Abstract
It is known that retinopathy of prematurity (ROP) alters the activation of rod photoreceptors, but the effect of ROP on deactivation has not been investigated. We studied deactivation using an electroretinographic (ERG) paired flash procedure in 22 subjects (12 infants and 10 older subjects) with a history of preterm birth and ROP. The amplitude of the rod-isolated a-wave response to a flash presented 2–120 s after a test flash was measured, and the time at which it reached 50% of the single flash amplitude (t 50) was determined by linear interpolation. Deactivation results were compared to those in former preterms who never had ROP (n = 6) and term-born controls. In infants, t 50 values of ROP subjects did not differ from those in subjects who never had ROP or term-born controls. Among mature ROP subjects, eight of 12 had t 50 values longer than any control subject. Prolonged deactivation in these mature ROP subjects may indicate lack of maturation of the deactivation process (t 50) or progressive compromise of retinal function with increasing age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abnormal activation of rod phototransduction has been reported in subjects with a history of retinopathy of prematurity (ROP) [1]. The more severe the ROP, the lower rod photoreceptor sensitivity, S ROD [1]. S ROD depends on the time constants of the biochemical steps from photon capture by rhodopsin to closure of the channels in the rod outer segment. Low S ROD indicates slow kinetics of the molecular processes in the activation of phototransduction, possibly due to subtle alteration in the rod outer segment [2].
Following activation, the rod must deactivate in a timely manner to prepare for response to the next stimulus. The rod recovers by stepwise deactivation of rhodopsin, transducin, and phosphodiesterase in a series of biochemically complex processes [3–7]. In term-born infants and infant rats, the kinetics of both activation and deactivation of the photoresponse are slower than in adults [5, 8–14]. If activation in ROP is slower than in age-matched term-born control subjects, is deactivation also abnormally slow? We used an electroretinographic (ERG) paired flash technique to study deactivation in ROP subjects in infancy and at older ages.
Methods
Subjects
Twenty-eight subjects with a history of preterm birth were studied (Table 1). All had been monitored in the newborn intensive care nursery following schedules for examination that were modeled on those used in the multi-center ROP treatment trials [15–17]. Based on a review of the results of these examinations, the subjects were categorized by ROP history as either mild ROP or no ROP. Those with mild, untreated ROP (n = 22) had Stage 2 or 3 in Zone II or III that resolved spontaneously [18]. The others (n = 6) had serial examinations but never had ROP. Gestational age at birth ranged from 23 to 32 (median 27) weeks and birth weight from 550 to 1,590 (median 910) grams. None had active ROP at the time of the ERG test. Thirteen subjects were tested as infants at median age 10 (range 7–18) weeks post term. Fifteen older subjects were tested at median age 13 (range 11–21) years. The parameters of rod activation in these preterm subjects have been reported [1]. Results from term-born 10-week-old infants (n = 15) and mature subjects (n = 10) provide control data for comparison. Data from the term-born infants and most of the mature control subjects (8 of 10) were reported previously [11].
This study conformed to the tenets of the Declaration of Helsinki and was approved by the Children’s Hospital Committee on Clinical Investigation. Informed consent was obtained from the parents of the infants and children, assent from the older children, and consent from those 18 years and older.
ERG procedure
Parents stayed with infants and children throughout the procedure. One pupil was dilated with cyclopentolate 1% and the subject dark-adapted for 30 min. Then, under dim red light, proparacaine 0.5% was instilled and a bipolar Burian–Allen electrode was placed on the cornea. A ground electrode was placed on the skin over the ipsilateral mastoid.
Twenty-four subjects were tested using a Compact 4 system (Nicolet, Madison, WI), and four subjects were tested using an Espion system (Diagnosys, Lowell, MA). Rod responses were recorded using a Wratten 47B filter (λ < 510 nm) in the Nicolet system and a 470 nm LED (half bandwidth 30 nm) in the Espion system. The gain was 1,000 and bandpass in both systems was 0.5–1000 Hz. The digitization rate was 2,564 Hz for the Nicolet and 2,000 Hz for the Espion. In adult control subjects, rod activation and deactivation parameters do not differ between the Espion and Nicolet systems [13, 19]. Therefore, data obtained using the two systems have been combined.
