Abstract
Purpose
To describe the deficits in four electroretinography (ERG) modalities in patients with neovascular age-related macular degeneration (AMD). To describe the changes in these parameters during a course of verteporfin photodynamic therapy (PDT).
Methods
Pattern (PERG), multifocal (mfERG) (19 segment simplified test protocol), flash ERG and flicker ERG were performed in patients with active neovascular AMD before PDT and compared to fellow eye controls using paired t-tests. Changes in ERG parameters during the 12 month treatment course were visualised using 95% confidence intervals of the median difference. The statistical significance of any changes was quantified using Wilcoxon signed ranks tests.
Results
Fifty patients were recruited and followed. At presentation all ERG amplitudes were reduced with greater reductions in focal as opposed to global test protocols (P < 0.05). Over the 12 month course of PDT, PERG P50 amplitude showed a general downward trend and latency remained unchanged. mfERG p1 amplitude density showed an upward trend at six months before returning to baseline by 12 months. mfERG ring 2 amplitude density was significantly increased at 12 months compared to baseline (P = 0.010). Flicker ERG latency was significantly increased at six months compared to baseline (P = 0.015).
Discussion
The simplified mfERG protocol was tolerated by this patient group, however, they found the full test protocol demanding. Large deficits in the retinal ERG function occur in neovascular AMD and involve retinal locations adjacent to as well as overlying choroidal neovascularisation (CNV). After PDT there is an improvement in electro-retinal function in retinal locations overlying the CNV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neovascular age-related macular degeneration (AMD) is characterised by the development of new blood vessels in the choroid (choroidal neovascularisation (CNV)) breaking through Bruch’s membrane and leaking blood, fluid, and lipids underneath and into the neuroretina. The most rapid deterioration in vision occurs when CNV involves the foveal centre.
The Treatment of AMD with Photodynamic therapy (PDT) study (TAP study) has shown that PDT with verteporfin limits visual loss from CNV [1–6]. The study investigated visual function using visual acuity (VA) and contrast sensitivity (CS) and described a wide variation in response during a course of treatment. The mechanisms of visual loss and response are poorly understood and there is a need to develop additional techniques to investigate the disease and its treatment.
Electroretinography (ERG) is one measure of central visual function. There have been previous studies on the effect of ageing [7–11] and AMD [12–15] on the ERG and the effect of PDT treatment for neovascular AMD on the multifocal electroretinogram (mfERG) [16–19]. These studies have used variable techniques, frequently with time consuming test protocols which can be challenging for elderly patients with poor central vision. The most recent study by Neveu et al. [20] observed PERG and mfERG improvements over two years in a group of bilateral neovascular AMD patients treated with PDT. We studied the deficits in responses to four ERG test modalities and their changes during a course of PDT in a group with both bilateral and unilateral cases.
PDT is a combined drug and laser treatment that limits the visual loss from CNV. Verteporfin is infused for ten minutes followed five minutes later by the application of a 689 nm diode laser through a slit lamp delivery system. Verteporfin has an absorption peak at 693 nm and on activation by the laser is thought to cause selective non-thermal photothrombosis [21].
Subjects and methods
Patient recruitment and preparation
Local ethics committee approval was given and all patients attending a regional PDT clinic with untreated active neovascular AMD comprising ≥50% classic CNV in one or both eyes and VA better than 20/200 (35 letters) were invited to participate in the study. The study was performed in accordance with the tenets of the Declaration of Helsinki. One subject with Parkinson’s disease and one substance abuser were excluded from the study and recruitment was stopped at 50 to allow follow-up assessments to be performed at three, six, and twelve months.
At each visit to the PDT clinic patients underwent refraction protocol VA assessment using the ETDRS chart, and the value at 1 meter recorded, CS using the Pelli-Robson chart [2], ERG and fundus fluorescein angiography (FFA) before PDT. Treatment was performed after the tests on the first visit and on subsequent visits if the CNV lesion was deemed active on clinical and angiographic criteria. ERGs were performed binocularly and recorded from each eye with DTL thread electrodes referenced to each outer canthus and a ground electrode placed at Fpz (middle of forehead). If fellow eyes had acuity of 6/9 or better and no clinical or angiographic evidence of choroidal neovascularisation they were used as controls. Patients wore their own spectacle correction if it aided fixation, or if refractive error was ≥6 diopters [22].
