Abstract
Ulcerative colitis (UC) is a prominent category of disease that is associated with bowel inflammation, it can occur at any period of life and is prevalently rising on a global scale. Dextran sulfate sodium (DSS) has been extensively used to develop colitis due to its ability to mimic human UC, providing consistent and reproducible inflammation, ulceration, and disruption of the epithelial barrier in the colon. Chronic inflammation in the gut can lead to alterations in the gut-liver axis, potentially impacting liver function over time, while direct evidence linking diversion colitis to liver damage is limited. Thus, the present study aims to assess the gut and liver damage against DSS and the possible molecular mechanisms. Forty-seven animals were randomly assigned to six groups. Ulcerative colitis was induced using 2.5% w/v DSS in three alternate cycles, each lasting 7 days, with 1-week remission periods in between. SOV (5 and 10 mg/kg, orally) and the standard drug 5-aminosalicylic acid (100 mg/kg, orally) were administered from the start of the 2nd DSS cycle until the end of the experiment. Biochemical parameters, ELISA, histopathological, and immunohistochemical analyses have been conducted to assess damage in the colon and liver. SOV significantly reduced colitis severity by lowering the DAI score, oxidative stress markers (LPS, IL-1β, MPO, nitrite), and restoring liver biomarkers (SGPT, SGOT). Histopathological findings supported these protective benefits in the liver and gut. Moreover, immunohistochemical analysis showed SOV enhanced the expression of the cytoprotective mediator Nrf2/Keap-1 and reduced the expression of inflammatory mediators NF-κB and IL-6. Present findings concluded that SOV demonstrated a dose-dependent effect against UC through anti-inflammatory and antioxidant pathways, with the highest dose of SOV 10 mg/kg having more significant (p < 0.001) results than the low dose of 5 mg/kg.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a bowel-associated inflammatory disease that affects the rectum and colon to various extents throughout the life of an individual. The typical symptoms of UC include chronic diarrhea, often accompanied by blood or pus, abdominal pain and cramping, rectal bleeding, unintentional weight loss, fatigue, and occasional fever (Ungaro et al. 2017) (Danese et al. 2018). This condition mainly involves periodic inflammation of the mucous lining that starts in the rectal area and extends continuously upward, terminating abruptly where it transits into healthy colon tissues (Burisch et al. 2023). Without effective treatment, persistent inflammation can degrade quality of life, often requiring surgical intervention and frequent hospital visits, and it can also elevate the risk of colorectal cancer (Viola et al. 2024). The annually reported incidences of UC contain up to 20 cases per 100,000 persons and prevail up to 291 cases per 100,000 persons (Santiago et al. 2022), and the estimation for the year 2023 is 5 million cases throughout the globe (Le Berre et al. 2023). The advancement of UC is influenced by genetics and environment. Environmental and geographical factors, along with unhealthy eating habits, alcohol consumption, smoking, pollution, and the use of medications, can alter gut microflora resulting in inflammatory conditions (Molodecky and Kaplan 2010). Similarly, the genetic factors are partially involved in disease progression. A recent meta-analysis of genome-wide association studies (GWAS) has identified 163 single nucleotide polymorphism loci associated with inflammatory bowel disease (IBD) (Ek et al. 2014). These genetic loci include genes involved in key biological processes such as autophagy, microbial recognition, lymphocyte signaling, response to endoplasmic reticulum stress, and cytokine signaling (Liu et al. 2015; Jostins et al. 2012). The pathogenesis of UC remains uncertain, but it is widely acknowledged that the colonic mucin, gut microbiome composition, and barrier integrity result in potentially heightened absorption of luminal antigens, that further initiate the various molecular pathways (Tatiya et al. 2018). It has been reported lipopolysaccharide (LPS) is primarily produced inside the gut when the barrier of the gut is interrupted (Li et al. 2023), and LPS through the portal vein enters into the liver, then stimulates the hepatic inflammatory mediator causing liver damage (Liu et al. 2017). It mainly binds to TLR4 on the surface of macrophages and dendritic cells (Boirivant et al. 2008). This binding activates intracellular signaling pathways including NF-κB, NLRP3 and Nrf2, and proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 that tend to propagate, amplify the inflammatory response, and contribute to systemic and local inflammation in the gut (Currie et al. 2001). Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) acts as a regulatory mediator for survival, initiation, and diversification of innate immune and inflammatory cells by potentiating the expression of pro-inflammators that contributes to chronic inflammation and tissue damage in UC (Liu et al. 2018). Furthermore, the Nrf2 (nuclear factor erythroid 2-related factor 2) acts as a cyto-defensive element, that relocates into the nucleus and prompts the activation of phase II detoxification and antioxidant genes including NQO1, HO-1, GCL, and SOD (Khor et al. 2006). This activation enhances the cell’s ability to neutralize reactive oxygen species and mitigate intestine inflammation. So, Nrf2/Keap1 and NF-κB pathways present novel avenues for managing inflammatory bowel disease, particularly UC. Majorly 5-aminosalicylic acid (5-ASA), which is utilized in inflammatory gut management and UC remission maintenance, is suggested to exert its effects through the Nrf2/Keap1 axis (Kang et al. 2017).
