methyltransferase in the defense against N-nitroso compounds and colorectal cancer. Carcinogen 34(11):2435–2442. https://doi.org/10.1093/carcin/bgt275
Kaina B, Christmann M, Naumann S, Roos WP (2007) Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 6:1079–1099
Li F, Fernandez PP, Rajendra P, Hui KM, Sethi G (2010) Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett 292:197–207
Lin RC, Hanquet B, Mareh-Aleth L (1996) Aferoside A, a steroidal saponin from Costus afer. Phytochem 43:665–668
Lin RC, Lacaille-Dubois M-A, Hanquet B, Correia M, Bruno C (1997) New diosgenin glycosides from Costus afer. J Nat Prod 60(11):1165–1169. https://doi.org/10.1021/np9702190
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G (2016) Hepatocellular carcinoma. Nat Rev Dis Primers 2:16018. https://doi.org/10.1038/nrdp.2016.18
Lourenço SC, Moldão-Martins M, Alves VD (2019) Antioxidants of natural plant origins: from sources to food industry applications. Molecules 24:4132. https://doi.org/10.3390/molecules24224132
Marengo B, Ciucis CD, Ricciarelli R, Romano P, Passalacquaet M et al (2010) DNA oxidative damage of neoplastic rat liver lesions. Oncol 23:1241–1246
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzyme function for erythrocuprein. J Biol Chem 244:6049–6055
McCune LM, Johns T (2002) Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmcol 82:197–205
Mittal S, El-Serag HB (2013) Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 47:2–6
Nair SVG, Hettihewa M, Rupasinghe HP (2014) Apoptotic and inhibitory effects on cell proliferation of hepatocellular carcinoma HepG2 cells by methanol leaf extract of Costus speciosus. Biomed Res Int 2014. https://doi.org/10.1155/2014/637098
National Institutes of Health (2011) Guide for the Care and Use of Laboratory Animals, 8th edn. The National Academies Press, Washington DC
Organization for Economic Cooperation Development (2001) The OECD Guideline for Testing of Chemical. The Organization of Economic Co-operation Development, Paris
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243
Park SJ, Jan JY, Jeong SW, Cho YK, Lee SH et al (2017) Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 96:e5811. https://doi.org/10.1097/MD.0000000000005811
Prashant T, Bimlesh K, Mandeep K, Gurpreet K, Harleen K (2011) Phytochemical screening and extraction: a review. Int Pharm Sci 1:98–106
Prieto RL, Pineda M, Aquilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Ann Biochem 269:337–341
Salvatore R, Giuseppe F (2018) Role, targets and regulation of (de)nitrosylation in malignancy. Front Oncol 8:334. https://doi.org/10.3389/fonc.2018.00334
Sarikaya E, Dogan S (2020) Glutathione peroxidase in health and disease. Intechopen. https://doi.org/10.5772/intechopen.91009
Sasaki Y, Yamada T, Tanaka H, Ohigashi H, Eguchi H, Yano M, Ishikawa O, Imaoka S (2006) Risk of recurrence in a long term follow-up after surgery in 417 patients with hepatitis B or hepatitis C related hepatocellular carcinoma. Ann Surg 244:771–780
Selim S, Al-Jaouni S (2015) Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm. BMC Complement Altern Med 15:301. https://doi.org/10.1186/s12906-0150836-8
Sethi G, Shanmugan MK, Warrier S, Merarchi M, Arfuso F et al (2018) Pro-apoptotic and anti-cancer properties of diogenin: a comprehensive and critical review. Nutrients 10:645. https://doi.org/10.3390/nu100050645
Shiraishi A, Sakumi K, Sekihuchi M (2000) Increased susceptibility to chemotherapeutic alkylating agents of mice deficient in DNA repair methyltransferase. Carcinogenesis 21:1879–1883
Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA (2019) ROS generation and antioxidant defense systems in normal and malignant cells. Ox Med Cell Lonev 2019 6175804 https://doi.org/10.1155/2019/61758046175817.
Soll JM, Sobol RW, Mosammaparast N (2016) Regulation of DNA alkylation damage repair: lessons and therapeutic opportunities. Trends Biochem Sci 42(3):206–218. https://doi.org/10.1016/j.tibs.2016.10.001
Su XY, Zhao JQ, Li N, Kumar M, Yang AU (2019) Chemoprotective effects of resveratrol against diethylnitrosamine induced hepatocellular carcinoma in wistar rats. Int J Pharmacol 15:549–559. https://doi.org/10.3923/ijp.2019.549.559
Sudan R, Bhagat M, Gupta S, Singh J, Koul A (2014) Iron chelation, ferric reducing antioxidant power and immune modulating potential of Arisaema jacquemontii (Himalayan Cobra Lily). Biomed Res Int 2014:179865–179867. https://doi.org/10.1155/2014/179865
Tcheghebe OT, Tala VR, Fouodjouo M (2018) Ethnobotanical uses, phytochemical and pharmacological profiles and toxicity of Costus afer ker Gawl. J Sci Res Allied Sci 4:1–11
Tolba R, Kraus T, Liedtke C, Schwarz M, Weiskirchen R (2015) Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab Anim 49:59–69. https://doi.org/10.1177/0023677215570086
Umoh IU, Aquaisua AN, Udo NM (2019) The effect of fresh stem juice extract of Costus afer on the cytohistomorphology of the kidney in aspirin-treated wistar rats. J Appl Biol Biotechnol 7:78–81. https://doi.org/10.7324/JABB.2019.70214
Vairappan B (2015) Endothelial dysfunction in cirrhosis: role of inflammation and oxidative stress. World J Hepatol 7(3):443–459. https://doi.org/10.4254/wjh.v7.i3.443
Vargas-Mendoza N, Madrigal-Santillan E, Morales-Gonzalez A, Esquivel-Soto J, Esquivel-Chirino C, Garcia Luna M, Gonzalez-Rubio MG, Gayosso-de-Lucio JA, Morales-Gonzalez JA (2014) Hepatoprotective effect of silymarin. World J Hepatol 6:144–149
Veeraraghavan VP, Mohan SK, Jainu M, Seshadri C (2015) Ameliorating effects of Garcinia mangostana Linn pericarp extract on hepatic antioxidants in diethylnitrosamine (DEN) induced hepatocellular carcinoma (HCC). Indian J Pharm Educ Res 49:344–352
Verna L, Whysner J, Williams GM (1996) N-Nitrodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 71:57–81
Xu JH, Chang WH, Fu HW, Yuan T, Chen P (2018) The mRNA, miRNA and lncRNA networks in hepatocellular carcinoma: an integrative transcriptomic analysis from gene expression omnibus. Mol Med Rep 17:6472–6482
Acknowledgments
The authors express deep gratitude to the Director, Research Innovation and International Cooperation, Babcock University with grant number BU/RIIC/2018/008. We appreciate the Department of Biochemistry, Babcock University for providing support and research facility that enabled the completion of this study. We thank Mrs. Chiamaka Anyasor for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial and nonfinancial competing interest concerning this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anyasor, G.N., Idowu, D.P. & Nabofa, W. Evaluation of the hepatoprotective effect of oral administration of aqueous fraction of methanolic extract of Costus afer leaves during induction of hepatocellular carcinoma with diethylnitrosamine in rats. Comp Clin Pathol 29, 733–744 (2020). https://doi.org/10.1007/s00580-020-03124-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-020-03124-w