Abstract
Background
Chronic inflammation disrupts the colonic epithelial layer in patients afflicted by ulcerative colitis (UC). The use of inhibitors of glycogen synthase kinase three beta (GSK3β) has proven efficacious to mitigate disease symptoms in rodent models of UC by reducing the pro-inflammatory response. Less is known about whether these inhibitors promote colonic regeneration by stimulating proliferation of colonic epithelial cells.
Aims
We investigated whether delivery of the GSK3β inhibitor, lithium chloride (LiCl), during the recovery period from acute DSS-induced colitis in mice promoted colonic regeneration and ameliorated disease symptoms. We also tested whether the c-MYC transcription factor (MYC) was involved in this response.
Methods
Acute colitis was induced by administration of 2.5 % dextran sodium sulfate (DSS) to wild-type C57BL/6 mice for 5 days. During the recovery period, mice received a daily intraperitoneal (IP) injection of LiCl or 1X PBS as a control. Mice were weighed, colon lengths measured, disease activity index (DAI) scores were assessed, and histological analyses were performed on colonic sections. We analyzed transcripts and proteins in purified preparations of the colonic epithelium. We delivered the MYC inhibitor 10058-F4 via IP injection to assess the role of MYC in colonic regeneration.
Results
Lithium treatments promoted recovery from acute DSS-induced damage by increasing expression of Myc transcripts, MYC proteins, and expression of a subset of Wnt/MYC target genes in the colonic epithelium. Inhibiting MYC function with 10058-F4 blunted the lithium response.
Conclusions
By inducing Myc expression in the colonic epithelium, lithium promotes colonic regeneration after DSS-induced colitis. Therefore, the use of lithium may be of therapeutic value to manage individuals afflicted by UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a form of inflammatory bowel disease (IBD) that is characterized by chronic inflammation of the colon [1]. In individuals afflicted with UC, a deterioration of the colonic mucosa leads to an inappropriate immune response against commensal microflora [2]. Depending on the severity of the disease, patients are treated with aminosalicylates, immunosuppressive agents such as anti-tumor necrosis factor alpha (TNF-α) antibodies, and/or glucocorticosteroids [3, 4]. Even when effective management is achieved and the disease goes into remission, nearly 70 % of patients suffer a disease relapse, and up to 40 % of UC patients will ultimately undergo a colectomy at some point in their lifetimes [5]. Clearly there is an urgent need to develop better treatment options for individuals suffering from UC.
In the colon, a single layer of epithelial cells protects underlying tissues from the toxic contents of the lumen and enteric bacteria [6]. These cells are arranged in a smooth surface marked by invaginations, or crypts, and the entire epithelial layer is replaced every five or six days making the colon one of the most rapidly regenerating organs in the body [6, 7]. This level of regeneration requires a constant cycle of cellular proliferation, which is driven by the Wnt/β-catenin signaling pathway [8]. Glycogen synthase kinase three beta (GSK3β) is a central regulatory protein in the Wnt/β-catenin pathway, and this kinase resides in a cytoplasmic “destruction complex” of proteins that controls cellular levels of the β-catenin transcriptional co-activator [8]. In the absence of Wnt, phosphorylation of β-catenin by GSK3β targets it for degradation via the proteasome. In the presence of Wnt, the destruction complex is inactivated, and β-catenin translocates into the nucleus where it binds T cell factor/Lymphoid-enhancer factor (TCF/Lef; hereafter TCF) sequence specific transcription factors [9]. β-Catenin/TCF complexes associate with Wnt responsive DNA elements (WREs) and activate expression of target genes including the c-Myc proto-oncogene (Myc) [10–12]. While the Wnt/MYC axis is involved in regenerating the colonic epithelium following γ-irradiation [13], less is known about whether this pathway plays a role in repairing damage caused by UC.
In previous work, we identified a MYC 3′ WRE that maps 1.4-kb downstream from the MYC transcription stop site [12, 14]. Mice harboring a germ line deletion of this element expressed 2.5-fold higher levels of MYC protein in their colons, demonstrating that the physiological role of the Myc 3′ WRE in this tissue is to repress Myc gene expression [15]. As a consequence of elevated MYC, the colonic crypts of Myc 3′ WRE−/− mice contained an expansion of the proliferative compartment and a reduction in differentiated cells comprising the colonic epithelium. When Myc 3′ WRE−/− mice were subjected to an acute model of UC damage involving feeding them with dextran sodium sulfate (DSS) in their drinking water, these mice displayed a more rapid recovery in comparison with wild-type littermates subjected to the same protocol [15, 16]. This phenotype was accompanied by increased epithelial cell proliferation and a more rapid repopulation of goblet cells. Thus, a modest increase in MYC levels correlated with an enhanced recovery from damage caused by DSS-induced colitis, suggesting that pharmacological stabilization of MYC may promote colonic regeneration.
Previous studies have examined the role of GSK3β in regulating the response to UC damage in rodents [17–20]. In rats, pre-treatment with GSK3β inhibitors, such as TDZD-8, SB415286, or lithium, reduced damage to the colonic epithelium by suppressing the inflammatory response [17, 20]. In a chronic model of UC in mice, delivery of lithium or SB216763 during the recovery period lessened the severity of disease symptoms by inhibiting pro-inflammatory immune cells [18]. However, using an acute model of UC in mice, van der Logt et al. [19] found that lithium pre-treatment had no effect on DSS-induced damage. These studies primarily focused on the inflammatory responses associated with GSK3β inhibition and not on the regeneration or proliferation of the colonic epithelium. In addition, the impact of GSK3β inhibition on colonic MYC expression was not addressed in these reports.