Recent research indicates that activating intestinal Nrf2 can inhibit the NF-κB pathway, suggesting a promising therapeutic approach for treating UC and its complications (Horowitz et al. 2023). Clinical studies indicate that 5–10% of cases of liver injury is a common extra-intestinal complication in persons having UC, often seen as a secondary effect of gut barrier disruption (Thin et al. 2014). It is undeniable that managing hepatic problems can also be prioritized within the treatment regimen for UC. Therefore, gut microflora regeneration through the nutritional and natural manner in UC advancement is a rational preference (Herrera et al. 2023). However, the UC management is multifaceted, but the cornerstone medication is 5-aminosalicylic acid (5-ASA), also known as mesalamine, and is a therapeutic agent used in the treatment of UC. It primarily reduces colon inflammation by inhibiting the production of inflammatory mediators like prostaglandins and leukotrienes, key components in the inflammatory process of UC (Sandborn and Hanauer 2003). But acute 5-ASA intolerance syndrome typically presents with worsened diarrhea, fever, and abdominal pain. Patients who stop 5-ASA due to intolerance are at greater risk of adverse outcomes like hospitalization, colectomy, needing more advanced therapies, and reduced response to TNF biologics (Mikami et al. 2023). Besides this, other treatment options include glucocorticosteroids, thiopurine, TNF-α antagonists, methotrexate, vedolizumab, and antibiotics. Unfortunately, these drugs also have limitations in effectiveness and safety. Corticosteroid intake increases the risk of infections, osteoporosis, hyperglycemia, hypertension, weight gain, and mood swings, and in long-term use, adrenal suppression (Timmer et al. 2016) and immunomodulator therapies cause bone marrow suppression, liver toxicity, and pancreatitis (Reddy and Loftus 2006). Thus, finding of drug with more potency, effectiveness, and minimal adverse effects is imperative (Fu et al. 2010). Notably, sodium orthovanadate (SOV), a compound originating from inorganic moiety related to the vanadium group, acts as a competitive inhibitor of protein tyrosine phosphatases (PTPs) (Harrington et al. 2021; Joshi et al. 2022). In reported studies on cancer cells, vanadium compounds showed a protective response by inhibiting the cell cycle at checkpoints G0/G1, G1/S, and G2/M (He et al. 2021). Besides this, it is involved in multiple physiological processes including fertilization (Gunther et al. 2013), apoptosis (Khalil and Jameson 2017), cell cycle, and neuronal death through the PI3K/AKT and MAPK pathway (Kim et al. 2019), which further initiates the cyto-defensive mechanism (Yu et al. 2019). The previous reports have various pharmacological properties of the SOV including the antidiabetic (Mehdi et al. 2006; Begum et al. 1991), antioxidant, anti-inflammatory (Willsky et al. 2013), and neuroprotective activities (Khalil and Jameson 2017). Earlier studies reported that the antioxidant and anti-inflammatory properties of vanadates by inhibiting PTPs work as a key mechanism in the treatment of UC (Omayone and Olaleye 2021). SOV might be helpful by protecting the cells in the colon lining, promoting healing, regulating apoptosis, and maintaining cellular integrity that reduces the inflammation in the colon. Moreover, PTPs regulate the cell cycle by controlling the phosphorylation status of key proteins involved in cell division (He et al. 2021). So, SOV can promote the survival and proliferation of epithelial cells in the gut lining, aiding in the repair of ulcerated and mucosal healing and regeneration. Therefore, the current study is designed to explore the effectiveness of SOV against the DSS and its gut-associated liver damage.
Materials and methods
Drugs and chemicals
Dextran sulfate sodium salt (Cat. # 76203, 30–50 kDa molecular weight) was procured from Sisco Research Laboratories, and sodium orthovanadate (Cat. #S6508 purity = ≥ 90%) was procured from Sigma Aldrich. The lipopolysaccharide (Cat# E2214Mo) and IL-1 β (Cat# E-EL-M0037) ELISA Kits were procured from Elabsciences, China, and Erba kits for SGPT (Cat# 120207) and SGOT (Cat# 120616) were procured from the Transasia Biomedical Ltd. The antibody NF-Bp65 (Cat# SC-8008), Nrf2 (Cat#SC-365949), and IL-6 (Cat# SC-28343) soured from the Santa Cruz biotechnology. Additionally, Oncogene Research Product USA detection kit FragEL™ DNA Fragmentation (Cat. # QIA39; Calbiochem), Vector labs staining kit the impress excel (Cat. # MP-7601), and other chemical reagents and stains were acquired from Sigma-Aldrich, USA.
Rationale of study and dose selection
The dextran sulfate sodium (DSS) induced colitis model is widely used for studying inflammatory bowel disease (Eichele and Kharbanda 2017). It provides key insights into histopathological and morphological changes in intestinal barrier function, microbial balance shifts, and metabolome alterations associated with human UC development (Singla et al. 2023). Various studies have explored a spectrum of 1–5% w/v DSS dosages for 5–7 days durations (Wetwittayakhlang et al. 2021). Clinically, a treat-to-target strategy has been adopted in ulcerative colitis, focusing on inducing and maintaining remission to replicate human pathological conditions (Bressenot 2018). The dosage selected for this study was determined by referencing earlier preclinical studies. The dosage regimen for sodium orthovanadate dose, 5 and 10 mg/kg (Joshi et al. 2022), and standard drug 5-aminosalicylic acid, 100 mg/kg (Roy et al. 2022), has been selected, both orally administered after being dissolved in distilled water from the 1st day of 2nd DSS cycle continued till sacrifice (Fig. 1).
Experimental protocol
Forty-seven male BALB/c mice, weighing between 25 ± 2 g, were procured from a CCSEA registered breeder (AIIMS, New Delhi). The study’s research plan was sanctioned by the Institutional Animal Ethics Committee (IAEC) of ASBASJSM College of Pharmacy, Bela (Ropar), under the allotted ASCB/IAEC/18/23/180 number. The animals were kept in standard laboratory conditions, with temperature maintained at 23 ± 2 °C, humidity at 40 ± 10%, and a 12-h light–dark cycle. All experimental procedures conducted between 9:00 AM and 4:00 PM daily were in accordance with the Indian Government New Delhi, regulatory principles by the Committee for the Control and Supervision of Experiments on Animals (CCSEA), and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Animals were assigned randomly into six different groups.
-
Group 1: CON received 0.9% saline.
-
Group 2: PERSE mice received a high dose of sodium orthovanadate (SOV 10 mg/kg). Group 3: DSS mice received DSS (2.5% w/v) with drinking water in 3 cycles of 7 days with a remission period of 7 days in between each cycle (Singla et al. 2023).
-
Group 4: DSS + 5-ASA mice received DSS (2.5% w/v) and standard drug 5-amino salicylic acid 100 mg/kg.
-
Group 5: DSS + SOV 5 mice received DSS (2.5% w/v) intervention sodium orthovanadate low dose 5 mg/kg.
-
Group 6: DSS + SOV 10 mice received DSS (2.5% w/v) and intervention sodium orthovanadate high dose 10 mg/kg.
After the completion of the experimental work, animals were sacrificed for the biochemical and fixed with formalin for histological investigations.
A visual representation outlining the experimental framework, demonstrating the treatment protocols and dosage regimens allocated to different groups: CON: received normal drinking water. PERSE: received SOV 10 mg/kg started from 3rd week and continued till sacrifice. DSS: received DSS 2.5% w/v in drinking regular water for three consecutive cycles of 7 days from the first week of the study and continued till sacrifice. DSS + 5-ASA: received DSS in 3 alternative cycles and 5-ASA 100 mg/kg from 2nd cycle of DSS administration persists until the point of sacrifice. DSS + SOV 5: received 2.5% DSS in 3 alternative cycles and SOV 5 mg/kg from 2nd cycle of DSS exposure extends until the animals were sacrificed. Group 6; DSS + SOV 10: received DSS and SOV 10 mg/kg from 2nd cycle of DSS exposure continue up to sacrifice. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg
Collection of samples
On the last day of the experimental study, mice (overnight fasting) were anesthetized with pentobarbitone (50 mg/kg, i.p.) and samples were acquired through retro-orbital plexus for biochemical investigation. The liver and colon were dissected and washed with chilled PBS. The tissue was stored at − 80 °C for further biochemical and molecular investigations and fixed with formalin for histopathology.
