Abstract
A broad spectrum of retinal diseases affects both the retinal vasculature and the neural retina, including photoreceptor and postreceptor layers. The accepted clinical hallmarks of acute retinopathy of prematurity (ROP) are dilation and tortuosity of the retinal vasculature. Additionally, significant early and persistent effects on photoreceptor and postreceptor neural structures and function are demonstrated in ROP. In this paper, we focus on the results of longitudinal studies of electroretinographic (ERG) and vascular features in rats with induced retinopathies that model the gamut of human ROP, mild to severe. Two potential targets for pharmaceutical interventions emerge from the observations. The first target is immature photoreceptors because the status of the photoreceptors at an early age predicts later vascular outcome; this approach is appealing as it holds promise to prevent ROP. The second target is the interplay of the neural and vascular retinal networks, which develop cooperatively. Beneficial pharmaceutical interventions may be measured in improved visual outcome as well as lessening of the vascular abnormalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
As a system, the mammalian retina is vulnerable to diseases that affect the exquisitely balanced interplay of the neural retina and the vasculature that nourishes it. Visual loss occurs when this balance is disturbed. On the one hand, diseases such as photoreceptor degenerations that primarily affect the neural retina also affect the retinal vasculature. On the other hand, diseases that are clinically characterized by abnormality in the choroidal or retinal vasculature, such as age related macular degeneration, diabetic retinopathy, and retinopathy of prematurity (ROP), also affect the retinal neurons. Thus, all such diseases fall within the broad group of hypoxic ischemic disorders of neural tissue. Photoreceptors, specialized cells that have the highest oxygen requirements of any cell in the body [1], are likely important in all hypoxic ischemic diseases of the retina.
Translation from animal models to patients
The photoreceptors are nestled closely to the choroidal vasculature (Fig. 1). Highly organized postreceptor retinal neurons form layers that are supplied by the retinal vessels. Although the choroid is the principal supply to the photoreceptors, degeneration of the photoreceptors is, nonetheless, associated with attenuation of the retinal arterioles [2]. Because the photoreceptor layer is such an extraordinary oxygen sink, it is presumed that, as photoreceptors degenerate, their metabolic demands wane and the retinal vasculature becomes attenuated consequent to the neural retina’s chronic lower requirement for oxygen [2].
Diagram of the neural retina and its vascular supplies (not to scale). The layers of the neural retina (ganglion cell, inner plexiform, inner nuclear, outer plexiform, outer nuclear) are indicated. Blood flow through the choroidal vessels is swift. The retinal vasculature, visible by ophthalmoscopy, lies among the ganglion cells on the vitreal surface of the retina and extends capillary networks deep into the postreceptor layers. The caliber of the retinal arterioles adjusts to perturbations in blood oxygen levels (“autoregulation”)
A tight link between the photoreceptors and the retinal vascular network is evident in the developing retina. Postreceptor cells differentiate before the photoreceptors, which are the last retinal cells to mature. As the formation of rod outer segments advances in a posterior to peripheral gradient, so too does vascular coverage. Thus, concurrent and cooperative development of the neural and vascular components characterize normal retinal maturation.
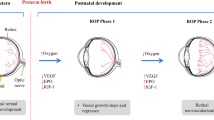
In the preterm infant, the age of onset of ROP is around the age of rapid developmental increase in rod outer segment length and consequent increase in rhodopsin content (Fig. 2). This observation is circumstantial evidence of a role for the rod photoreceptors in the ROP disease process. In addition to immature photoreceptors and retinal vasculature, the preterm infant has immature lungs that create a precarious respiratory status with attendant risk of hypoxic injury to immature cells. This is countered by administration of supplemental oxygen. Both high and low oxygen levels are known to injure the immature photoreceptors [3, 4]. Indeed, rat models of ROP are induced by rearing pups in habitats with alternating periods of relatively high and low oxygen during the critical period of rod outer segment elongation [5–10]. Following induction, abnormalities of the retinal vasculature ensue, as do abnormalities of the structure and function of the neural retina (Fig. 3) [3, 5–9, 11]. The abnormalities in the morphology of the retinal vasculature and in the function of the neural retina in ROP rats are similar to those found in pediatric ROP patients (Fig. 4) [7–9, 11–16]. Thus, translation from the rat models to the human condition may be justified.
