Abstract
Background
The Wnt/β-catenin pathway regulates intestinal development, homeostasis, and regeneration after injury. Wnt/β-catenin signaling drives intestinal proliferation by activating expression of the c-Myc proto-oncogene (Myc) through the Myc 3′ Wnt responsive DNA element (Myc 3′ WRE). In a previous study, we found that deletion of the Myc 3′ WRE in mice caused increased MYC expression and increased cellular proliferation in the colon. When damaged by dextran sodium sulfate (DSS), the increased proliferative capacity of Myc 3′ WRE−/− colonocytes resulted in a more rapid recovery compared with wild-type (WT) mice. In that study, we did not examine involvement of the immune system in colonic regeneration.
Purpose
To characterize the innate immune response in Myc 3′ WRE−/− and WT mice during and after DSS-induced colonic injury.
Methods
Mice were fed 2.5 % DSS in their drinking water for five days to induce colonic damage and were then returned to normal water for two or four days to recover. Colonic sections were prepared and neutrophils and macrophages were analyzed by immunohistochemistry. Cytokine and chemokine levels were analyzed by probing a cytokine array with colonic lysates.
Results
In comparison with WT mice, there was enhanced leukocyte infiltration into the colonic mucosal and submucosal layers of Myc 3′ WRE−/− mice after DSS damage. Levels of activated neutrophils were substantially increased in damaged Myc 3′ WRE−/− colons as were levels of the neutrophil chemoattractants C5/C5a, CXCL1, and CXCL2.
Conclusion
The Myc 3′ WRE regulates neutrophil infiltration into DSS-damaged colons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The entire intestinal epithelium is replaced every 3–5 days, making the intestine one of the most rapidly regenerating organs in the body [1]. This level of regeneration requires a fine balance of cellular proliferation, differentiation, and apoptosis. The surface of the colon is characterized by a single layer of epithelial cells marked by thousands of invaginations, or crypts. Stem cells occupy the lower regions of each crypt and produce highly proliferative transit amplifying (TA) precursor cells [2]. As TA cells divide, they migrate up the crypt axis and differentiate into absorptive colonocytes, mucus-secreting goblet cells, or hormone-secreting enteroendocrine cells. Ultimately, these differentiated cells undergo apoptosis and are shed into the lumen. When the colon is severely injured, the colonic architecture initially deteriorates and stem cells are depleted [3–6]. During the repair process, stem cells repopulate the crypts and drive a proliferative program to restore the colonic crypt microenvironment.
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) in which the colonic epithelium is compromised and luminal bacteria penetrate into underlying mucosal and submucosal layers where they elicit a host immune response [7, 8]. Administration of dextran sodium sulfate (DSS) is a widely used model of UC in mice; it is also used as a model to study colonic regeneration after injury [9–11]. DSS perforates the colonic epithelium and leukocytes from the innate arm of the immune system, for example neutrophils and macrophages, respond to the tissue damage and invading bacteria. This process severely damages the colonic crypts; when DSS is removed, however, stem cells drive a regenerative program that stimulates cellular proliferation and repair.
The Wnt/β-catenin signaling pathway drives proliferation of the intestinal epithelium [12–14]. The key effector of the Wnt pathway is the β-catenin transcriptional co-activator. When Wnt binds Frizzled/LRP cell surface receptors, β-catenin translocates into the nucleus and associates with members of the T cell factor/lymphoid enhancer factor (TCF/Lef) family of sequence specific transcription factors. β-Catenin/TCF complexes bind Wnt-responsive DNA elements (WREs) and activate expression of Wnt/β-catenin target genes including the c-MYC protooncogene (MYC) [15–17]. MYC is a transcription factor that promotes cellular proliferation and growth by regulating expression of target genes that control ribosome biogenesis, metabolism, and progression through the cell cycle [18, 19]. The MYC 3′ WRE maps approximately 1.4 kb downstream from the MYC transcription stop site and this element controls MYC expression in human and mouse intestinal cells [17, 20, 21]. Deletion of the Myc 3′ WRE in mice led to increased MYC expression, increased cellular proliferation, and a decrease in differentiated cells within their colons [22].
As a key driver of intestinal cell proliferation, the Wnt/β-catenin signaling pathway is crucial for intestinal repair after injury [23, 24]. Ashton et al. [23] found that Wnt/β-catenin signaling was required for intestinal repair of damage caused by a sub-lethal dose of gamma-irradiation. In addition, they demonstrated that MYC was required for the Wnt/β-catenin-induced regeneration. Koch et al. [24] demonstrated that Wnt/β-catenin signaling promoted colonic regeneration in the DSS model; however, the role of MYC in this response was not evaluated. We have previously reported an enhanced regenerative phenotype of the colonic crypts of Myc 3′ WRE−/− mice in response to DSS-induced damage [22]. Because Myc 3′ WRE−/− mice contain 2.5-fold higher levels of MYC protein in the colon than WT mice, we concluded that MYC expression correlates with a greater regenerative propensity. Each of these aforementioned studies focused primarily on the Wnt/β-catenin stimulation of intestinal epithelial cells and did not consider the role of the innate immune system in mediating regeneration.