ERG recording
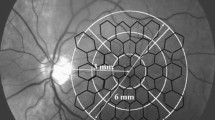
The Roland Consult Retiscan system was used for both stimulation and acquisition of all ERGs. PERGs were acquired bandpass filtered 1–100 Hz in response to 50′ checks of maximum photopic luminance 120 cd/m2 reversing four times a second. The stimulus field size was 17°× 23°. A drop of 0.4% benoxinate was used if necessary. Pupils were then maximally dilated using 2.5% phenylephrine and 1% tropicamide. mfERGs were acquired bandpass filtered 2–300 Hz in response to an array of 19 hexagons scaled 4:1 grouped in three rings (radii 2.7°, 2.7–10.8°, 10.8–21.8°) as in Fig. 1. Flashes of photopic 2.0 cd.s/m2 were delivered using a CRT monitor at 60 Hz with four blank frames between each step of a short pseudo-random sequence [22]. A fixation cross that extended to all four corners of the screen facilitated central fixation. The patient was also coached for steady fixation and observed throughout the test. Full field flash ERGs (1–300 Hz) and 30 Hz flicker ERGs (5–100 Hz) were acquired in response to xenon flashes of photopic luminance 3.0 cd.s/m2 against a background light of 34 cd/m2 inside a Ganzfeld bowl.
ERG signal processing
PERGs were digitally filtered 0–50 Hz or 1–50 Hz in the presence of a blink artefact. mfERGs were filtered 0–80 Hz, averaged into three concentric rings containing one, six and 12 segments respectively (Fig. 1). Where it was not possible to identify mfERGs and PERGs they were deemed too small to be recorded and we imputed an amplitude value half as large as the smallest recordable amplitude. For latency we imputed the group mean value. Flash ERGs were high-pass filtered at 1 Hz.
Statistical analysis
A subgroup of thirteen patients had their fellow eyes included in a control group. The mean difference between these thirteen fellow eyes and their corresponding AMD eyes was calculated for each ERG parameter and a paired t-test used to identify statistically significant differences. For those patients who were seen on four consecutive occasions, 95% confidence intervals of the median change from baseline were used to visualise changes over the course of treatment. Any clear changes had significance quantified using Wilcoxon signed ranks tests.
Results
Demography
Table 1 describes the details of the patient and age matched control groups. Most patients had classic no occult subfoveal CNV and 96% (25/26) of those who attended had a second PDT treatment at three months. Pre-treatment ERGs in affected and control fellow eyes are summarised in Fig. 2.
PERG
The mean difference in PERG P50 and N95 amplitudes were each 1.7 μV (t = −4.83, P = 0.01 and t = −3.92, P = 0.01) when AMD eyes were compared to controls. The mean difference in P50 latency was 6.7 ms (t = 2.10, P = 0.09). The PERG amplitude and latency at baseline recording and the succeeding assessment months were summarised as independent groups (Table 2). No statistically significant changes with treatment were observed, however the P50 component appeared to reduce in amplitude and latency during the course of treatment.
MfERG
The mean deficit in mfERG central segment, ring 2 and ring 3 amplitude density was 59.7 nV/degree2, 13.6 nV/degree2 and 3.2 nV/degree2 respectively (t = −4.86, P < 0.01; t = −2.99, P = 0.01; t = −2.95, P = 0.01) when AMD eyes were compared to controls. We did not find a statistically significant difference in latency for any ring; there may have been a small difference that this comparison was simply underpowered to show. For complete cases (n = 12) there was a trend to increase in mfERG amplitude density for the central segment at six months with a return to baseline at twelve months. Ring 2 was significantly larger than baseline at 12 months (z = −2.59, P = 0.01) (Fig. 3b). Ring 3 showed no significant difference to baseline at any follow up visit. Amplitude density remained smaller than normal in all three rings at twelve months. No statistically significant delays in latency were observed for any ring over the course of treatment. Latency remained shorter than normal in all three rings at twelve months.