In the present study, we sought to determine whether lithium treatment promoted recovery from DSS-induced colitis in mice, and whether MYC was required for this response. We found that administering lithium during the recovery phase from acute colitis lessened disease symptoms and facilitated regeneration of the colonic epithelium. Lithium stimulated epithelial cell proliferation and led to a more rapid restoration of goblet cell populations. Lithium promoted β-catenin and RNA Polymerase II (RNAP) recruitment to the Myc proximal promoter region, increased Myc expression, and stabilized MYC protein levels in the colonic epithelium, which led to an increase in expression of a subset of Wnt/MYC target genes. Impairing the function of MYC as a transcription factor, through co-administration of the MYC inhibitor 10058-F4 [21], dampened the beneficial response of lithium treatments. These findings not only demonstrate the critical role for MYC in colonic regeneration after acute injury, but also indicate that lithium treatment may be a viable alternative to the more toxic and current therapies used for the management of individuals afflicted by UC.
Methods
DSS-Induced Colitis
Colitis was induced in 7-week-old male and female C57BL/6 mice (Jackson laboratories) by administering 2.5 % DSS (TdB Consulting; AB DB001-229) in their drinking water for five days [22]. Following DSS treatment, the animals were returned to normal drinking water and allowed to recover for up to four days. For lithium treatments, animals received a single 200-μl intraperitoneal injection of 4 mg LiCl (EMD, 5910) dissolved in 1X PBS, or 1X PBS alone as a control, daily during the recovery period [18]. For experiments involving MYC inhibition, animals were given 25 mg/kg 10058-F4 dissolved in 200 μl of 1X PBS/0.1 % DMSO via intraperitoneal injection daily [23]. At each day during recovery, mice were weighed and disease activity index (DAI) scores were assessed [24]. DAI scores were based on the following: changes in body weight, consistency of stool, and hemoccult or visible bleeding. These parameters were assessed on a scale from 0 to 4, with 4 being the most severe. The DAI data are presented as an average score of these parameters taken daily. Colonic tissue samples were also collected during each day of the recovery period, fixed in formaldehyde and paraffin-embedded or stored in a −80 °C freezer until analysis. The Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee (IACUC) approved the animal protocols used in this study.
Histology and Immunohistochemistry
Tissue collection, processing, and immunohistochemistry procedures were performed as described previously [15]. For histological analyses, periodic acid-Schiff (PAS)-stained slides were scored by a board certified pathologist, blinded to treatment, using modified parameters described in Ju et al. [25] (Supplemental Table S2). Briefly, inflammation severity, ulceration, and inflammation areas were assessed. For inflammation severity, slides received a score of 0–3, with 3 assigned to tissues with the most severe degree of inflammation. For ulceration, slides received a score of 0 (normal) or 1, with 1 assigned to slides containing ulcers. For inflammation area, slides received a score of 0–4 (0, no inflammation; 1, 1–25 % inflamed; 2, 26–50 % inflamed; 3, 51–75 % inflamed; 4, 76–100 % inflamed). A minimum of n = 3 slides were assessed per condition, the scores were tallied for each slide, and results were presented as averages ±SD. For additional details, see Supplemental Table S2. For alcian blue staining of goblet cells, following rehydration, the slides were incubated in a 0.1 % alcian blue solution for 30 min at room temperature and rinsed in water. The samples were counterstained with nuclear fast red, rinsed, and then dehydrated prior to mounting. Slides were incubated with anti-Ki67 antibodies (Vector laboratories, VP-RM04, 1:200 dilution), or anti-cleaved caspase-3 (Cell Signaling Technologies, 9961, 1:200 dilution). The slides were examined at 200X magnification and images were captured using an Olympus microscope and analyzed using ToupeView software (Amscope, version X86.3.2.1168).
Transcript Analysis
A 100 mg section of the colon was resuspended in 1 ml of TRIzol reagent (Invitrogen, 15596-018), and total RNAs were isolated following the manufacturer’s instructions. cDNAs were synthesized using the iScript cDNA synthesis kit (Bio-Rad, 170-8891), and quantitative PCRs (qPCRs) were conducted using the SensiFAST kit (Bioline, Bio-96020) using conditions described previously [26]. For experiments conducted in IEC6 cells, total RNAs were isolated from the cells in TRIzol reagent and cDNAs were synthesized 24 h after treatment with LiCl and/or 10058-F4. The data are presented relative levels of target gene expression using the 2ΔCT method with 18 s ribosomal RNA (18 s rRNA) serving as the internal reference. The primer sequences used to measure transcript levels of the indicated genes are listed in Supplemental Table S1.
Chromatin Immunoprecipitation (ChIP)
We used a protocol modified from Mahmoudi et al. to perform ChIP assays on purified samples of mouse colonic epithelia as outlined in detail in our previous report [27, 28]. Briefly, the colons were harvested, opened longitudinally, rinsed with 1X PBS, cut into 5 mm pieces, and these pieces were incubated for 30 min at 4 °C in ice-cold chelation buffer (5.6 mM Na2HPO4, 8 mM KH2PO4, 96.2 mM NaCl, 1.6 mM KCl, 43.3 mM D-sorbitol, 0.5 mM DTT) containing 2 mM EDTA. The colonic epithelial layer was separated mechanically by pipetting multiple times using a 10-ml serological pipet and allowing the underlying tissues to settle in a tube by gravity. This process was repeated 8 times, after which the pooled supernatants were passed through a 70-μm cell strainer and centrifuged at 200×g for 3 min at 4 °C to collect the epithelial layer. The samples were treated with 1 % formaldehyde for 30 min at room temperature, after which 125 mM glycine was added to stop the cross-linking. At this point, ChIP assays were conducted as previously described [27]. The following antibodies were used to precipitate the cross-linked and sonicated chromatin with 3 μg of each added per sample; anti-β-catenin (BD Transduction, 610154), anti-TCF4 (Millipore, 05-511), and anti-RNAP (Covance, 8WG16). After reversing the cross-links, the DNA was purified and precipitated in ethanol. The DNA pellets were resuspended in 150 μl of TE, and the Myc promoter and control region were analyzed in qPCRs as previously described [26] using primer sequences listed in Supplemental Table S1. A standard curve that was generated with serial dilutions of purified input DNA was used as a reference to quantify Myc and control fragments in the precipitated DNA. Specifically, these reactions contained 50, 10, 2, 0.4, or 0.08 ng of sonicated and purified input DNA to ensure that the ChIP signal was within the lineage range of detection. The data are presented as relative levels obtained using the standard curve.