Rat model of retinopathy of prematurity. (a) Scanning laser ophthalmoscope (SLO) images obtained using blue (488 nm) laser stimulation [42] after injection of fluorescein in 22 day old control and ROP rats. (Pigmented rats were used to facilitate SLO imaging.) The integrated curvature of each retinal arteriole is expressed as a proportion of the mean (IC A) in the control. The higher IC A value for the ROP rat reflects the greater tortuosity of its arterioles. The choroidal appearance is similar in the control and ROP fundi. (b) Sample ERG responses to full-field stimuli [43] in control and ROP rats. Both rats were tested with the same flash intensities, as indicated. The vertical gray lines indicate the time at which the flash was presented
Arteriolar integrated curvature (IC A) and rod photoreceptor sensitivity (SROD) in infants with a history of ROP [13–15, 44] and in rat models [5], plotted as percent of normal for age (SEM). In both the human and animal subjects, mean IC A is nearly two times higher in ROP, and rod sensitivity (SROD) is reduced by ∼25%
We use albino rat models of ROP to study the neural and vascular characteristics of the retina during development [5, 6, 8, 9]. Different schedules of oxygen exposure induce a range of effects on the retinal vasculature and the neural retina that model the gamut of retinopathy, mild to severe, observed in human ROP cases. As shown in Fig. 5, the oxygen exposures are timed to impact the retina during the ages when the rod outer segments are elongating and the rhodopsin content of the retina is increasing. Longitudinal measures of electroretinographic (ERG) responses and retinal vascular features are obtained in infant (∼20 days old), adolescent (∼30 days old), and adult (∼60 days old) rats. Our results from longitudinal investigations in ROP rats suggest two retinal targets for drug therapies.
Rat models of ROP. Two models, the “50/10 model” and the “75 model,” are induced by exposing infant rats to alternating periods of relatively high and low ambient oxygen; room air is 21%. For both models the exposures are delivered at ages during which the rod photoreceptors are immature, as indicated by the low rhodopsin content. ERGs and fundus images are obtained longitudinally in infant (20 ± 1 days old), adolescent (30 ± 1 days old), and adult (60 ± 1 days old) rats (gray bars)
Assessment of neural function
We use the ERG to characterize neural function. ERG responses to full-field stimuli over a range of intensities are recorded from the dark-adapted animal as previously described in detail [5]. To summarize rod photoreceptor activity, a model of the activation of phototransduction is fit to the a-waves [17–20] and the resulting sensitivity (SROD) and saturated amplitude (RROD) parameters are calculated. Postreceptor activity is represented by the b-wave. The stimulus/response functions are summarized by the saturated amplitude (Vmax) and the stimulus producing a half-maximum response (log σ); these parameters are derived from the Michaelis–Menten function fit to the b-wave amplitudes [5]. Although we find significant deficits in the amplitude parameters (RROD and Vmax), it is the sensitivity parameters (SROD and log σ) that, as described in the following sections, direct us to the postulated intervention sites.
Assessment of vascular characteristics
We derive retinal vascular parameters using image analysis software applied to digital fundus photographs [5, 21, 22]. Integrated curvature (IC), which agrees well with subjective assessment of vascular tortuosity reported by experienced clinicians [14], is used to specify the vascular status of each fundus. Both arterioles and venules are significantly affected by ROP. We find, however, that the arterioles are markedly affected, while the venules are less so [5, 9, 14], and therefore use the arteriolar parameter IC A in our analyses.
Relation of retinal sensitivity and vasculature
Rod photoreceptor sensitivity (SROD) at a young age (20 days) predicts retinal vascular outcome as specified by IC A . Better sensitivity at an early age is associated with better (less tortuous) vascular outcome (Fig. 6) [5]. After cessation of the inducing oxygen exposure, recovery of postreceptor neural retinal sensitivity (b-wave log σ) and decrease of vascular tortuosity occur hand in hand (Fig. 7). However, at a given age, sensitivity and vascular status are not correlated. Thus, simple compromise of vascular supply to the postreceptor neurons is, at most, an incomplete explanation for the low sensitivity. We note that the regulation of developing retinal neurons and blood vessels is complex, being under the cooperative control of several growth factors, such as vascular endothelial growth factor (VEGF), semaphorin, and their neuropilin receptors [23]. In rat models of ROP, expression of these growth factors is altered [24].