Disease Activity Index (DAI)
DAI was calculated by averaging the total scores of body weight loss assessed as 0, no loss; 1, 1–5%; 2, 5–10%; 3, 10–15%; and 4, > 15%; stool consistency score as 0, normal; 2, loose stool; 4, diarrhea; and rectal bleeding assessed as 0, no bleeding; 1, hemoccult positive; 2, hemoccult positive and visual pellet; and 4, gross bleeding. The Disease Activity Index was calculated as %weight loss + Stool consistency + Bleeding/3 (Vashist et al. 2018).
Peripheral blood micronucleus assay
The purpose of the peripheral micronucleus assay is to evaluate the genotoxic potential of a substance by detecting and measuring micronuclei inside the cell. As per standard protocol, mice tail’s tip blood sample was collected before sacrifice (Morita et al. 1997). Further pre-cleaned slides were used for smear preparation. Following drying, the slides were treated with absolute methanol for 5 min and subsequently stained with acridine orange. The Olympus fluorescent microscope (Model BX 51) was utilized for observing the slides at the objective (100 ×) under oil immersion with the digital photomicrograph software (OLYSIA BioReport, 2001).
Biochemical analysis
Biomarkers for inflammation and oxidative stress in serum
The levels of lipopolysaccharide and IL-1 β levels were evaluated by employing the ELISA technique in accordance with the guidelines provided by the manufacturer. The levels of SGPT and SGOT were analyzed through the ERBA commercial kits as per standard protocol.
Biomarkers for oxidative stress and inflammation in the colon
To assess inflammation, myeloperoxidase (MPO) (Ohkawa et al. 1979) and nitrite concentrations (Green et al. 1982) were assessed by adhering to established procedures with colon tissue homogenate with phosphate buffer and then underwent three cycles of freezing and thawing, and then centrifugation was performed by rpm 4000 g at 4 °C up to 20 min. The concentration of MPO in the sample was determined by adding o-dianisidine. The MPO activity was assessed spectrophotometrically at 460 nm wavelength. For conducting the nitrite assay, supernatant and Griess reagent were mixed in equal volumes, and for completing this reaction 10–15 min in the dark incubated mixture, the value of absorbance was measured at 540 nm. Biuret assay is used for estimating protein concentration.
TUNEL assay for detection of apoptotic cells
The colon and liver were fixed in 10% v/v formalin, blocks were prepared in paraffin, and slides were prepared with microtome (Leica RM2145, Germany) 4 µm sections. DNA disintegration was assessed through terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) (Calbiochem, Oncogene Research Product, USA) assay. As per the standard protocol, the assay was conducted, and the software “Isis” was utilized for image analysis of the total cell population (Carl Zeiss, AxioImager M1, Germany) and TUNEL-positive cell count. A charged coupled device (CCD) camera was used for image capturing.
Histopathological examination
The animals were euthanized, and the colon and liver were isolated, then fixed these organs with formalin 10% v/v. Dehydration of tissue was done with increasing concentrations of ethanol, and then paraffin blocks were prepared, 5 µm thick sections of tissue through Mayer’s albumin mounted over glass slides. Sections underwent staining with standard stains which include hematoxylin and eosin for morphological examination (Pai et al. 2018) (score scale was used for the colon and liver histology damage), picrosirius red-acid fast green for collagen deposition (Segnani et al. 2015), alcian blue for goblet cell (Otali et al. 2016), and ortho-toluidine blue for mast cell, and then further observation of the slides was done at 20 × and 40 × magnifications under the Olympus BX51, Tokyo, Japan, microscope. The impact of SOV on the production of mucous-secreting goblet cells and collagen deposition quantified with ImageJ software (version 1.54h, National Institutes of Health, Bethesda, MD).
Immunohistochemistry
Paraffin sections were cleared and rehydrated with xylene and grade concentrations of alcohol. Further, citrate buffer was utilized for antigen retrieval at 95–100 °C incubation. The primary antibodies of anti-NF-κBp65, anti-Nrf2, and anti-IL-6 (1:50 dilution with TBS) were added on sections and incubated throughout the night. Further, slides were treated with goat anti-mouse IgG and goat anti-rabbit IgG or depending upon the 1° antibody and stained the remaining background with haematoxylin. The percentage of immunopositive areas was measured utilizing the “Image J” software.
Statistical analyses
GraphPad Prism 8 software was utilized for analyzing the obtained data, and the mean ± SEM was used to express the results. Statistical differences among all distinct groups were assessed by Bonferroni post hoc testing for two-way ANOVA and Tukey’s test for one-way ANOVA multiple comparisons within groups, and p < 0.05 represents statistical significance.
Results
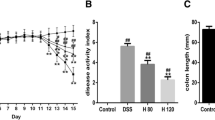
Effect of SOV on Disease Activity Index (DAI) and colon length
The Disease Activity Index (DAI) demonstrates a direct association with the severity of ulcerative colitis. Animals exposed to DSS exhibited a significant (p < 0.001) decline in body weight percentage, colon length, and DAI score significantly higher as compared to the CON group. In contrast to this, the administration of DSS + SOV 5 and SOV 10 led to a significant (p < 0.001) upturn in body weight percentage, colon length, and DAI score significantly (p < 0.001) decreased as compared to diseased group DSS. The DSS + SOV 5 intervention group demonstrated a significant variation in body weight percentage (p < 0.001), length of colon (p < 0.01), and DAI score showed a non-significant difference, whereas the DSS + SOV 10 group did not display any notable difference in body weight percentage, length of colon, and DAI score in comparison to the DSS + 5-ASA group (Fig. 2a, b, c, d, and e).
a Pictorial representation demonstrates the stool consistency as indicated in a dotted circle black, rectal bleeding mark as a black square. b Pictorial representation demonstrates the colon length. c Histogram showing Disease Activity Index. d Colon length. e Percentage change in body weight. All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, ##p < 0.01, ###p < 0.001 vs. DSS, **p < 0.01, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented by one-way ANOVA analysis using Turkey’s test and two-way analysis of variance (ANOVA) with post hoc test Bonferroni. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg
Effect of SOV on the oxidative stress markers and inflammation
The oxidative burden and inflammatory biomarkers including LPS, IL-1β, MPO, nitrite, SGPT, and SGOT (p < 0.001) significantly elevated in DSS-exposed groups in comparison of CON group mice. However, the DSS + SOV 5 and SOV 10 showed a significant reduction in LPS, IL-1β, MPO, nitrite, SGPT, and SGOT levels as compared to the DSS. Besides this, the intervention group DSS + SOV 5 showed a significant difference in LPS (p < 0.01), IL-1β (p < 0.001), MPO (p < 0.001), nitrite (p < 0.001), SGPT (p < 0.001), and SGOT (p < 0.05), and DSS + SOV 10 showed a significant difference in LPS (p < 0.05), SGPT (p < 0.01), and SGOT (p < 0.001) levels whereas non-significant difference in MPO, IL-1β, and nitrite levels when compared to DSS + 5-ASA (Fig. 3a, b, c, d, e, f).