Western Blotting
We prepared protein extracts and conducted western blot analysis as previously described [15] with 25 μg of protein loaded per lane on the polyacrylamide gel. The following antibodies and concentrations were used for detection; anti-MYC (Santa Cruz, SC-40; 1:250), anti-pT58-MYC (ABM, Y011034; 1:1000), and anti-β-actin (Sigma, A5541; 1:1000).
In Vitro Wound Assays
The IEC6 rat intestinal epithelial cell line (ATCC, CRL-1592) was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % fetal bovine serum (FBS), 50 units/ml penicillin, 0.1 mg/ml streptomycin, and 2 mM Glutamax at 37 °C in 5 % CO2. The cells were grown to confluence in 6-well dishes, and a vertical scratch was drawn across the monolayer using a standard pipet tip. For experiments involving LiCl, cells were serum-starved and then treated with 20 mM LiCl for 24 h prior to inflicting the scratch wound. For experiments involving the MYC inhibitor 10058-F4, the inhibitor was added at a concentration of 100 μM concurrently with LiCl. Cells treated with vehicle alone served as controls, and the cells were allowed to migrate into the wounded area for 24 h. After migration, the percentage of wounded area remaining was quantified using ImageJ software.
Statistical Analysis
Each experiment was repeated a minimal of three times. For experiments involving IEC6 cells, four wells per treatment were assessed. In the DSS-colitis experiments, a minimum of five mice per condition was used to assess differences in weight loss, colon lengths, DAI scores, and for histochemical and immunohistochemical analyses at each day during the recovery. For qRT-PCR and ChIP-qPCR experiments, each of three independent samples was analyzed in quadruplicate PCRs and statistical significance was determined using the Student’s t test. For analysis of histological sections, statistical significance was determined using the Mann–Whitney U test.
Results
Lithium Treatment Improves Recovery of Mice from Acute Colitis
UC can be modeled in mice through DSS administration [22, 29]. DSS is toxic to colonic epithelial cells, and it disrupts the epithelial barrier allowing inappropriate penetration of commensal bacteria into the underlying mucosal and submucosal layers. This insult elicits an innate immune response, which neutralizes the bacteria and also facilitates colonic repair. To determine whether lithium treatment could improve recovery from DSS-induced damage, mice were given 2.5 % DSS in their drinking water for five days and then returned to normal drinking water for four days to allow recovery (Fig. 1a). At each day during the recovery period (recovery days 0-4; hereafter referred to as R0-R4), mice were given a single intraperitoneal injection of lithium chloride (LiCl) or PBS as a control. We monitored weight loss and shortening of the colons, which are hallmarks of colitis in humans and are also seen in mice subjected to DSS-induced damage [24, 30]. Whereas lithium treatment did not influence weight loss, it did facilitate colonic lengthening during recovery (Fig. 1b, c).
Lithium treatment improves recovery of mice from acute colitis. a Diagram of the DSS-colitis protocol. Mice were untreated (UT) or given 2.5 % DSS in their drinking water for 5 days, after which they were returned to normal drinking water. On each day during the recovery period (R0–R4), mice received a single intraperitoneal injection of lithium chloride (LiCl), or 1X PBS (PBS) as a control. b Assessment of weight loss and, c colon lengths, of mice subjected to DSS-damage and then treated with LiCl or PBS during the recovery period. d Parameters used to assess the Disease Activity Index (DAI) score. e Average DAI scores of lithium-treated or control mice during the recovery period. For these experiments, n = 5 mice were used for each group and error bars represent standard errors of the mean (** P < 0.01; *** P < 0.001)
The disease activity index (DAI) is a scoring system that evaluates the severity of colitis, and we used the DAI system established by Cooper et al. [24] to evaluate lithium-treated and control mice. Based on the degree of weight loss, stool consistency, and rectal bleeding, mice are assigned a score of 1−4, with 4 representing the most severe state of the disease (Fig. 1d). The scores were tallied and presented as an average DAI. At each day during the recovery period, lithium treatment mitigated disease symptoms, which were most apparent on days R2 through R4 (Fig. 1e).
Lithium Promotes Restoration of the Colonic Epithelium
We next conducted a series of histological analyses to assess the architecture and cellular composition of the colons of lithium-treated and control mice during the recovery period. The slides were first stained with periodic acid-Schiff (PAS) to assess goblet cells, which are depleted in UC patients [31] and mouse models of UC [32], and counterstained with hematoxylin to visualize colonic architecture (Fig. 2a). The slides were assessed for the degree of inflammation, the presence of ulcerations, and for the percentage of tissue displaying inflamed areas. We followed histological scoring criteria defined by Ju et al. [25] where a score of nine denotes tissues with the most severe inflammation and damage (Supplemental Table S2). Whereas lithium treatments did not affect histological scores at R1 and R2, lithium-treated mice at R3 and R4 displayed a marked reduction in these scores relative to controls (Fig. 2b). An assessment of PAS+ cells found that a reestablishment of goblet cell populations was evident at R2 and R3 in lithium-treated mice, whereas this level of repair was not seen in control mice until R4 (Fig. 2a, c). The effect of lithium treatment on goblet cell re-population was also seen when these cells were stained with alcian blue (Supplemental Fig. S1). By staining sections with antibodies directed against the proliferation marker Ki67 [33], we noted that an increase in Ki67+ epithelial cells accompanied the more rapid restoration of the colonic crypts in lithium-treated mice (Fig. 2d, e). To determine whether the numbers of cells undergoing apoptosis were affected, we stained sections with antibodies directed against cleaved caspase-3 (Casp3). This analysis found that lithium decreased the number of Casp3+ cells within the colonic crypts at R2 and R3 (Fig. 2f, g). Together, these results indicate that lithium treatment promotes recovery of mice from DSS-induced colitis by diminishing inflammation and tissue damage, facilitating goblet cell repopulation, stimulating epithelial cell proliferation, and reducing the number of apoptotic cells in the regenerating colonic epithelium.