Arteriolar curvature (IC A) and photoreceptor sensitivity (SROD) in 50/10 model, 75 model, and control rats expressed as a proportion of the mean in controls (dotted lines). For each subject, IC A measured at ∼60 days is plotted as a function of that subject’s SROD measured earlier, at ∼20 days. The solid line is a linear regression through all the data. Early rod sensitivity predicted vascular outcome (r = −0.332; P = 0.024). Adapted from Fig. 8, Akula et al. [5]; copyright of Association for Research in Vision and Ophthalmology
Mean (SEM) rate of change in postreceptor sensitivity (log σ) and arteriolar curvature (IC A) for 50/10 model, 75 model, and control rats. Adapted from Fig. 7, Akula et al. [5]; copyright of Association for Research in Vision and Ophthalmology
Identification of targets for pharmaceutical interventions
The longitudinal data from rat models of ROP identify two targets for pharmaceutical intervention: (1) the immature photoreceptors and (2) the molecular cross talk between neurons and retinal vasculature. We outline the rationale for each.
VEGF promotes development of retinal vasculature. Hypoxia promotes expression of VEGF. The rods are the most demanding of aerobic energy of any cells in the body [1]. Thus, rod-instigated hypoxia must lead to up-regulation of growth factors that promote retinal vascular development. Low rod sensitivity at an early age predicts the poorer vascular outcome at older ages (Fig. 6). Because low rod sensitivity appears to be indicative of early injury to the rod [3, 11], therapy designed to relieve the immature rod of its burgeoning oxygen-based energy requirements should be beneficial. It is at the ages during which the developing rod outer segments elongate with consequent escalation of energy demands that ROP has its onset (Fig. 2). Arden et al. [25] proposed treatment with light to reduce the ROP rods’ energy requirements by suppressing the circulating current. Light has a mild beneficial effect on a rat model of ROP [26]. Careful regulation of supplemental oxygen so that arterial oxygen levels are neither too high nor too low may also have some beneficial effect on ROP [27–30]. One can argue that optimal timing for light and oxygen interventions has yet to be specified, but, to date, adjustments in light and oxygen have not been a panacea for ROP. Thus, pharmaceutical approaches are warranted. We theorize that improved management, or, better yet, prevention of ROP may be achieved through timely pharmaceutical suppression of rods’ energy demanding processes such as turnover of outer segment material [31] and generation of the circulating current [19].
The second target for pharmaceutical intervention, the molecular cross talk between the retinal vasculature and postreceptor neurons, deserves attention first because abnormal retinal vasculature provides the usual clinical definition of ROP, and also because the postreceptor neurons have a great capacity to remodel [32] in the face of irreversible damage to the photoreceptors [33]. Damage to photoreceptors in ROP may be unavoidable as there is evidence of persistent damage to the rods even in mild cases [13, 34]. Conceivably, treatment of the retinal vasculature could result in improved visual function (e.g., contrast sensitivity) by an associated beneficial effect on the postreceptor neurons. However, treatment of ROP by manipulating growth factors must be approached with considerable caution because the same growth factors regulate the developing photoreceptors and postreceptor retinal neurons [35, 36]. Indeed, particular caution in use of anti-VEGF treatment in immature retina has been advised specifically because of the potential for adverse effects on the developing neurons [37]. Further knowledge about neurovascular interactions in the immature retina, coupled with analysis of the function of those networks, is essential to delineate the potential therapeutic role of growth factors in ROP.
Conclusions
A systems biology approach is applicable not only to animal models but also to the human retina. Such an approach is nicely supported by the combination of full-field ERG analysis of the neural retina and image analysis of the retinal vasculature. Using the multifocal ERG and high resolution optical coherence tomography (OCT), we are now also applying the systems approach to study of disease of the macula in ROP [38, 39]. In summary, the perspective that we illustrate by study of ROP may also yield novel perspectives on other hypoxic ischemic retinal disorders, including diabetic retinopathy and age related macular degeneration, diseases which are characterized by both vascular and neural abnormalities of the retina.