a Histogram represents plasma LPS levels (EU/L). b IL-1β levels (pg/ml). c Histogram represents myeloperoxidase levels (U/100 mg protein). d Nitrite levels (µM/mg protein). e Histogram represents SGPT levels (IU /dl). f SGOT levels (IU /dl). All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, ##p < 0.01, ###p < 0.001 vs. DSS, *p < 0.05, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented using one-way ANOVA analysis using Turkey’s test. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg
Effect of SOV on peripheral blood micronucleus
A significant rise in the count of micronucleated erythrocytes (MNERYs) was detected in the DSS exposure group (p < 0.001) in comparison to CON animals. A notable significant decrease in the number of MNERYs was noted in the DSS + SOV 5 (p < 0.01) and DSS + SOV 10 (p < 0.001) treatment group as compared to the DSS group. Furthermore, a significant difference was noted in DSS + SOV 5 (p < 0.001) and a non-significant difference with the DSS + SOV 10 group in a number of MNERYs compared with 5-ASA (Fig. 4a and b).
a Pictorial representation shows peripheral blood micronucleus in the red circle. b Histogram represents micronucleated erythrocytes per 1000 cells. All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, #p < 0.05, ###p < 0.001 vs. DSS, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented using one-way ANOVA analysis using Turkey’s test. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg
Effect of SOV on histological of the colon
Colon sections of all groups were studied under the microscopic through H&E staining where the DSS group exhibited crypt distortion, infiltration, and high histopathological score in comparison to the CON group (Fig. 5 1a and 1b). Microscopically examination with PSRFG staining (Fig. 5 2a and 2b), the DSS group displayed a significant (p < 0.001) rise in the percentage of fibrotic content and a significant decline in the percentage of goblet cell area in comparison to the CON group (Fig. 5 3a and 3b). Further, the mast cell number (Fig. 54a and 4b) is significantly elevated in DSS-exposed mice as compared to the CON group. However, the group DSS + SOV 5 and 10 effectively recovered tissue morphological changes, showed a (p < 0.05, p < 0.001) significant decline in the histological score, fibrosis score, and mast cell number significantly, and improved the goblet cell as compared to the DSS group. Whereas, a significant (p < 0.05, p < 0.001) difference was observed DSS + SOV 5 group in all histopathological examinations, and group DSS + SOV 10 showed a non-significant difference in all observed histopathological scores except the fibrosis score (p < 0.001) as compared with 5-ASA.
1a Pictorial representation shows crypt disruption (red star) leukocyte infiltration (red triangle). 1b Histogram represents histological score. 2a Pictorial representation shows PSRFG staining in colon sections; fibrotic area shows with red arrow. 2b Histogram represents fibrotic index. 3a Pictorial representation shows alcian blue staining of colon sections; loss in goblet cell area shows with red arrow. 3b Histogram represents percent goblet cell area. 4a Pictorial representation shows toluidine blue staining of colon sections; mast cell shows with dotted red arrow. 4b Histogram represents mast cell. All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, ##p < 0.01, ###p < 0.001 vs. DSS, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented using one-way ANOVA analysis using Turkey’s test. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg
Effect of SOV on histological of the liver
In the liver of normal and perse mice when examined microscopically through H&E staining, no abnormal view or architecture change was noted. The animal exposed to DSS initiates the damage in the liver that was indicated by inflammatory cell infiltration in the central vein, sinusoid dilation with (p < 0.001) a significant high histological (Fig. 6 1a and 1b), fibrosis score (Fig. 6 2a and 2b), and mast cell number (Fig. 6 3a and 3b), as compared to the DSS-exposed mice. Besides this, the 5-ASA, DSS + SOV 5 and 10 mice exhibit binuclear hepatocytes (BNs), which displayed promoting the proliferation of hepatocyte and histological (p < 0.01), fibrosis score (p < 0.001), and mast cell number significantly (p < 0.001) reduced which indicated the treatments facilitated recuperation against DSS induced liver damage. Besides this, the intervention group DSS + SOV 5 significant differences in histological (p < 0.001), fibrosis score (p < 0.001), and non-significant differences in mast cell number significantly and DSS + SOV 10 showed non-significant differences in the histological score in comparison to DSS + 5-ASA.
1a Pictorial representation shows H&E staining; inflammatory cell infiltration in the central vein represents with black arrow, sinusoid dilation leukocyte infiltration with black star, and binuclear hepatocyte with black dotted circle. 1b Histogram represents histological score. 2a Pictorial representation shows PSRFG staining of liver sections; fibrotic area shows with dotted black square. 2b Histogram represents percent fibrotic area. 3a Pictorial representation shows toluidine blue staining of liver sections; mast cell shows with dotted red arrow. 3b Histogram represents mast cell number. All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, ##p < 0.01, ###p < 0.001 vs. DSS, **p < 0.01, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented using one-way ANOVA analysis using Turkey’s test. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg
Effect of SOV on Nrf2, NF-κB, and IL-6 expression in the colon
Immunohistochemical analysis revealed that DSS significantly (p < 0.001) activates the inflammation by reducing the Nrf2 and exhibited a lessened proportion of immunopositive staining (Fig. 7 1a and 1b), whereas the expression of p < 0.001 significantly arises the NF-κB (Fig. 7 2a and 2b) and IL-6 (Fig. 7 3a and 3b) and showed a higher proportion of immunopositive staining in comparison of CON group. Conversely, the DSS + SOV 5 treatment group displayed a significant (p < 0.001) high expression of Nrf2 and low expression of NF-κB (p < 0.01) and IL-6 (p < 0.001) protein, as compared to the DSS group, whereas the DSS + SOV 10 treatment groups displayed (p < 0.001) significant upturn in the expression of Nrf2 and significant (p < 0.001) suppress the expression of NF-κB and IL-6 protein, as compared to the DSS group. Besides this, the intervention group DSS + SOV 5 showed a significant (p < 0.001) difference in all protein expression, and DSS + SOV 10 showed a significant difference in (p < 0.05) NF-κB, whereas the non-significant difference in Nrf2, IL-6 percentage immunopositive area in comparison to the standard group DSS + 5-ASA.