Lithium promotes restoration of the colonic epithelium. a PAS-stained sections of colons prepared from control (PBS) or lithium-treated mice during the indicated days of recovery (R1–R4) from DSS-induced colitis. The sections were counter-stained with hematoxylin. b Histological scores evaluating the degree of damage and inflammation in control and lithium-treated colonic sections. c Quantification of PAS+ cells in the regenerating colonic crypts of control and lithium-treated mice. d Slides were stained with anti-Ki67 antibodies to visualize proliferating cells in the colonic crypts in control or lithium-treated mice during the indicated days of recovery. e Quantification of Ki67+ cells. f Slides were stained with anti-cleaved caspase-3 (Casp3) antibodies to detect cells undergoing apoptosis. g Quantification of Casp3+ cells. In b, n = 3 slides per condition were evaluated. In c, e, and g, positively stained cells were quantified in 15 independent fields of view per day per condition examined. In these experiments, n = 5 mice were used for each group and error bars represent standard errors of the mean (* P < 0.01; ** P < 0.01; *** P < 0.001)
Lithium Induces Myc and Expression of Wnt/MYC Target Genes in Regenerating Colons
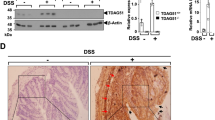
We next analyzed Myc expression and Wnt/MYC target gene expression in purified preparations of colonic epithelia from control and lithium-treated mice on recovery days three and four (R3 and R4). In these preparations, approximately 80 % of the cells are colonocytes and 16 % are goblet cells [34, 35]. First, using qRT-PCR analysis on cDNAs synthesized from isolated RNAs, we found that lithium increased Myc mRNA levels 1.5-fold (Fig. 3a). Within the Myc proximal promoter maps a WRE to which TCF/β-catenin transcription complexes bind to control Myc expression [10, 12, 14, 36]. Using chromatin immunoprecipitation (ChIP) assays, we found that while lithium treatment stimulated RNAP and β-catenin recruitment to Myc promoter, it did not affect TCF4 binding to this site (Fig. 3b). Because lithium increased Myc mRNA expression, we next determined whether this effect was also seen at the protein level. In western blot analyses of protein extracts prepared from the colons of control and lithium-treated mice, we found that lithium induced MYC protein levels (Fig. 3c). MYC contains a phosphodegron at its amino terminus, and when GSK3β phosphorylates threonine 58 (T58) within this region, MYC is subsequently ubiquitinated and targeted for proteasomal degradation [37–39]. Using anti-T58 phospho-specific antibodies, we found that lithium treatment reduced levels of pT58-MYC, indicating that lithium is promoting MYC stabilization by inhibiting GSK3β (Fig. 3c). Thus, by inhibiting GSK3β, lithium activates Myc expression indirectly by inducing β-catenin and RNAP binding to the Myc 5′ proximal promoter, and directly by reducing phosphorylation of residue T58.
Lithium induces Myc and expression of Wnt/MYC target genes in regenerating colons. a qRT-PCR analysis of Myc mRNA levels in colonic sections prepared from mice treated with LiCl or PBS as a control on day 3 (R3) and day 4 (R4) during the recovery period from DSS-induced colitis. The data is normalized to 18s rRNA levels. b The proliferative portions of the colonic crypts were isolated from lithium-treated and control mice on R3 and R4. Chromatin immunoprecipitation (ChIP) assays were conducted with antibodies directed against RNA Polymerase II (RNAP), β-catenin, or TCF4, and purified DNAs were analyzed in qPCR reactions with oligonucleotides that annealed to the Myc promoter (Myc) or a distal control region (dist.) that maps 13-kb downstream from the Myc transcription stop site. c Western blot analysis of MYC and pT58-MYC levels in colonic lysates prepared from mice on R3 and R4. Blots were re-probed with anti-β-actin antibodies as a control for loading. d qRT-PCR analysis of Wnt/MYC target gene expression in the colons of control or lithium-treated mice at R3 and R4. Expression is normalized to 18s rRNA levels. Error bars are standard errors of the mean (* P < 0.05; ** P < 0.01; *** P < 0.001)
To determine whether lithium treatment influenced Wnt/β-catenin- and MYC-co-regulated target gene expression, we evaluated a panel of 25 genes whose expression were shown previously to be regulated by these factors in the small intestines [40]. In a prior study, we demonstrated that a subset of these genes was also regulated by β-catenin and MYC in the colonic epithelium [27]. Using qRT-PCR analysis, we found that expression of 22 of these targets (88 %) was induced by lithium treatment on either R3 or R4 (Fig. 3d). These results suggest that lithium promotes colonic regeneration following damage by stabilizing β-catenin and MYC and increasing expression of a subset of Wnt/MYC target genes.