Flash and flicker ERG
The mean difference in amplitude was 11.5 μV and 10.0 μV for flash and flicker ERGs respectively (t = −2.1, P = 0.07; t = −2.31, P = 0.05) (Figs. 2 and 4). There was no statistically significant difference in latency for either stimulus. We did not show any statistically significant change in flash or flicker ERG amplitude during treatment, however flicker ERG latency was increased by a median of 2 ms at six months (z = −2.43, P = 0.02). There were no changes in flash ERG latency.
Discussion
Pre-treatment
Our study shows deficits in ERG responses at presentation to a wide range of stimuli when compared to control fellow eyes. Our 19-segment stimulus and recording protocol has been validated [23] and its findings support those of others. Our study also extends the evidence across other stimuli (Figs. 2 and 4). Previous studies report amplitude reductions in the central 40 degree field [12, 13, 15, 24–26] and latency increases across 120 degrees of the field [27]. For global stimuli (flash, flicker) there was an approximate 10% reduction in amplitude, not detected by previous work [28]. However deficits were more profound for the focal stimuli we studied, especially the central segment of the mfERG. Our findings of mfERG deficits in ring 2 and especially ring 3 show that the effect on the retina extends beyond the retina overlying the CNV.
We have shown that mfERGs are feasible in this population and that affected eyes show amplitude deficits across the macula when compared with their fellow eye. Patients with neovascular AMD have scotomas in the central visual field making fixation unstable or eccentric depending on the stage of the disease [29]. Fixation monitoring goes some way to addressing this problem, however, topographic integrity of responses cannot be guaranteed [30, 31]. Responses of low amplitude mean that signal to noise ratio is also prohibitively small in these patients. We chose a 19 segment stimulus over the central 42 degree diameter field to maximise signal to noise ratio and believe that this is more appropriate than a higher resolution stimulus for patients with severely reduced central vision. Elsner et al. [32] used microperimetry to measure fixation area in a similar patient group and from their results it can be postulated that by six months fixation is only occasionally outside the central segment. We did not observe fixation that was systematically outside the central segment during any of our mfERG recordings.
The mechanism of visual loss in neovascular AMD is unclear but many factors have been implicated. Our findings show profound photoreceptor dysfunction on mfERG, flash and flicker ERGs while ganglion cell function on PERG was relatively preserved. The deficits in ERG b-wave and mfERG p1 response in our patients reflect bipolar and other second order neurone dysfunction. Muller cell integrity is also an important aspect of ERG b-wave and mfERG p1 response which is affected in the later stages of the disease when cystoid macular edema develops. ERG testing may help determine groups of patients with better prognosis and shed further light on underlying disease processes.
Changes after PDT
Our results show that during a course of PDT the deficits seen in the ERG remain largely unchanged. We did detect some significant improvements (mfERG ring 2 amplitude density), trend improvements (ring 1 amplitude density) and significant deficits (flicker ERG latency) after treatment. Not all of the original 50 subjects completed all four testing sessions; they gave long test procedures as their reason for not attending. This fits with the intermittent nature of our missing data and suggests that no bias was present. Conversely, if there was a common reason why the 12 patients who attended four consecutive sessions did so then it was likely to be that they felt positive about PDT and had responded relatively well. This may positively bias the data.
Consideration of clinical factors
Mennel et al. [33] reported increased lesion thickness and an accumulation of sub-retinal fluid on optical coherence tomography (OCT) in the days after PDT. The presence of fluid resulted in hypemetropia, however pre-treatment VA was regained by increasing the refractive correction. After one week, VA had further improved with this new refractive correction [ibid] and presumably some re-absorption of fluid. Sub-retinal fluid (SRF) is re-absorbed at different rates in different subjects but usually commences one week after PDT [19, 34]. Transient losses in VA following PDT were also shown in the TAP and VIP studies. MfERG amplitude deficits have been found one week after treatment [19], but were not considered to be related to hypermetropia because of their persistence with the new refractive correction. The presence of cystoid macular oedema relates to both the stage of the disease and the lesion characteristics, the effects of which are discussed in a separate paper in preparation.