Deactivation of phototransduction in rods
The recovery of the rod’s response to light was evaluated using a paired flash paradigm [5, 9, 20]. After a +3.3 log scotopic troland second (scot td s) test flash, an equal intensity probe flash was presented at seven selected inter-stimulus intervals (ISI 2–120 s). For each test–probe pair, the amplitude (R) of the rod-isolated a-wave response to the probe was measured at 8 ms. The response to the probe flash provides a measure of the circulating current in the rods [9, 10, 20]. For each ISI, R was expressed as proportion of R MAX, the amplitude of the a-wave to the probe alone. Linear interpolation was used to determine the time, t 50, at which a-wave amplitude was half R MAX. Between each test–probe pair, 2 min in the dark was allowed. Control experiments in term-born subjects showed that 2 min were sufficient for full recovery to the amplitude of the dark-adapted response [11]. To obtain the rod-isolated response, the amplitude of the a-wave to a photopically matched red flash was subtracted from the response to the probe flash.
Rod ERG
Activation was also studied in all subjects. Responses to full-field, brief (<3 ms), blue stimuli ranging from +2.1 to +3.3 log scot td s were recorded. The rod photoresponse parameters (S ROD and R ROD) were calculated by fit of the Hood and Birch [21] formulation of the Lamb & Pugh model [22, 23] to the a-waves. The equation is
where I is the flash (scot td s), S ROD a sensitivity parameter [(scot td)−1 s−3] that depends on the time constants of the steps in activation of phototransduction [23], R ROD the saturated response amplitude (μV), and t d a brief delay (ms). All three parameters, (S ROD, R ROD, and t d) were free to vary [24].
Calibrations
Stimuli were measured with a detector and appropriate photopic or scotopic filter (IL 1700, International Light, Newburyport, MA) placed at the position of the subject’s cornea. Retinal illuminance varies directly with area of the pupil and the transmissivity of the ocular media and inversely with the square of the posterior nodal distance [25]. We used the area of each infant’s dilated pupil, published estimates of the ocular media density, and measures of the axial length of the eye. In summary, equal intensity stimuli produced approximately equal retinal illuminance in both infants and control subjects [1, 11, 26–30]. The maximum intensity stimulus produced a retinal illuminance of approximately +3.3 log scot td s in both infants and mature subjects.
Statistical analyses
For each age group, the Mann–Whitney test was used to compare the deactivation parameter (t 50) of the ROP and no ROP subjects. Spearman rank order correlation was used to evaluate the relationship between t 50 and S ROD. The level of significance was P < 0.01.
Results
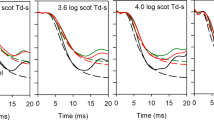
On records such as those in Fig. 1 (left panel), a-wave amplitude was measured 8 ms after the stimulus. For each test–probe flash pair, R/R MAX was plotted as a function of inter-stimulus interval (right panel).
Sample results from a 14-year-old subject with a history of mild ROP and t 50 value (10 s) close to the ROP median. Rod-isolated ERG a-wave responses are shown in the left panel; the responses to the single flash and to the probe flash at the indicated inter-stimulus intervals (ISI) are shown. In the right panel, a-wave amplitude (R) expressed as a proportion of the response to the single flash (R MAX) is plotted as a function of ISI. The ISI (arrow) that would produce a half maximum response was determined by linear interpolation
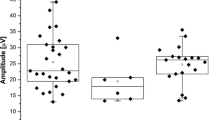
The t 50 values in infants and mature subjects are plotted in Fig. 2. Table 2 summarizes the median deactivation (t 50) and activation (S ROD) parameters of the former preterms and control subjects. The t 50 values in mature ROP subjects were significantly longer (Mann–Whitney U = 0, P < 0.01) than in those who never had ROP. In eight of the 12 mature ROP subjects, t 50 was longer than in any of the mature controls. The t 50 values in ROP infants did not differ (Mann–Whitney U = 15, ns) from those in prematurely born infants who never had ROP and were similar to those previously reported for term-born infants. In both age groups, the t 50 values of the former preterm subjects who never had ROP were similar to those of the control subjects. The t 50 values in the mature controls are similar to those in the two subjects tested by Friedburg et al. [10] who also used equal intensity test and probe flashes (~+3 log td s).