1a Pictorial representation shows Nrf2 expressions in colon sections of different groups. 1b Histogram represents percentage immunopositive area of Nrf2 protein. 2a Pictorial representation of the NF-κB expressions in colon sections of different groups. 2b Histogram represents percentage immunopositive area of NF-κB protein. 3a Pictorial representation shows IL-6 expressions in colon sections of different groups. 3b Histogram represents percentage immunopositive area of IL-6 protein. All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, ##p < 0.01 ###p < 0.001 vs. DSS, *p < 0.05, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented using one-way ANOVA analysis using Turkey’s test. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg; Nrf2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL-6, Interleukin 6
Effect of SOV on Nrf2, NF-κB, and IL-6 expression in the liver
Immunohistochemical analysis revealed that DSS significantly activates the inflammation by (p < 0.001) decreasing the Nrf2 (Fig. 8 1a and 1b) and increasing (p < 0.001) NF-κB (Fig. 8 2a and 2b) and IL-6 (Fig. 8 3a and 3b) expression and exhibiting a higher proportion of immunopositive staining in inflamed areas of liver tissue in comparison of the CON group. Conversely, the DSS + SOV 5 treatment group displayed a significant (p < 0.01) increase in the expression of Nrf2 and reduction in NF-κB (p < 0.01) and IL-6 (p < 0.001) protein and expressed less immunopositive region as compared to DSS treated mice, whereas the DSS + SOV 10 treatment group exhibited a significant (p < 0.001) increase in the expression of Nrf2 and more immunopositive region and significant reduction (p < 0.001) in NF-κB and IL-6 protein expression and expressed less immunopositive region as compared to DSS treated mice. Besides this, the intervention group DSS + SOV 5 showed a significant (p < 0.01, p < 0.001) difference, and DSS + SOV 10 showed a non-significant difference in the percentage immunopositive area in comparison to the standard group DSS + 5-ASA.
1a Pictorial representation shows Nrf2 expressions in colon sections of different groups. 1b Histogram represents percentage immunopositive area of Nrf2 protein. 2a Pictorial representation of the NF-κB expressions in colon sections of different groups. 2b Histogram represents percentage immunopositive area of NF-κB protein. 3a Pictorial representation shows IL-6 expressions in colon sections of different groups. 3b Histogram represents percentage immunopositive area of IL-6 protein. All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, ##p < 0.01, ###p < 0.001 vs. DSS, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented using one-way ANOVA analysis using Turkey’s test. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg; Nrf2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL-6, Interleukin 6
Effect of SOV on DNA damage
Colonic and hepatic sections were examined for DNA damage; the DSS group displayed a (p < 0.001) significant rise in the percentage of TUNEL-positive cells in comparison to the CON group. Conversely, the DSS + SOV 5 displayed significant (p < 0.01) and DSS + SOV 10 (p < 0.001) significant decline in the % of TUNEL-positive cells in comparison to the DSS group. However, the intervention group DSS + SOV 5 showed a significant (p < 0.001) difference, and DSS + SOV 10 showed a non-significant difference in the TUNEL-positive cells percentage in comparison to the standard group DSS + 5-ASA (Fig. 9 1a and 1b, 2a and 2b).
1a Pictorial representation demonstrates the colon (DAPI, FITC, and merged) of TUNEL-positive cells (represented with the red circle). 1b Histogram showing percentage of TUNEL-positive cells in different groups. 2a Pictorial representation demonstrates the liver (DAPI, FITC, and merged) of TUNEL-positive cells (represented with the red circle). 2b Histogram showing percentage of TUNEL-positive cells in different groups. All values are expressed as mean ± SEM (n = 7–8). @@@p < 0.001 vs. CON, ##p < 0.01, ###p < 0.001 vs. DSS, ***p < 0.001 vs. DSS + 5-ASA. The statistical data analysis was presented using one-way ANOVA analysis using Turkey’s test. DSS, dextran sulfate sodium 2.5% w/v; 5-ASA, 5-aminosalicylic acid 100 mg/kg; SOV 5, sodium orthovanadate 5 mg/kg; SOV 10, sodium orthovanadate 10 mg/kg; DAPI, 4′;6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling
Discussion
The interdependence of the gut and the liver disturbances worsen a range of hepatic diseases. Roughly 25–30% of patients experience impaired liver function, and about 4–5% will progress to chronic liver disease (Seidelin and Nielsen 2009). Research suggests that colon inflammation and ulcers cause rectal bleeding, mice fed 2–10% DSS developed acute colitis, with symptoms including diarrhea, bleeding from the rectal, and loss of body weight throughout the 10–15 days (Babu et al. 2023). However, the acute nature of the DSS model limits its relevance for studying long-term disease progression and chronic inflammatory processes characteristic of human IBD (Chassaing et al. 2014). Despite this, DSS remains valuable for initial screening of anti-inflammatory drugs, studying the innate immune response, and understanding basic mechanisms of epithelial injury and repair (Wirtz et al. 2017). The primary reason for these symptoms is the presence of multiple erosions and inflammatory alterations (Juluri et al. 2011). In contrast to this, our current result showed a reduction in ulcerative colitis symptoms such as weight loss, diarrhea, and bloody stools, which resulted overall significant (p < 0.001) decline in DAI score in SOV 5 and 10 and 5-ASA treatment groups as compared to the DSS-exposed animals. The inflammatory environment inside the intestines helps to transfer the colitis into systemic inflammation. The primary significance lies in LPS directly initiating liver injury via the gut-liver axis, thereby raising in pro-inflammatory cytokines originating from the liver and worsening the progression of UC (Taehwan et al. 2024). Researchers have reported the reactive species of oxygen and nitrogen triggered oxidative burden, driven by inflammation that leads to lipid peroxidation, and molecular damage in colitis patients (Chassaing et al. 2014; Tuzun et al. 2002). In the current research, an overproduction of free radicals was suggested by the significant (p < 0.001) increase in levels of oxidative stress markers including LPS, IL-1β, MPO, nitrite, and hepatic markers SGPT and SGOT in colitis-induced animals, whereas our intervention, SOV, low and high doses restore the levels of these markers which demonstrated anti-inflammatory and anti-oxidative property of present intervention model. Research has indicated that intestine inflammation or dysplasia tends to occur in multiple locations across the colon (Levi and Harpaz 2006). Additionally, the disruptions in the mucus layer can reduce intercellular adhesion, making individuals more vulnerable to pathogens and thereby triggering inflammation and dysbiosis (Riddell et al. 1983). In our recent study, the DSS-exposed mice exhibited intense colonic mucosal and crypts disruption, whereas in liver inflammatory cell infiltration in the central vein, sinusoid dilation, leukocyte infiltration, and less binuclear hepatocyte. Moreover, the high fibrotic index, reduced goblet cell percentage, and increased mast cell count were observed in both of the organs. Conversely, the groups DSS + SOV 5 and 10 demonstrated significant (p < 0.01, p < 0.001) improvement in all these histopathological changes by improving the cell architecture, reducing the collagen deposition, regenerating the goblet cells, hepatocyte, and reducing the mast cell number. The oxidative stress encourages the molecular pathways that initiate genetic damage which includes DNA single-nucleobase lesions, strand breaks, and impairment cross linking of inter- and intra-strand (Melis et al. 2013). This effect is correlated with our study results, and micronucleated cell count is significantly (p < 0.001) high in the case of the colitis-induced mice that indicates the possibility of DNA damage, whereas our DSS + SOV 5 and 10 significantly reduce micronucleated cells. The Nrf2/Keap1axis primarily influences DNA damage repair pathways including BER (base excision repair), NER (nucleotide excision repair), MMR (mismatch repair), HR, and NHEJ (homologous recombination and non-homologous end joining). It is hypothesized SOV might alter the Nrf2/Keap1axis for maintaining cellular redox balance and reducing DNA lesions by activating supports DNA repair (Jayakumar et al. 2015). Besides this, Nrf2/Keap1 axis is important for both the development and upkeep of optimal functioning of GI (Liu et al. 2023). The researcher reported and published evidence of this axis in the progression of UC (Khor et al. 2006).