References
Steinberg R (1987) Monitoring communications between photoreceptors and pigment epithelial cells: effects of “mild” systemic hypoxia. Invest Ophthalmol Vis Sci 28:1888–1903
Hansen RM, Eklund SE, Benador IY, Mocko JA, Akula JD, Yao L, Martinez-Perez ME, Fulton AB (2008) Retinal degeneration in children: dark adapted visual threshold and arteriolar diameter. Vis Res 48:325–331
Fulton AB, Reynaud X, Hansen RM, Lemere CA, Parker C, Williams TP (1999) Rod photoreceptors in infant rats with a history of oxygen exposure. Invest Ophthalmol Vis Sci 40:168–174
Wellard J, Lee D, Valter K, Stone J (2005) Photoreceptors in the rat retina are specifically vulnerable to both hypoxia and hyperoxia. Vis Neurosci 22:501–507
Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB (2007) Rod photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest Ophthalmol Vis Sci 48:4351–4359
Akula JD, Mocko JA, Moskowitz A, Hansen RM, Fulton AB (2007) The oscillatory potentials of the dark-adapted electroretinogram in retinopathy of prematurity. Invest Ophthalmol Vis Sci 48:5788–5797
Dembinska O, Rojas L, Varma D, Chemtob S, Lachapelle P (2001) Graded contribution of retinal maturation to the development of oxygen induced retinopathy in rats. Invest Ophthalmol Vis Sci 42:1111–1118
Liu K, Akula JD, Hansen RM, Moskowitz A, Kleinman MS, Fulton AB (2006) Development of the electroretinographic oscillatory potentials in normal and ROP rats. Invest Ophthalmol Vis Sci 47:5447–5452
Liu K, Akula JD, Falk C, Hansen RM, Fulton AB (2006) The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 47:2639–2647
Penn JS, Henry MM, Wall PT, Tolman BL (1995) The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci 36:2063–2070
Reynaud X, Hansen RM, Fulton AB (1995) Effect of prior oxygen exposure on the electroretinographic responses of infant rats. Invest Ophthalmol Vis Sci 36:2071–2079
Barnaby AM, Hansen RM, Moskowitz A, Fulton AB (2007) Development of scotopic visual thresholds in retinopathy of prematurity. Invest Ophthalmol Vis Sci 48:4854–4860
Fulton AB, Hansen RM, Petersen RA, Vanderveen DK (2001) The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol 119:499–505
Gelman R, Martinez-Perez M, Vanderveen D, Moskowitz A, Fulton A (2005) Diagnosis of plus disease in retinopathy of prematurity using retinal image multi-scale analysis (RISA). Invest Ophthalmol Vis Sci 46(12):4734–4738
Moskowitz A, Hansen RM, Fulton AB (2005) Early ametropia and rod cell function in retinopathy of prematurity. Optom Vis Sci 82:307–317
Fulton AB, Hansen RM, Moskowitz A (2008) The cone electroretinogram in retinopathy of prematurity. Invest Ophthalmol Vis Sci 49:814–819
Hood DC, Birch DG (1994) Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci 35:2948–2961
Lamb TD, Pugh ENJ (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol (Lond) 449:719–758
Pugh EN Jr, Lamb TD (1993) Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta 1141:111–149
Pugh EN Jr, Lamb TD (2000) Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga DG, de Grip WJ, Pugh EN Jr (eds) Handbook of biological physics, vol 3. Molecular mechanisms of visual transduction. Elsevier Science, pp 183–255
Martinez-Perez ME (2001) Computer analysis of the geometry of the retinal vasculature. Imperial College, London
Martinez-Perez ME, Hughes AD, Stanton AV, Thom SA, Chapman N, Bharath AA, Parker KH (2002) Retinal vascular tree morphology: a semi-automatic quantification. IEEE Trans Biomed Eng 49:912–917
Gariano RF, Hu D, Helms J (2006) Expression of angiogenesis-related genes during retinal development. Gene Expr Patterns 6:187–192
Mocko JA, Akula JD, Benador IY, DiNardo A, Hansen RM, Fulton AB (2008) Expression of ‘neural’ growth factors directs angiogenesis early in the course of ROP. ARVO Abstract
Arden GB, Sidman RL, Arap W, Schlingemann RO (2005) Spare the rod and spoil the eye. Brit J Ophthalmol 89:764–769
Fulton AB, Hansen RM, Roberto K, Penn JS (1998) Persistent dysfunction in a rat model of ROP. Invest Ophthalmol Vis Sci 39:S820
Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM (2003) Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med 349:959–967
Vanderveen DK, Mansfield TA, Eichenwald EC (2006) Lower oxygen saturation alarm limits decrease the severity of retinopathy of prematurity. J AAPOS 10:445–448
Chow LC, Wright KW, Sola A (2003) Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics 111:339–345
Saugstad OD (2006) Oxygen and retinopathy of prematurity. J Perinatol 26 Suppl 1:S46–50, discussion S63–4
Tamai M, Mizuno K, Chader GJ (1982) In vitro studies on shedding and phagocytosis of rod outer segments in the rat retina: effect of oxygen concentration. Invest Ophthalmol Vis Sci 22:439–448
Jones BW, Watt CB, Frederick JM, Baehr W, Chen C, Levine EM, Milam AH, Lavail MM, Marc RE (2003) Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol 464:1–16
Lu M, Hansen RM, Cunningham MJ, Eklund SE, Fulton AB (2007) Effects of desferoxamine on retinal and visual function. Arch Ophthalmol 125:1581–1582
Reisner DS, Hansen RM, Findl O, Petersen RA, Fulton AB (1997) Dark adapted thresholds in children with histories of mild retinopathy of prematurity. Invest Ophthalmol Vis Sci 38:1175–1183
Yourey PA, Gohari S, Su JL, Alderson RF (2000) Vascular endothelial cell growth factors promote the in vitro development of rat photoreceptor cells. J Neurosci 20:6781–6788
Hashimoto T, Zhang XM, Chen BY, Yang XJ (2006) VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development 133:2201–2210
Nishiguchi KM, Nakamura M, Kaneko H, Kachi S, Terasaki H (2007) The role of VEGF and VEGFR2/Flk1 in proliferation of retinal progenitor cells in murine retinal degeneration. Invest Ophthalmol Vis Sci 48:4315–4320
Fulton AB, Hansen RM, Moskowitz A, Barnaby AM (2005) Multifocal ERG in subjects with a history of retinopathy of prematurity. Doc Ophthalmol 111:7–13
Hammer DX, Iftimia NV, Ferguson RD, Bigelow CE, Ustun TE, Barnaby AM, Fulton AB (2008) Foveal fine structure in retinopathy of prematurity: an adaptive optics fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci. doi:10.1167/iovs.07-1228
Fulton AB, Dodge J, Hansen RM, Williams TP (1999) The rhodopsin content of human eyes. Invest Ophthalmol Vis Sci 40:1878–1883
Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB, Tung B (1991) Incidence and early course of retinopathy of prematurity. Ophthalmology 98:1628–1640
Seeliger MW, Beck SC, Pereyra-Munoz N, Dangel S, Tsai JY, Luhmann UF, van de Pavert SA, Wijnholds J, Samardzija M, Wenzel A, Zrenner E, Narfstrom K, Fahl E, Tanimoto N, Acar N, Tonagel F (2005) In vivo confocal imaging of the retina in animal models using scanning laser ophthalmoscopy. Vis Res 45:3512–3519
Seeliger MW, Grimm C, Stahlberg F, Friedburg C, Jaissle G, Zrenner E, Guo H, Reme CE, Humphries P, Hofmann F, Biel M, Fariss RN, Redmond TM, Wenzel A (2001) New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet 29:70–74
Fulton AB, Hansen RM (2006) Rod photoreceptor function in ROP. Mol Vis 12:548–549
Acknowledgments
This work was supported by grants from The National Institutes of Health (EY 10597), The Massachusetts Lions Eye Research Fund, Fight for Sight, The Knights Templar, The March of Dimes (6-FY06-330), European Community Grant IP LSHG-CT-2005-512036 `EVI-Genoret,’ and The Ministry of Science, Research and the Arts, Baden-Wuerttemberg, Germany (NeME).
Author information
Authors and Affiliations
Corresponding author
Additional information
While categorized as a review article, this paper presents the authors’ perspective on potential pharmaceutical interventions for retinal degenerative and hypoxic ischemic retinal diseases; it is not offered as a review of research into these conditions.
Rights and permissions
About this article
Cite this article
Fulton, A.B., Akula, J.D., Mocko, J.A. et al. Retinal degenerative and hypoxic ischemic disease. Doc Ophthalmol 118, 55–61 (2009). https://doi.org/10.1007/s10633-008-9127-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-008-9127-8