Values of t 50 and S ROD. Upper panel Values of t 50 are plotted for the mild ROP subjects (filled circles), the no ROP subjects (filled triangles), and term-born control subjects (open circles). The horizontal bars indicate the median for each group. Lower panel Values of S ROD are plotted for the same subjects; all features are as described for the upper panel. S ROD values for these subjects were reported previously [1]
In term-born subjects, higher values of S ROD are associated with shorter t 50 values [11]. However, this is not the case in the ROP subjects (ρ = −0.262; ns). In Fig. 3, deactivation (t 50) and activation (S ROD) values for each ROP subject are shown. In the majority of mature ROP subjects (5 of 7) with normal S ROD (>69.7 [(scot td)−1 s−3]), deactivation was slower than normal (t 50 > 6.2 s); t 50 was also abnormal in three of the five mature ROP subjects who had low S ROD. Two had normal t 50 and low S ROD. Both parameters were normal in only two. Thus, a mismatch occurred in seven of the 12 mature ROP subjects. In contrast, all ten infant ROP subjects had normal t 50 for age and S ROD was normal in six. For preterm subjects, neither t 50 nor S ROD varied significantly with either gestational age at birth or birth weight.
Deactivation parameter (t 50) versus activation parameter (S ROD). In both panels, the shaded bands indicate the range of t 50 and S ROD values in control subjects; the cross-hatched area represents the locus of control data [11]. Data from infant ROP subjects (n = 10) are plotted in the upper panel and from mature ROP subjects (n = 12) in the lower panel. The same scales are used in both panels to facilitate comparison across age groups
Discussion
The results are evidence that the kinetics of deactivation (t 50) in the majority of the mature ROP subjects were abnormally slow, even in cases with normal kinetics of activation (S ROD). Furthermore, two with normal t 50 had low S ROD. In normal development, t 50 and S ROD are correlated; higher S ROD is associated with more rapid recovery, that is, shorter t 50. This is not necessarily the case in disease. For example, re-analysis of data in patients with Smith-Lemli-Opitz Syndrome and mitochondrial disease [31, 32] indicated no relationship between t 50 and S ROD. In the present sample of mature ROP subjects, we found no significant correlation. These data raise the possibility that the ROP rod’s capacity to recover from light stimulation did not mature in eight of the subjects with mild ROP, even among those in whom S ROD was normal (Fig. 3). Alternatively, the deactivation of the rods in some older ROP subjects may have suffered progressive compromise; long t 50 is associated with photoreceptor degeneration [9]. S ROD was abnormally low in five subjects due to either lack of maturation or progressive loss. The data do not distinguish between these two explanations. There is evidence of progressive compromise of rod driven post-receptor retinal function in subjects with a history of severe ROP [1, 33]. All ROP infants had deactivation kinetics that were normal for age; their t 50 values were within the range of age-matched controls.
In healthy control subjects, activation and deactivation are coupled; higher S ROD values are associated with lower t 50 values [11, 34]. Studies of deactivation in healthy infants [11, 34] and adults [5, 9, 11, 34, 35] have shown that the brighter the stimuli, the slower the recovery. In healthy infant and adult rat retina, t 50 varies with the proportion of rhodopsin isomerized [8]. A similar relationship appears to pertain to deactivation in human rods. We have measured [36] the length of peripheral human rod outer segments (ROS) shown in Hendrickson [37]. The peripheral ROS length in the 5-day-old infant was 43% of that in the adult. Assuming that there are 7 × 107 molecules of rhodopsin in the adult ROS [23], there are ~3 × 107 molecules of rhodopsin in the 5-day-old ROS. If the axial density of rhodopsin in the ROS is proportional to the rhodopsin content of the retina, the ratio of infant to adult isomerizations is proportional to (1–10−Dinfant)/(1–10−Dadult). The rhodopsin content of the retina in a 10-week-old infant is ~68% of that in the adult retina [38]. Therefore, D infant is ~0.68 × D adult and 1 scot td would isomerize 6.6 molecules of rhodopsin in the infant retina [11]. The +3.3 log scot td s flash would isomerize ~13,200 molecules of rhodopsin (0.044%) in the infant retina compared to ~17,000 (0.024%) in the adult. Thus, isomerization of a higher proportion of rhodopsin in the infants is consistent with the longer t 50 values found in infants. This explanation may pertain to the ROP infants. The amount of rhodopsin extractable from the whole retina did not differ between ROP and control rats [2].