Stem cells occupy the base of the crypts and produce transit amplifying (TA) progenitor cells to maintain tissue homeostasis [7]. Upon injury to the colonic epithelium, stem cell populations proliferate to repair damage in the regenerating intestines [41]. To determine whether stem cell populations were induced in the regenerating colons, we evaluated expression of a subset of stem cell markers in RNAs isolated from purified preparations of colonic epithelium of control and lithium-treated mice on R3 and R4. Using qRT-PCR analysis, we analyzed expression of Lgr5 [42], Bmi1 [43, 44], Lrig1 [45], Olfm4 [46], and Hopx [47]. On R3, lithium treatment induced expression of each of these stem cell marker genes, whereas only Lrig1 and Olfm4 expression was induced by lithium on R4 (Supplemental Figure S2). Thus, lithium acts on stem cell populations to promote regeneration of the DSS-damaged colonic epithelium.
MYC Is Involved In Lithium-Stimulated Intestinal Epithelial Cell Wound Repair In Vitro
Our in vivo data suggest that colonic epithelial cells are the primary targets of lithium treatments. To directly assess whether lithium modulates the ability of intestinal epithelial cells to repair a wound, we subjected rat intestinal epithelial IEC6 cells to scratch-wound assays [48]. In this assay, IEC6 cells are grown to confluence and a wound is inflicted upon the monolayer using a pipet tip. Over time, IEC6 cells respond by migrating into the wounded area. In untreated samples, 24 h after the scratch injury, we found that approximately 85 % of the wound area was effectively repaired by migratory IEC6 cells (Fig. 4a, b). Lithium promoted this response as treated cells covered nearly all of the wounded area (Fig. 4a, b).
MYC is involved in lithium-stimulated intestinal epithelial cell wound repair in vitro. a Rat intestinal epithelial cells (IEC6s) were grown to confluence and treated with LiCl for 24 h. Cells treated with PBS served as a control. A scratch was drawn across the monolayer and migration of the cells into the denuded area was assessed 24 h later. b Quantification of the percent wound area remaining 24 h after the scratch was inflicted to the monolayer. c qRT-PCR analysis of Myc mRNA levels in IEC6 cells treated with PBS or LiCl. d Western blot analysis of MYC and pT58-MYC levels in control and LiCl-treated cells. Blots were re-probed with anti-β-actin antibodies to ensure equivalent loading. e qRT-PCR analysis of Ccnd2 and Cad mRNA levels in cells receiving the indicated treatments for 24 h. f Scratch-wound assays conducted in cells pretreated with LiCl or LiCl and 10058-F4 as indicated. g Quantification of the percent wound area remaining following a 24 h period of cell migration. In c and e, the data is normalized to β-actin whose levels were unchanged by the treatments. Error bars are standard errors of the mean (** P < 0.01; *** P < 0.001)
Using a similar system, Liu et al. [49] demonstrated that treatment of IEC6 cells with Wnt3A ligand potentiated wound repair and that Myc was required for this response. To determine whether Myc was involved in lithium-stimulated wound response, we first analyzed Myc transcripts in control and lithium-treated cells. We found that lithium increased Myc expression 1.75-fold (Fig. 4c). In addition, lithium blocked GSK3β-mediated phosphorylation of MYC on residue threonine 58 and increased overall MYC protein levels in treated cells (Fig. 4d). Thus, lithium induces Myc expression and also stabilizes MYC protein levels. These effects correlate with an increased capacity to repair a scratch wound.
To evaluate the role of MYC in lithium-stimulated wound repair, we used the MYC inhibitor 10058-F4 [21, 50]. This inhibitor binds to the basic helix-loop-helix-zipper domain of MYC, prevents its heterodimerization with MAX, and therefore blocks its function as a transcriptional regulator [21, 51, 52]. Treating cells with 10058-F4 reduced expression of the MYC target genes Cyclin D2 (Ccnd2) and carbamoyl phosphate synthase (Cad) [53], indicating that the inhibitor was functioning appropriately (Fig. 4e). Moreover, LiCl treatment activated expression of these targets, presumably through stabilizing MYC transcriptional regulatory complexes (Fig. 4e). Simultaneous treatment with LiCl and 10058-F4 reduced levels seen with LiCl alone, suggesting that MYC is a downstream regulator of the lithium response (Fig. 4e). Finally, 10058-F4 treatment impaired lithium-induced migration of IEC6 cells into the scratch wound (Fig. 4f , g). These findings indicate that functional MYC transcription complexes are required for lithium-stimulated repair of scratch wound by intestinal epithelial cells in vitro.
Inhibiting MYC Impairs Lithium-Induced Colonic Regeneration After Acute Colitis
The 10058-F4 inhibitor has previously been used in mice to demonstrate a role for MYC in fat droplet accumulation in cancer cells [23] and in osteoclast differentiation [54]. We therefore used this inhibitor to determine whether MYC plays a role in lithium-stimulated repair of the colonic epithelium. Following DSS-induced colitis, and during each day of the recovery period, mice were given intraperitoneal injections of LiCl, 10058-F4, or LiCl and 10058-F4. The mice had to be euthanized at R3, rather than R4, because those treated with the MYC inhibitor were moribund. While these treatments did not influence weight loss, MYC inhibition impaired the colonic lengthening phenotype that was stimulated by lithium on R3 (Fig. 5a, b). An assessment of disease progression using the DAI system found that during the course of these experiments, mice treated with 10058-F4 alone failed to recover (Fig. 5c). Moreover, 10058-F4 reduced the beneficial effects of lithium treatment during the recovery period (Fig. 5c). qRT-PCR analysis of transcripts expressed in colonic sections prepared on R3 found that 10058-F4 treatments reduced expression of Ccnd2 and Cad indicating that the inhibitor was targeting MYC-regulated genes when administered to the mice (Fig. 5d).