Nrf2 not only impacted NF-κB function and the production of inflammation-triggering cytokines like IL-1β, IL-6, and TNF-α, but also influenced the expression of ICAM-1 gene, intestinal permeability, and the level of endotoxin in the bloodstream (Qu et al. 2020; Gao et al. 2022). Earlier research indicated that DSS-treated Nrf2 knockout mice exhibited a threefold rise in lipid peroxidation and significant oxidative harm compared to their wild-type mice (Khor et al. 2006). Furthermore, the data reported that the mice with colitis symptoms had high expression of NF-κB; therefore, the silencing of these pathways could serve as a better treatment approach in colitis patients (Laurindo et al. 2023). In contrast to this, in the present investigation, the substantial significant (p < 0.001) reduction in Nrf2/Keap1 expression and overexpression of NF-κB and IL-6 was exhibited in the DSS group in comparison to control group animals. The treatment groups DSS + SOV 5, 10, and DSS + 5-ASA (p < 0.01, p < 0.001) significantly increase the area immunopositive cells in the case of Nrf2/Keap 1 expression and decline NF-κB and IL-6 proteins expression. Activation of Nrf2 could potentially correlate with heightened mobilization of NF-κB during an inflammatory response. Nevertheless, the direct correlation between inhibiting NF-κB and activating Nrf2 has not been conclusively established in colitis. Additional investigation is needed to clarify the specific impact of SOV by utilizing Nrf2 and NF-κB knockout mice. Research has shown that apoptosis serves as an indicator of active UC (Liu et al. 2017). One primary contributor to UC is the unfortunate occurrence of apoptosis, that is leading to the exposure of pathogenic attack to immune cell mucosal lining in the gut (Seidelin and Nielsen 2009). In this current investigation, the proportion of TUNEL-positive cells was notably greater in the group treated with DSS in comparison to the control group. However, after treatment with DSS + SOV 5 and SOV 10, as well as DSS + 5-ASA, there exists a significant reduction in the % TUNEL-positive cells. Additionally, PTPs control the cell cycle by managing the phosphorylation status of essential proteins involved in cell division. Consequently, SOV can enhance the survival and proliferation of epithelial cells in the gut lining, supporting the repair of ulcers and promoting mucosal healing and regeneration. Therefore, the current research demonstrates that SOV notably reduces colonic mucosal damage and associated liver dysfunctioning against colitis mice by suppressing ROS generation and release of inflammatory cytokines NF-κB and IL-6 and potentiating the cytodefensive mediator Nrf2/Keap1 expression. The molecular mechanism identified in this investigation recommends that sodium orthovanadate has the potency to be effective in treating colitis.
Conclusion
Comprehensive assessments, including DAI scores, biochemical markers of oxidative stress, neutrophil migration, histopathological findings, and immunohistochemical analysis confirmed that sodium orthovanadate significantly improved the anti-inflammatory; antioxidant activities against the DSS induced liver damage in male BALB/c mice. The high dose of 10 mg/kg shows more significant effects than the low dose of 5 mg/kg.
Data availability
No datasets were generated or analysed during the current study.
References
Babu SS, Singla S, Jena G (2023) Role of combination treatment of aspirin and zinc in DMH-DSS-induced colon inflammation, oxidative stress and tumour progression in male BALB/c mice. Biol Trace Elem Res 201(3):1327–1343. https://doi.org/10.1007/s12011-022-03241-3
Begum N, Sussman KE, Draznin B (1991) Differential effects of diabetes on adipocyte and liver phosphotyrosine and phosphoserine phosphatase activities. Diabetes 40(12):1620–1629. https://doi.org/10.2337/diab.40.12.1620
Boirivant M, Amendola A, Butera A, Sanchez M, Xu L, Marinaro M, Kitani A, Digiacinto C, Strober W, Fuss IJ (2008) A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology 135:1612–1623. https://doi.org/10.1053/j.gastro.2008.07.028
Bressenot MA (2018) Which evidence for a treat to target strategy in ulcerative colitis? Best Pract Res Clin Gastroenterol 32(33):3–8. https://doi.org/10.1016/j.bpg.2018.05.001
Burisch J, Zhao M, Odes S, De Cruz P, Vermeire S, Bernstein CN, Kaplan GG, Duricova D, Greenberg D, Melberg HO et al (2023) The cost of inflammatory bowel disease in high-income settings: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 8(5):458–492. https://doi.org/10.1016/S2468-1253(23)00003-1
Chassaing B, Aitken JD, Malleshappa M, Kumar VM (2014) Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 15(25):1–14. https://doi.org/10.1002/0471142735.im1525s104
Currie CG, Mccallum K, Poxton IR (2001) Mucosal and systemic antibody responses to the lipopolysaccharide of Escherichia coli O157 in health and disease. J Med Microbiol 50:345–354. https://doi.org/10.1099/0022-1317-50-4-345
Danese S, Banerjee R, Cummings JF, Dotan I, Kotze PG, Leong RL, Paridaens K et al (2018) Consensus recommendations for patient-centered therapy in mild-to-moderate ulcerative colitis: the i Support Therapy-Access to Rapid Treatment (iSTART) approach. Intest Res 16(4):522–528. https://doi.org/10.5217/ir.2018.00073
Eichele DD, Kharbanda KK (2017) Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol 23:6016–6029. https://doi.org/10.3748/wjg.v23.i33.6016
Ek WE, Damato M, Halfvarson J (2014) The history of genetics in inflammatory bowel disease. Ann Gastroenterol 27(4):294–303
Fu S, Wu D, Jiang W, Li J, Long J, Jia C, Zhou T (2010) Molecular biomarkers in drug-induced liver injury: challenges and future perspectives. Front Pharmacol 10(1):1–15. https://doi.org/10.3389/fphar.2019.01667
Gao W, Guo L, Yang Y, Wang Y, Xia S, Gong H, Zhang BK, Yan M (2022) Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Front Cell Dev Biol 9(2):1–21. https://doi.org/10.3389/fcell.2021.809952
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126(1):131–138. https://doi.org/10.1016/0003-2697(82)90118-x
Gunther TM, Kviecinski MR, Baron CC, Felipe KB, Farias MS, Silva FO, Bucker NC et al (2013) Sodium orthovanadate associated with pharmacological doses of ascorbate causes an increased generation of ROS in tumor cells that inhibits proliferation and triggers apoptosis. Biochem Biophys Res Commun 430(3):883–888. https://doi.org/10.1016/j.bbrc.2012.12.061
Harrington JM, Haines LG, Levine KE, Liyanapatirana C, Essader AS, Fernando RA, Robinson VG et al (2021) Internal dose of vanadium in rats following repeated exposure to vanadyl sulfate and sodium orthovanadate via drinking water. Toxicol Appl Pharmacol 412(1):115395. https://doi.org/10.1016/j.taap.2021.115395