The stimuli used in the present study bleach only a small fraction (<1%) of the rhodopsin in the ROS. The double flash method we have used [10] was designed to evaluate the time course of stepwise deactivation of rhodopsin, transducin, and phosphodiesterase. The rate-limiting step in recovery of the photoresponse (deactivation) is hydrolysis of guanosine triphosphate (GTP), which is catalyzed by a membrane-associated multi-molecular complex [7]. Slow diffusion of the activation and deactivation proteins in ROS could account for long t 50 and low S ROD values found in three of the mature ROP subjects. However, a mismatch (normal activation and abnormal deactivation or abnormal activation and normal deactivation) was found in seven of the mature ROP subjects. Normal kinetics of activation (normal S ROD) is evidence of normal molecular movements in the disc membrane and in the cytosol leading to closure of the channels in the outer segment membrane [39]. As for the kinetics of deactivation, possibly the re-supply of retinoid is sluggish. Alternatively, those molecules dedicated to recovery may be selectively affected in some ROP subjects. These molecular issues will be more readily approached in rats with oxygen-induced retinopathy [40–44], which is known to model key features of ROP [45]. To our knowledge, deactivation of phototransduction has yet to be studied in rat models of ROP.
Further study of the kinetics of deactivation in mature ROP subjects using a range of stimulus levels, along with measurement of rhodopsin density and kinetics of regeneration [46, 47] and dark adaptation, might help explain the abnormal results. The final dark-adapted thresholds of some ROP subjects are elevated [48]; the kinetics of dark adaptation and rhodopsin density and regeneration have not been studied.
References
Fulton AB, Hansen RM, Moskowitz A, Akula JD (2009) The neurovascular retina in retinopathy of prematurity. Prog Retin Eye Res 28(6):452–482; Corrigendum 29:94 (2010)
Fulton AB, Reynaud X, Hansen RM, Lemere CA, Parker C, Williams TP (1999) Rod photoreceptors in infant rats with a history of oxygen exposure. Invest Ophthalmol Vis Sci 40(1):168–174
Arshavsky VY (2002) Rhodopsin phosphorylation: from terminating single photon responses to photoreceptor dark adaptation. Trends Neurosci 25(3):124–126
Burns ME, Baylor DA (2001) Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci 24:779–805
Pepperberg DR, Birch DG, Hofmann KP, Hood DC (1996) Recovery kinetics of human rod phototransduction inferred from the two-branched a-wave saturation function. J Opt Soc Am A 13:586–600
Pugh EN Jr, Lamb TD (2000) Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga DG, de Grip WJ, Pugh EN Jr (eds) Handbook of biological physics. Vol 3. Molecular mechanisms of visual transduction. Elsevier, Amsterdam, pp 183–255
Burns ME, Pugh EN Jr (2009) RGS9 concentration matters in rod phototransduction. Biophys J 97(6):1538–1547
Fulton AB, Hansen RM (2003) Recovery of the rod photoresponse in infant rats. Vision Res 43(28):3081–3085
Birch DG, Hood DC, Nusinowitz S, Pepperberg DR (1995) Abnormal activation and inactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the pro-23-his mutation. Invest Ophthalmol Vis Sci 36:1603–1614
Friedburg C, Thomas MM, Lamb TD (2001) Time course of the flash response of dark- and light-adapted human rod photoreceptors derived from the electroretinogram. J Physiol 534(Pt 1):217–242
Hansen RM, Fulton AB (2005) Recovery of the rod photoresponse in infants. Invest Ophthalmol Vis Sci 46(2):764–768
Pepperberg DR, Cornwall MC, Kahlert M et al (1992) Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci 8:9–18
Fulton AB, Hansen RM (2000) The development of scotopic sensitivity. Invest Ophthalmol Vis Sci 41(6):1588–1596
Fulton AB, Hansen RM, Findl O (1995) The development of the rod photoresponse from dark-adapted rats. Invest Ophthalmol Vis Sci 36(6):1038–1045
Early Treatment For Retinopathy Of Prematurity Cooperative Group (2003) Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 121(12):1684–1694
Committee for the Classification of Retinopathy of Prematurity (1984) An international classification of retinopathy of prematurity. Arch Ophthalmol 102:1130–1134
Cryotherapy for Retinopathy of Prematurity Cooperative Group (1988) Multicenter trial of cryotherapy for retinopathy of prematurity: three-month outcome. Arch Ophthalmol 106:471–479
International Committee for the Classification of Retinopathy of Prematurity (2005) The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 123:991–999