Comparison to similar studies
The respective patient groups of Palmowski et al. [17] and Moschos et al. [16] had better and poorer VA than ours at baseline, which may explain the respective mfERG increases and decreases their studies showed. In agreement, Lai et al. [19] showed a transient mfERG amplitude density deficit then recovery by one month in a patient group with significantly better baseline VA than ours. The majority of patients studied by Neveu et al. [20] had unilateral disease, compared to our bilaterally affected group (Table 1). Their group also had slightly better baseline VA and both factors may contribute to the larger positive treatment effects they observed, particularly on the PERG. It is also noteworthy that our study group had a higher proportion of classic no occult lesions than most other studies.
The studies of Palmowski et al. [17] and Moschos et al. [16] both described scalar product (an index of cross-correlation) amplitudes using the patients’ global response as the template. Both response amplitude and latency contribute to this template. This may result in a small and late local response looking larger than it actually was. Comparisons between months are also variable as the template is constantly changing as well as the response.
Conclusions
We have found that we can follow neovascular AMD patients feasibly with our reduced test protocol. We have observed deficits in all ERGs in these patients and improvements in selected parameters over 12 months of treatment with PDT. The effect of CNV on the overlying retina is profound and spreads beyond the geographical location of the CNV.
Studying the mfERG using a simplified test protocol with consequent better follow-up may provide useful insights into the mechanisms of current and future therapies for AMD.
References
Rubin GS, Bressler NM (2002) Effects of Verteporfin therapy on contrast sensitivity – Results from the treatment of age-related macular degeneration with Photodynamic Therapy (TAP) investigation – TAP Report No. 4. Retina 22:536–544
TAP Study Group (1999) Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with verteporfin-TAP Report 1. Arch Ophthalmol 117:1329–1345
TAP Study Group (2001) Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with Verteporfin-TAP Report 2. Arch Ophthalmol 119:198–207
TAP Study Group (2002) Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with Verteporfin-TAP Report 3. Arch Ophthalmol 120:1443–1454
TAP Study Group (2002) Photodynamic therapy of subfoveal choroidal neovascularisation in age-related macular degeneration with Verteporfin-TAP Report 5. Arch Ophthalmol 120:1307–1314
TAP Study Group (2003) Improvement after Verteporfin therapy. Arch Ophthalmol 121:415
Celesia GG, Cone S, Kaufman D (1986) Effects of age and sex on pattern electroretinograms and and visual evoked potentials. Electroenceph Clin Neurophysiol 64:29
Jackson GR, Ortega JD, Girkin C et al (2002) Ageing-related changes in the multifocal electroretinogram. J Opt Soc Am A 19:185–189
Jackson GR, Leon-Ortega J, Rosenstiel CE et al (2001) Age-related changes in the multifocal electroretinogram of central vision. Invest Ophthalmol Vis Sci 42(4):381
Wright C, William D, Drasdo N et al (1985) The influence of age on the electroretinogram and visual evoked potential. Doc Ophthalmol 59:365–384
Seiple W, Vajaranant T, Szlyk J et al (2003) Multifocal electroretinography as a function of age: the importance of normative values for older adults. Invest Ophthalmol Vis Sci 44:1783–1792
Heinemann-Vernaleken B, Palmowski A, Allgayer R et al (2001) Comparison of different high resolution multifocal electroretinogram recordings in patients with age-related maculopathy. Graef Arch Clin Exp Ophthalmol 239:556–561
Huang S, Wu D, Jiang F et al (2001) The multifocal electroretinogram in age-related maculopathies. Doc Ophthalmol 101:115–124
Li J, Tso MOM, Lam TT (2001) Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br J Ophthalmol 85(3):287–290