He Z, Song J, Li X, Xiaoqian ZH, Wu C, Xiao W, Du X et al (2021) Bis(ethylmaltolato)oxidovanadium (IV) alleviates neuronal apoptosis through regulating peroxisome proliferator-activated receptor γ in a triple transgenic animal model of Alzheimer’s disease. J Biol Inorg Chem 26(5):551–568. https://doi.org/10.1007/s00775-021-01874-8
Herrera C, Varela E, Sarrabayrouse G, Pozuelo M, Alonso VR et al (2023) Gut Microbiota composition in long-remission ulcerative colitis is close to a healthy gut microbiota. Inflamm Bowel Dis 29(9):1362–1369. https://doi.org/10.1093/ibd/izad058
Horowitz A, Chanez P, Haest X, Turner JR (2023) Paracellular permeability and tight junction regulation in gut health and disease. Nat Rev Gastroenterol Hepatol 20(7):417–432. https://doi.org/10.1038/s41575-023-00766-3
Jayakumar S, Pal D, Sandur S (2015) Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res Mol Mech Mutagen 779:33–45. https://doi.org/10.1016/j.mrfmmm.2015.06.007
Joshi A, Akhtar A, Saroj P, Kuhad A, Sah SP (2022) Antidepressant-like effect of sodium orthovanadate in a mouse model of chronic unpredictable mild stress. Eur J Pharmacol 919:174798. https://doi.org/10.1016/j.ejphar.2022.174798
Jostins L, Ripke S, Weersma RK, Duerr RH, Mcgovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nat 491:119–124. https://doi.org/10.1038/nature11582
Juluri R, Vuppalanchi R, Olson J, Unalp A, Van Natta ML, Cummings OW, Tonascia J, Chalasani N (2011) Generalizability of the nonalcoholic steatohepatitis Clinical Research Network histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol 45(1):55–58. https://doi.org/10.1097/MCG.0b013e3181dd1348
Kang S, Kim W, Jeong S, Lee Y, Nam J, Lee S, Jung Y (2017) Oxidized 5-aminosalicylic acid activates Nrf2-HO-1 pathway by covalently binding to Keap1: implication in anti-inflammatory actions of 5-aminosalicylic acid. Free Radic Biol Med 108(1):715–724. https://doi.org/10.1016/j.freeradbiomed.2017.04.366
Khalil AA, Jameson MJ (2017) Sodium orthovanadate inhibits proliferation and triggers apoptosis in oral squamous cell carcinoma in vitro. Biochem 82(2):149–155. https://doi.org/10.1134/S0006297917020067
Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN (2006) Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66(24):11580–11584. https://doi.org/10.1158/0008-5472.CAN-06-3562
Kim UJ, Lee BH, Lee KH (2019) Neuroprotective effects of a protein tyrosine phosphatase inhibitor against hippocampal excitotoxic injury. Brain Res 1719:133–139. https://doi.org/10.1016/j.brainres.2019.05.027
Laurindo LF, Santos AR, Carvalho A, Bechara MD, Guiguer EL, Goulart R et al (2023) Phytochemicals and regulation of NF-kB in inflammatory bowel diseases: an overview of in vitro and in vivo effects. Metabolites 13(1):96. https://doi.org/10.3390/metabo13010096
Le Berre C, Honap S, Peyrin BL (2023) Ulcerative colitis. Lancet (london, England) 402:571–584. https://doi.org/10.1016/S0140-6736(23)00966-2
Levi GS, Harpaz N (2006) Intestinal low-grade tubuloglandular adenocarcinoma in inflammatory bowel disease. Am J Surg Pathol 30(8):1022–1029. https://doi.org/10.1097/00000478-200608000-00014
Li C, Peng K, Xiao S, Long Y, Yu Q (2023) The role of Lactobacillus in inflammatory bowel disease: from actualities to prospects. Cell Death Discov 9(1):361. https://doi.org/10.1038/s41420-023-01666-w
Liu JZ, Vansommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T (2015) Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 47:979–986. https://doi.org/10.1038/ng.3359
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Liu D, Huo X, Gao L, Zhang J, Ni H, Cao L (2018) NF-κB and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed Pharmacother 102:922–929. https://doi.org/10.1016/j.biopha.2018.03.130
Liu H, Chen R, Wen S, Li Q, Lai X, Zhang Z, Sun L, Sun S, Cao F (2023) Tea (Camellia sinensis) ameliorates DSS-induced colitis and liver injury by inhibiting TLR4/NF-κB/NLRP3 inflammasome in mice. Biomed Pharmacother 158:114136. https://doi.org/10.1016/j.biopha.2022.114136
Mehdi MZ, Pandey SK, Theberge JF, Srivastava AK (2006) Insulin signal mimicry as a mechanism for the insulin-like effects of vanadium. Cell Biochem Biophys 44(1):73–81. https://doi.org/10.1385/CBB:44:1:073
Melis JP, Vansteeg H, Luijten M (2013) Oxidative DNA damage and nucleotide excision repair. Antioxid Redox Signal 18(18):2409–2419. https://doi.org/10.1089/ars.2012.5036
Mikami Y, Tsunoda J, Suzuki S, Mizushima I, Kiyohara H, Kanai T (2023) Significance of 5-aminosalicylic acid intolerance in the clinical management of ulcerative colitis. Digestion 104:58–65. https://doi.org/10.1159/000527452
Molodecky NA, Kaplan GG (2010) Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol 6:339–346
Morita T, Asano N, Awogi T, Sasaki YF, Seiichi S, Shimada H, Sutou S et al (1997) Evaluation of the rodent micronucleus assay in the screening of IARC carcinogens (Groups 1, 2A and 2B): the summary report of the 6th collaborative study by CSGMT/JEMS·MMS. Mutat Res Toxicol Environ Mutagen 389(1):3–122. https://doi.org/10.1016/S1383-5718(96)00070-8
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Omayone TP, Olaleye SB (2021) Biochemical and histopathological effects of low dose vanadium in the healing of acetic acid-induced colitis in male wistar rats. J Basic Clin Physiol Pharmacol 33:273–283. https://doi.org/10.1515/jbcpp-2020-0246
Otali D, Fredenburgh J, Oelschlager DK, Grizzle WE (2016) A standard tissue as a control for histochemical and immunohistochemical staining. Biotech Histochem Off Publ Biol Stain Comm 91(5):309–326. https://doi.org/10.1080/10520295.2016.1179342
Pai RK, Jairath V, Casteele NV (2018) The emerging role of histologic disease activity assessment in ulcerative colitis. Gastrointest Endosc 88(6):887–898. https://doi.org/10.1016/j.gie.2018.08.018
Qu Z, Sun J, Zhang W, Yu J, Zhuang C (2020) Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radic Biol Med 159(1):87–102. https://doi.org/10.1016/j.freeradbiomed.2020.06.028
Reddy JG, Loftus EV (2006) Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am 35:837–855. https://doi.org/10.1016/j.gtc.2006.09.008
Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC (1983) Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 14(11):931–968. https://doi.org/10.1016/s0046-8177(83)80175-0
Roy S, Dhaneshwar S, Mahmood T (2022) Exploring the potential of IL-1β inhibitor diacerein and its combination with 5-aminosalicylic acid for the possible ameliorating effect in TNBS induced experimental colitis in Wistar rats. Cur Drug Ther 17(2):132–146. https://doi.org/10.2174/1574885517666220328142715
Vashist M , Samaan M, Mosli M, Parker C, Macdonald J etal (2018) Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis 1(1):CD011450. https://doi.org/10.1002/14651858.CD011450.pub2.