Inhibiting MYC reduces lithium-induced colonic regeneration after acute colitis. a Mice were subjected to the DSS-damage protocol and were then treated with LiCl, the MYC inhibitor 10058-F4, or a combination of LiCl and 10058-F4, during each day of the recovery period. The mice were weighed on each day of the recovery period and reported is the percentage of initial weight. b Measurements of colonic lengths at R3 in mice receiving the indicated treatments. c Average DAI scores in mice receiving the indicated treatments at each day of recovery. d qRT-PCR analysis of Ccnd2 and Cad expression in the colons of mice receiving the indicated treatments. The data is normalized to 18s rRNA levels. e PAS-stained colonic sections prepared from mice on R3 receiving the indicated treatments. The slides were counterstained with hematoxylin. f Histological scores with n = 3 slides evaluated per condition. g Quantification of PAS+ cells. h Ki67-stained colonic sections and i, quantification of Ki67+ cells in mice receiving the indicated treatments on R3. j Cleaved caspase-3 (Casp3)-stained colonic sections and k, quantification of Casp3+ cells. In g, i, and k, positively stained cells were quantified in 15 independent fields of view per day per condition examined. For these experiments, n = 5 mice were examined per group and error bars are standard errors of the mean (* P < 0.05; ** P < 0.01; *** P < 0.001)
Next conducted histochemical and immunohistochemical analyses to determine whether MYC inhibition abrogated the beneficial affects of lithium treatment on the architecture and cellular composition of the colons of mice on R3 following DSS-induced damage. An assessment of PAS-stained sections found that in comparison with mice treated with lithium, the colonic epithelia of mice receiving 10058-F4 alone were severely damaged with widespread inflammation (Fig. 5e, f). Whereas lithium co-treatment appeared to partially rescue the detrimental effects of 10058-F4 administrations on the colonic epithelium (Fig. 5e), this phenotype was not reflected in the histological scores (Fig. 5f). Moreover, in comparison with mice receiving only lithium, the colons of mice injected with the MYC inhibitor alone contained a severe reduction in PAS+ goblet cells, a reduced number of Ki67+ cells, and more Casp3+ apoptotic cells (Fig. 5e, g, h–k). When MYC function was inhibited in lithium-treated mice through co-injection of 10058-F4, we noted that the number of Ki67+ and PAS+ cells were reduced and the number of apoptotic cells were increased in the colonic epithelial relative to mice receiving lithium alone (Fig. 5e, g, h–k). Therefore, these results demonstrate that MYC is a critical downstream target of lithium-stimulated repair of the colonic epithelia of mice after DSS-induced damage.
Discussion
A single layer of epithelial cells lines the intestines and forms a barrier protecting underlying tissues from enteric bacteria and toxic contents of the lumen [6]. In UC, chronic inflammation compromises this barrier function and leads to a deterioration of the colonic crypt architecture [1, 2]. In response, Wnt/β-catenin signaling induces cell proliferation to repair the damage [55]. GSK3β is a component of the Wnt/β-catenin signaling pathway, and it serves to negatively regulate β-catenin and MYC protein levels in a cell. In this study, we demonstrate that inhibiting GSK3β activity during the recovery phase from DSS-induced colitis dampens disease symptoms by promoting proliferation and regeneration of the colonic epithelium. In addition, we demonstrate that MYC is a critical downstream effector of this response.
Pharmacological inhibition of GSK3β has been studied previously in rodent models of UC; however, differences in experimental protocols and model systems complicate comparison of results [17–20]. In rats, GSK3β inhibitors were shown to lessen the severity of acute colitis by reducing the inflammatory response [17, 20]. In these studies, the GSK3β inhibitors were delivered prior to the onset of colitis. Prior to our study, two groups evaluated the efficacy of lithium to mitigate disease symptoms associated with DSS-colitis in mice [18, 19]. Using a chronic colitis model where Balb/c mice were treated with multiple cycles of DSS with intermittent recovery periods, Hofmann et al. [18] found that intraperitoneal injection of lithium, or the GSK3β inhibitor SB216763, for five days after completion of the last cycle attenuated the pro-inflammatory immune response and facilitated recovery. In a second study, van der Logt et al. [19] used the acute model of DSS-colitis, similar to the protocol we used in this study, and they found that delivery of lithium prior to the onset of DSS-colitis did not provide a therapeutic benefit. The primary feature that distinguishes our studies from these is that we administered lithium during the recovery phase after damage caused by acute DSS-induced damage in C57BL/6 mice. Our findings indicate that lithium treatment during the recovery period from DSS-induced damage facilitated a more rapid recovery of mice by promoting colonic repair and regeneration. These findings support those reported by Hofmann et al. [18], and together, indicate that inhibiting GSK3β offers a beneficial therapeutic response from damage to the colonic epithelium caused by acute and chronic UC.
In response to damage to the intestinal epithelium caused by exposure to γ-irradiation, Wnt signaling activates Myc expression to promote cellular proliferation to repair the damage [13]. Furthermore, MYC was required for full repair, arguing that the Wnt/MYC axis is a critical pathway involved in intestinal regeneration in response to damage [13]. During the recovery phase from DSS-induced colitis, we found that lithium activated Myc expression in the colons by stimulating binding of β-catenin and RNAP to the Myc proximal promoter (Fig. 3a, b). TCF4 binding was not affected, consistent with a role of TCF proteins serving as a platform to exchange co-regulatory complexes [11]. We also found that lithium treatment stabilized MYC protein levels by reducing GSK3β-mediated phosphorylation on residue T58 of MYC (Fig. 3c). In addition, we show that lithium treatment during the recovery period activates expression of a subset of β-catenin/MYC target genes (Fig. 3d) [27, 40]. These findings support a model whereby lithium treatment activates a genetic program controlled by MYC and β-catenin transcription complexes to drive cellular proliferation and restore the colonic epithelium. Therefore, and in agreement with Ashton et al. [13], our report demonstrates that MYC is required to regenerate the intestinal epithelium in response to damage.