Jurklies B, Weismann M, Husing J et al (2002) Monitoring retinal function in neovascular maculopathy using multifocal electroretinography- early and long-term correlation with clinical findings. Graef Arch Clin Exp Ophthalmol 240:244–264
Moschos MM, Panayotidis D, Theodossiadis G et al (2004) Assessment of macular function by multifocal electroretinogram in age-related macular degeneration before and after photodynamic therapy. J Fr Ophthalmol 27:1001–1006
Palmowski AM, Allgayer R, Heinemann-Vernaleken B et al (2002) Influence of photodynamic therapy in choroidal neovascularization on focal retinal function assessed with the multifocal electroretinogram and perimetry. Ophthalmology 109:1788–1792
Jiang L, Jin C, Wen F et al (2003) The changes of multifocal electroretinography in the early stage of photodynamic therapy for choroidal neovascularisation. Doc Ophthalmol 107(2):165–170
Lai TYY, Chan W, Lam D (2004) Transient reduction in retinal function revealed by multifocal electroretinogram after photodynamic therapy. Am J Ophthalmol 137:826–833
Neveu MM, Tufail A, Dowler JG, Holder GE (2006) A comparison of pattern and multifocal electroretinography in the evaluation of age-related macular degeneration and its treatment with photodynamic therapy. Doc Ophthalmol 113(2):71–81
Michels S, Schmidt-Erfurth U (2003) Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci 44:2147–2154
Marmor M, Hood DC, Keating D et al (2003) Guidelines for basic multifocal electroretinography (mfERG). Doc Ophthalmol 106:105–115
Hagan RP, Fisher AC, Brown MC (2006) Examination of short binary sequences for mfERG recording. Doc Ophthalmol 113(1):21–27
Heinemann-Vernaleken B, Palmowski AM, Allgayer R, Jurklies B (1999) Comparison of different high resolution multifocal ERG (MF-ERG) recordings in age related macular degeneration (AMD). Invest Ophthalmol Vis Sci 40(4):3773
Jurklies B, Weismann M, Bornfeld N (2000) Multifocal ERG in age-related macular degeneration with hard and soft drusen. Invest Ophthalmol Vis Sci 41(4):4749
Jurklies B, Weismann M, Bornfeld N (1999) Multifocal electretinography in age-related macular degeneration- changes in amplitude of the 1st order kernel. Invest Ophthalmol Vis Sci 40(4):1668
Parks S, Keating D, Evans AL (2000) Peripheral retinal dysfunction in Age-related macular degeneration. Invest Ophthalmol Vis Sci 41(4):847
Holopigian K, Seiple W, Greenstein V et al (1997) Relative effects of aging and age-related macular degeneration on peripheral visual function. Optom Vis Sci 74:152–159
Schmidt-Erfurth UM, Elsner H, Terai N et al (2004) Effects of verteporfin on central visual field function. Ophthalmology 111:931–939
Jurklies B, Sutter E (2001) Reply to G. Rudolph and P. Kalpadakis: the role of fixation for reliable mfERG results. Graef Arch Clin Exp Ophthalmol 240:876
Rudolph G, Kalpadakis P (2002) The role of fixation for reliable mfERG results. Graef Arch Clin Exp Ophthalmol 240:874–875
Elsner H, Schmidt-Erfurth UM (2004) Analysis of fixation results after photodynamic therapy of subfoveal choroidal neovascularisations. Ophthalmologe 99:620–624
Mennel S, Meyer CH (2005) Transient visual disturbance after photodynamic therapy. Am J Ophthalmol 139:748–749
Rogers AH, Martidis A, Greenberg PB et al (2002) Optical coherence tomography findings following photodynamic therapy of choroidal neovascularization. Am J Ophthalmol 134:566–576
Acknowledgements
The authors would like to thank the Royal Liverpool University Hospital Research and Development Fund and the St. Paul’s Eye Appeal for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mackay, A.M., Brown, M.C., Hagan, R.P. et al. Deficits in the electroretinogram in neovascular age-related macular degeneration and changes during photodynamic therapy. Doc Ophthalmol 115, 69–76 (2007). https://doi.org/10.1007/s10633-007-9056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-007-9056-y