Hansen RM, Fulton AB (2005) Development of the cone ERG in infants. Invest Ophthalmol Vis Sci 46(9):3458–3462 (PMC1467576)
Lyubarsky AL, Pugh EN Jr (1996) Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci 16:563–571
Hood DC, Birch DG (1994) Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci 35(7):2948–2961
Lamb TD, Pugh EN Jr (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol 449:719–758
Pugh EN Jr, Lamb TD (1993) Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta 1141(2–3):111–149
Fulton AB, Hansen RM, Petersen RA, Vanderveen DK (2001) The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol 119(4):499–505
Pugh EN Jr (1988) Vision: Physical and retinal physiology. Steven’s handbook of experimental psychology. Wiley, New Work
Brown AM, Dobson V, Maier J (1987) Visual acuity of human infants at scotopic, mesopic and photopic luminances. Vision Res 27:1845–1858
Hansen RM, Fulton AB (1993) Development of scotopic retinal sensitivity. In: Simons K (ed) Early visual development, normal and abnormal. Oxford University Press, New York, pp 130–142
Hansen RM, Fulton AB, Harris SJ (1986) Background adaptation in human infants. Vision Res 26(5):771–779
Mactier H, Maroo S, Bradnam M, Hamilton R (2008) Ocular biometry in preterm infants: implications for estimation of retinal illuminance. Invest Ophthalmol Vis Sci 49(1):453–457
Malcolm CA, Hamilton R, McCulloch DL, Montgomery C, Weaver LT (2003) Scotopic electroretinogram in term infants born of mothers supplemented with docosahexaenoic acid during pregnancy. Invest Ophthalmol Vis Sci 44(8):3685–3691
Cooper LL, Hansen RM, Darras BT et al (2002) Rod photoreceptor function in children with mitochondrial disorders. Arch Ophthalmol 120(8):1055–1062
Elias ER, Hansen RM, Irons M, Quinn NB, Fulton AB (2003) Rod photoreceptor responses in children with Smith-Lemli-Opitz syndrome. Arch Ophthalmol 121(12):1738–1743
Harris ME, Hansen RM, Moskowitz A, Fulton AB (2009) Long term effects of retinopathy of prematurity (ROP) on rod and rod-driven function. Invest Ophthalmol Vis Sci: ARVO E-Abstract 5310
Pepperberg DP, Birch DG, Hood DC (2000) Electroretinographic determination of human rod flash response in vivo. Methods Enzymol 316:202–223
Pepperberg DR, Birch DG, Hood DC (1997) Photoresponse of human rods in vivo derived from paired flash electroretinograms. Vis Neurosci 14:73–82
Hansen RM, Fulton AB (1999) The course of maturation of rod-mediated visual thresholds in infants. Invest Ophthalmol Vis Sci 40(8):1883–1886
Hendrickson AE (1994) The morphologic development of human and monkey retina. In: Albert DM, Jakobiec FA (eds) Principles and practice of ophthalmology: basic sciences. WB Saunders, Philadelphia, pp 561–577
Fulton AB, Dodge J, Hansen RM, Williams TP (1999) The rhodopsin content of human eyes. Invest Ophthalmol Vis Sci 40(8):1878–1883
Lamb TD, Pugh EN Jr (2006) Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci 47(12):5137–5152
Akula JD, Favazza TL, Mocko JA et al (2010) The anatomy of the rat eye with oxygen-induced retinopathy. Doc Ophthalmol 120(1):41–50
Barnett JM, Yanni SE, Penn JS (2010) The development of the rat model of retinopathy of prematurity. Doc Ophthalmol 120(1):3–12
Berkowitz BA, Roberts R (2010) Evidence for a critical role of panretinal pathophysiology in experimental ROP. Doc Ophthalmol 120(1):13–24
Fletcher EL, Downie LE, Hatzopoulos K et al (2010) The significance of neuronal and glial cell changes in the rat retina during oxygen-induced retinopathy. Doc Ophthalmol 120(1):67–86
Hartnett ME (2010) The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol 120(1):25–39
Fulton AB, Akula JD, Mocko JA et al (2009) Retinal degenerative and hypoxic ischemic disease. Doc Ophthalmol 118(1):55–61
Alpern M, Pugh EJ (1974) The density and photosensitivity of human rhodopsin in the living retina. J Physiol 237:341–370
Fulton A, Hansen R (1988) The relation of rhodopsin and scotopic retinal sensitivity in sector retinitis pigmentosa. Am J Ophthalmol 105:132–140
Hansen RM, Fulton AB (2000) Background adaptation in children with a history of mild retinopathy of prematurity. Invest Ophthalmol Vis Sci 41(1):320–324
Acknowledgment
This work was supported by National Eye Institute Grant R01 EY010597.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansen, R.M., Harris, M.E., Moskowitz, A. et al. Deactivation of the rod response in retinopathy of prematurity. Doc Ophthalmol 121, 29–35 (2010). https://doi.org/10.1007/s10633-010-9228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-010-9228-z