While we demonstrate that colonic epithelial cells are likely the primary targets for lithium action, we cannot rule out the possibility that lithium is also acting on the immune system to mitigate disease phenotypes. Indeed, our histological analyses of the colons from control and lithium-treated mice (Figs. 2, 5) indicate that lithium suppresses inflammation, as evident by the overall lower histological scores relative to controls (Supplemental Figure S2) [25]. However, our studies using the MYC inhibitor 10058-F4 support our conclusions that lithium is acting through MYC in the colonic epithelium to promote colonic regeneration. In comparison with lithium-treated mice on R3, the colons of mice receiving 10058-F4 alone remained severely damaged (Fig. 5f). Co-treatment with lithium and 10058-F4 failed to improve the histological scores seen in the colons of mice treated with 10058-F4 alone (Fig. 5f). If MYC was functioning in leukocytes to promote inflammation, and lithium was dampening this response, then we would have expected that MYC inhibition in these lithium-stimulated cells would decrease inflammation and improve histological scores. Moreover, co-administration of the MYC inhibitor reduced the number of PAS+ and Ki67+ cells seen in the colons of lithium-treated mice (Fig. 5e, g−i), and also increased the number of apoptotic cells (Fig. 5j, k). Therefore, these data support a model whereby by lithium stabilizes MYC in the colonic epithelium to promote recovery from damage caused by acute colitis.
Surprisingly, only a few studies have examined MYC expression in intestinal tissues obtained from patients afflicted by inflammatory disorders such as Crohn’s disease, diverticulitis, and UC [56–58]. Of these, only the study by Ciclitira et al. [57] analyzed MYC by immunohistochemistry, and they reported higher MYC levels in inflamed tissue when compared to levels seen in uninvolved colonic mucosa. While elevated MYC expression is usually correlated with an adverse outcome, we propose that interpretation of results, such as these, should be made with caution. Our findings would suggest that elevated MYC expression may demarcate epithelial cells that are actively engaged in regeneration and that these MYC+ cells would therefore correlate with a positive prognosis. Clearly, our study indicates that a more complete and through evaluation of patient samples is required to properly interpret the role of MYC in IBD. For example, it would be of interest to determine whether elevated MYC expression in the colonic epithelium at ulcerated regions undergoing repair characterizes the colons of UC patients whose disease is being managed by current immunosuppressive strategies.
In summary, our findings support the possibility of using lithium as a therapeutic to manage individuals suffering from UC. In support of this strategy, a case report from over four decades ago found that administration of lithium to a patient with manic-depressive psychosis and UC alleviated the symptoms associated with his colitis [59]. While the underlying mechanisms to his recovery were not elucidated as the molecular targets of lithium were unknown at that time, the author of his study concluded that “further investigation may prove rewarding.” Results presented in this current study, and those described by others, support this conclusion. Because lithium is an FDA-approved drug, clinical trials are needed to determine whether lithium treatment offers an alternative approach for treating individuals with UC. In addition, our results suggest that small molecules designed to transiently stabilize MYC levels may be efficacious in promoting colonic regeneration in response to damage caused by chronic inflammation.
References
Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619.
Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20.
Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut. 2012;61:918–932.
Danese S, Fiocchi C. Ulcerative colitis. New Engl J Med. 2011;365:1713–1725.
Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative-colitis—analysis of changes in disease-activity over years. Gastroenterology. 1994;107:3–11.
Noah TK, Donahue B, Shroyer NF. Intestinal development and differentiation. Exp Cell Res. 2011;317:2702–2710.
Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205.
Archbold HC, Yang YX, Chen L, Cadigan KM. How do they do Wnt they do? regulation of transcription by the Wnt/beta-catenin pathway. Acta Physiol (Oxf). 2011;204:74–109.
He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512.
Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286.
Yochum GS, Cleland R, Goodman RH. A genome-wide screen for beta-catenin binding sites identifies a downstream enhancer element that controls c-Myc gene expression. Mol Cell Biol. 2008;28:7368–7379.
Ashton GH, Morton JP, Myant K, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269.
Yochum GS, McWeeney S, Rajaraman V, Cleland R, Peters S, Goodman RH. Serial analysis of chromatin occupancy identifies beta-catenin target genes in colorectal carcinoma cells. Proc Natl Acad Sci USA. 2007;104:3324–3329.
Konsavage WM Jr, Jin G, Yochum GS. The Myc 3′ Wnt-responsive element regulates homeostasis and regeneration in the mouse intestinal tract. Mol Cell Biol. 2012;32:3891–3902.
Konsavage WM Jr, Roper JN, Ishmael FT, Yochum GS. The Myc 3′ Wnt responsive element regulates neutrophil recruitment after acute colonic injury in mice. Dig Dis Sci. 2013;58:2858–2867.
Daneshmand A, Rahimian R, Mohammadi H, et al. Protective effects of lithium on acetic acid-induced colitis in rats. Dig Dis Sci. 2009;54:1901–1907.
Hofmann C, Dunger N, Scholmerich J, Falk W, Obermeier F. Glycogen synthase kinase 3-beta: a master regulator of toll-like receptor-mediated chronic intestinal inflammation. Inflamm Bowel Dis. 2010;16:1850–1858.
van der Logt EM, Blokzijl T, Diepstra A, et al. Lithium induces intestinothrophic effects in the healthy colon, but does not ameliorate dextran sulfate sodium-induced colitis in mice. e-SPEN J. 2011;7:e16–e22.
Whittle BJ, Varga C, Posa A, Molnar A, Collin M, Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta. Br J Pharmacol. 2006;147:575–582.
Hammoudeh DI, Follis AV, Prochownik EV, Metallo SJ. Multiple independent binding sites for small-molecule inhibitors on the oncoprotein c-Myc. J Am Chem Soc. 2009;131:7390–7401.
Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546.
Zirath H, Frenzel A, Oliynyk G, et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc Natl Acad Sci USA. 2013;110:10258–10263.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investigation J Tech Methods Pathol. 1993;69:238–249.