Sandborn WJ, Hanauer SB (2003) The pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol & Ther 17:29–42. https://doi.org/10.1046/j.1365-2036.2003.01408.x
Santiago M, Stocker F, Ministro P, Goncalves R, Carvalho D, Portela F, Correia L, Lago P, Trindade E, Dias CC, Magro F (2022) Incidence trends of inflammatory bowel disease in a southern European country: a mirror of the western world? Clin Transl Gastroenterol 13:e00481. https://doi.org/10.14309/ctg.0000000000000481
Segnani C, Ippolito C, Antonioli L, Pellegrini C, Blandizzi C, Dolfi A, Bernardini N (2015) Histochemical detection of collagen fibers by sirius red/fast green is more sensitive than van Gieson or sirius red alone in normal and inflamed rat colon. PLoS ONE 10(12):e0144630. https://doi.org/10.1371/journal.pone.0144630
Seidelin JB, Nielsen OH (2009) Epithelial apoptosis: cause or consequence of ulcerative colitis? Scand. J Gastroenterol 44(12):1429–1434. https://doi.org/10.3109/00365520903301212
Singla S, Kumar V, Jena G (2023) 3-Aminobenzamide protectst against colitis associated diabetes mellits in male BALB/c mice: role of PARP-1, NLRP3, SIRT-1 AMPK. Biochim 211:96–109. https://doi.org/10.1016/j.biochi.2023.03.009
Taehwan A, Li S, Kim Y, You Y, Park Y (2024) Food preference-based screening method for identification of effectors of substance use disorders using Caenorhabditis elegans. Life Sci 345:122580. https://doi.org/10.1016/j.lfs.2024.122580
Tatiya N, Chatuphonprasert W, Jarukamjorn K (2018) Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol 30(1):1–10. https://doi.org/10.1515/jbcpp-2018-0036
Thin LY, Lawrance IC, Spilsbury K, Kava J, Olynyk JK (2014) Detection of liver injury in IBD using transient elastography. J Crohn’s Colitis 88(7):671–677. https://doi.org/10.1016/j.crohns.2013.12.006
Timmer A, Patton PH, Chande N, Mcdonald JW, Macdonald JK (2016) Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2016:CD000478. https://doi.org/10.1002/14651858.CD000478.pub4
Tuzun A, Erdil A, Inal V, Aydin A, Bagci S, Yesilova Z, Sayal A, Karaeren N, Dagalp K (2002) Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem 35(7):569–572. https://doi.org/10.1016/s0009-9120(02)00361-2
Ungaro R, Mehandru S, Allen PB, Peyrin BL, Colombel JF (2017) Ulcerative colitis. Lancet 402(10401):1756–1770. https://doi.org/10.1016/S0140-6736(16)32126-2
Viola A, Fiorino G, Costantino G, Fries W (2024) Epidemiology and clinical course of late onset inflammatory bowel disease. Minerva Gastroenterol 70:52–58. https://doi.org/10.23736/S2724-5985.21.02890-4
Wetwittayakhlang P, Lontai L, Gonczi L, Golovics PA, Hahn GD, Bessissow T, Lakatos PL (2021) Treatment targets in ulcerative colitis: is it time for all in, including histology? J Clin Med 10(23):5551. https://doi.org/10.3390/jcm10235551
Willsky GR, Halvorsen K, Godzala ME, Chi LH, Most MJ, Kaszynski P, Crans DC et al (2013) Coordination chemistry may explain pharmacokinetics and clinical response of vanadyl sulfate in type 2 diabetic patients. Metallomics 5(11):1491–1502. https://doi.org/10.1039/c3mt00162h
Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner FS, Neurath MF (2017) Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 12:1295–1309. https://doi.org/10.1038/nprot.2017.044
Yu Q, Jiang W, Li D, Gu M, Liu K, Dong L, Wang C, Jiang H, Dai W (2019) Sodium orthovanadate inhibits growth and triggers apoptosis of human anaplastic thyroid carcinoma cells in vitro and in vivo. Oncol Lett 17:4255–4262. https://doi.org/10.3892/ol.2019.10090
Acknowledgements
The authors express gratitude to the Director and Management committee members of Amar Shaheed Baba Ajit Singh Jujhar Singh Memorial College of Pharmacy in BELA (Ropar) and IK Gujral Punjab Technical University, Kapurthala, Jalandhar Punjab, for providing necessary research facilities.
Author information
Authors and Affiliations
Contributions
GK conducted experiments, collected and analyzed data, and wrote the initial draft. ASK conceptualized the study, designed the methodology, and validated the data. Both authors (GK and ASK) reviewed and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
The experiments were conducted in accordance with ethical guidelines for animal experimentation established by the Committee for Control and Supervision of Experiments on Animals (CCSEA), Government of India, New Delhi. The research plan was approved by the Institutional Animal Ethics Committee (IAEC) of Amar Shaheed Baba Ajit Singh Jujhar Singh Memorial College of Pharmacy, Bela 140111, Ropar, Punjab, India, under the designated ASCB/IAEC/18/23/180 reference number.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, G., Kushwah, A.S. Sodium orthovanadate protects against ulcerative colitis and associated liver damage in mice: insights into modulations of Nrf2/Keap1 and NF-κB pathways. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03335-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03335-w