Ju J, Hao X, Lee MJ, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer prevention research. 2009;2:143–152.
Bottomly D, Kyler SL, McWeeney SK, Yochum GS. Identification of {beta}-catenin binding regions in colon cancer cells using ChIP-Seq. Nucleic Acids Res. 2010;38:5735–5745.
Konsavage WM Jr, Yochum GS. The myc 3′ wnt-responsive element suppresses colonic tumorigenesis. Mol Cell Biol. 2014;34:1659–1669.
Mahmoudi T, Li VS, Ng SS, et al. The kinase TNIK is an essential activator of Wnt target genes. Embo J. 2009;28:3329–3340.
Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliver Rev. 2007;59:1073–1083.
Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571–1579.
Greco V, Lauro G, Fabbrini A, Torsoli A. Histochemistry of the colonic epithelial mucins in normal subjects and in patients with ulcerative colitis. A qualitative and histophotometric investigation. Gut. 1967;8:491–496.
Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol. 2005;288:G1328–1338.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715.
Chang WW, Leblond CP. Renewal of the epithelium in the descending colon of the mouse. I. Presence of three cell populations: vacuolated-columnar, mucous and argentaffin. Am J Anat. 1971;131:73–99.
Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286–298.
Yochum GS, Sherrick CM, Macpartlin M, Goodman RH. A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5′ and 3′ Wnt responsive enhancers. Proc Natl Acad Sci USA. 2010;107:145–150.
Amati B. Myc degradation: dancing with ubiquitin ligases. Proc Natl Acad Sci USA. 2004;101:8843–8844.
Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612.
Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514.
Sansom OJ, Meniel VS, Muncan V, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679.
Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature reviews. Mol Cell Biol. 2014;15:19–33.
Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007.
Reinisch C, Kandutsch S, Uthman A, Pammer J. BMI-1: a protein expressed in stem cells, specialized cells and tumors of the gastrointestinal tract. Histol Histopathol. 2006;21:1143–1149.
Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920.
Wong VW, Stange DE, Page ME, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nature Cell Biol. 2012;14:401–408.
van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17.
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424.
Mccormack SA, Viar MJ, Johnson LR. Migration of Iec-6 cells—a model for mucosal healing. Am J Physiol. 1992;263:G426–G435.
Liu L, Rao JN, Zou TT, et al. Activation of Wnt3a signaling stimulates intestinal epithelial repair by promoting c-Myc-regulated gene expression. Am J Physiol-Cell. 2012;302:C277–C285.
Wang H, Hammoudeh DI, Follis AV, et al. Improved low molecular weight Myc-Max inhibitors. Mol Cancer Ther. 2007;6:2399–2408.
Muller I, Larsson K, Frenzel A, et al. Targeting of the MYCN protein with small molecule c-MYC inhibitors. PloS One. 2014;9:e97285.
Zinin N, Adameyko I, Wilhelm M, et al. MYC proteins promote neuronal differentiation by controlling the mode of progenitor cell division. EMBO Rep. 2014;15:383–391.
Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264.
Park-Min KH, Lim E, Lee MJ, et al. Inhibition of osteoclastogenesis and inflammatory bone resorption by targeting BET proteins and epigenetic regulation. Nat Commun. 2014;5:5418.
Koch S, Nava P, Addis Cet al. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology. 2011;141:259–268, 268 e251–258.
Alexander RJ, Panja A, Kaplan-Liss E, Mayer L, Raicht RF. Expression of protooncogene-encoded mRNA by colonic epithelial cells in inflammatory bowel disease. Dig Dis Sci. 1996;41:660–669.
Ciclitira PJ, Macartney JC, Evan G. Expression of c-myc in non-malignant and pre-malignant gastrointestinal disorders. J Pathol. 1987;151:293–296.
Macpherson AJ, Chester KA, Robson L, Bjarnason I, Malcolm AD, Peters TJ. Increased expression of c-myc proto-oncogene in biopsies of ulcerative colitis and Crohn’s colitis. Gut. 1992;33:651–656.
Zisook S. Ulcerative-colitis—case responding to treatment with lithium carbonate. J Am Med Assoc. 1972;219:755.
Acknowledgments
This work was supported by institutional funds provided by the Pennsylvania State University College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2015_3852_MOESM3_ESM.jpg
Supplemental Fig. S1 Lithium promotes restoration of goblet cells after DSS-induced colitis. a Mice subjected to acute DSS-induced colitis were given an IP injection of LiCl or PBS as a control during each day of the recovery period (R1–R4). The sections were stained with alcian blue to detect goblet cells. b Quantification of alcian blue+ cells. For these experiments, n = 5 mice were used per condition. Cells were quantified in 15 fields of view per day per condition examined. Error bars are standard errors of the mean (***, P < 0.001). (JPEG 1675 kb)
10620_2015_3852_MOESM4_ESM.jpg
Supplemental Fig. S2 Lithium induces expression of intestinal stem cell markers in the regenerating colonic epithelium after DSS-induced colitis. The colonic epithelia were purified from lithium-treated and control mice on recovery days R3 and R4 after DSS-induced colitis. RNAs were extracted, and qRT-PCR was used to measure expression of the intestinal stem cell genes Lgr5, Bmi1, Lrig1, Olfm4, and Hopx. The data are normalized to 18s rRNA levels. Error bars are standard errors of the mean (**, P < 0.01 and ***, P < 0.001). (JPEG 104 kb)
Rights and permissions
About this article
Cite this article
Raup-Konsavage, W.M., Cooper, T.K. & Yochum, G.S. A Role for MYC in Lithium-Stimulated Repair of the Colonic Epithelium After DSS-Induced Damage in Mice. Dig Dis Sci 61, 410–422 (2016). https://doi.org/10.1007/s10620-015-3852-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3852-0