In our previous analysis, we noted that DSS treatment caused more dramatic thickening of the colonic submucosal layer in Myc 3′ WRE−/− mice [22]. This observation suggested that the Myc 3′ WRE might regulate the immune response to injury. In this study, we find that colonic damage stimulates a greater influx of activated neutrophils into the mucosal and submucosal layers of Myc 3′ WRE−/− versus WT mice. In addition, DSS treatment causes both increased numbers of macrophages and increased expression of cytokines and chemokines known to recruit neutrophils within Myc 3′ WRE−/− colons. Together, our data indicate that the Myc 3′ WRE is a critical regulator of neutrophil infiltration into damaged colons.
Methods
Mice and Dextran Sodium Sulfate-Induced Injury
Generation of Myc 3′ WRE−/− mice is described elsewhere [22]. Colonic injury was induced in seven-week-old mice by treating them with 2.5 % dextran sodium sulfate (DSS; TdB Consulting; AB DB001-229) in their drinking water for five days, after which the animals were returned to normal water and allowed to recover for up to four days. Tissue samples were collected before treatment, after five days of DSS treatment, and after two or four days of recovery. The Pennsylvania State University College of Medicine Institutional Animal Care and Use Committee approved the animal protocols used in this study.
Histology and Immunohistochemistry
Tissue collection, processing, and immunohistochemistry were performed as described elsewhere [22]. Briefly, tissue was collected from mice subjected to the DSS treatment protocol and fixed in 3.7 % paraformaldehyde overnight. Tissue was then paraffin-embedded and 4-μm sections were cut for slides. For immunohistochemistry, slides were dewaxed in xylenes and rehydrated in graded ethanol washes. Antigen retrieval was performed by heating slides in 10 mM citrate buffer, pH 6.0, for 20 min in a standard rice steamer. Slides were first blocked for 1 h with 10 % goat serum (Jackson Immunoresearch; 005-000-121), followed by avidin/biotin blocking (Vector Laboratories; SP-2001). The slides were incubated overnight at 4 °C with anti-Ly6/G (Santa Cruz Biotechnology; sc-53515) or anti-F4/80 (Santa Cruz Biotechnology; sc-59171) antibodies that were diluted 1:200 in 1 % bovine serum albumin. The slides were then washed in 1× TBST and incubated with secondary antibody (Vector; BA-9400) at 1:300 dilution in 1 % bovine serum albumin for 1 h at room temperature. The slides were treated with ABC reagent (Vector; PK-6100), developed with DAB (Vector; SK-4100), counterstained with hematoxylin (Fisher Scientific; CS401-1D), and mounted with cytoseal (Thermo Scientific; 8310-16). Images were taken on an Olympus scope by use of Toupeview software (Amscope; version x86 3.2.1168).
For Hanker–Yates staining, slides were dewaxed and rehydrated as above. A vial of peroxidase indicator reagent (Sigma; 390-1) was added to 50 mL prewarmed 100 mM Tris pH 6.8 containing 0.012 % hydrogen peroxide (Fisher Scientific; M-12141). Slides were incubated at 37 °C in the dark with Hanker–Yates reagent for 30 min. The slides were then washed with 1× PBS, counterstained with methylene green (Vector; H-3402), dehydrated, and mounted with cytoseal. Images were taken on an Olympus scope by use of Toupeview software.
Intestinal Barrier Function
To assess intestinal barrier function, mice were briefly anesthetized by use of isoflurane and gavaged with 0.4 mg/g TRITC-dextran (molecular mass 4.4 kDa; Sigma; T1037) in 1× PBS [6]. After 4 h, blood was collected by cardiac puncture and samples were measured in duplicate on a Spectramax Paradigm plate reader (Molecular Devices). The values reported are normalized to levels measured in WT mice.
Flow Cytometry
Blood was collected by cardiac puncture from untreated animals, and red blood cells were lysed by incubating the samples in pre-warmed FCM lysing solution (Santa Cruz; sc-3621) at room temperature for 5 min. White blood cells were collected by centrifugation at 300×g for 5 min and resuspended in cold 1× PBS. FC receptors were blocked by incubating the samples with 0.5 μg FCγ RIIb/CD16-2 antibody (Santa Cruz; sc-18867) at room temperature for 10 min. Next, 1 μg Ly6/G antibody was added and the samples were incubated for 20 min at room temperature. The leukocytes were pelleted by centrifugation for 5 min at 300×g and washed three times with 1× PBS to remove unbound antibody. The samples were then incubated with 0.5 μg goat anti-rat PE-CY5 conjugated secondary (Santa Cruz; sc-3830) for 20 min at room temperature, followed by three additional washes with 1× PBS. The cells were then resuspended in 300 μl 1 % paraformaldehyde, and the samples were analyzed on a Guava PCA flow cytometer (Millipore).
Myeloperoxidase Activity Assays
Approximately 3 cm of a distal colon section was first Dounce homogenized in 500 μl 50 mM potassium phosphate buffer (pH 6.0) containing 0.5 % hexadecyltrimethylammonium bromide (Sigma; H9268); the sample was then briefly sonicated on ice for 10 s [25, 26]. The lysates were subjected to three freeze–thaw cycles, and the samples were cleared by centrifugation at 14,000×g for 15 min at 4 °C. A milligram of soluble colonic lysate was then added to 1 ml 50 mM phosphate buffer (pH 6.0) containing 0.167 mg/ml o-dianisidine dihydrochloride (TCI; D1657) and 0.005 % hydrogen peroxide. Myeloperoxidase (MPO) activity was measured spectrophotometrically and is reported as the change in absorbance at 450 nm over time.
Cytokine Analysis
Mouse cytokine array panels were purchased from R&D systems (ARY006) and analyzed in accordance with the manufacturer’s recommended procedure. Colon samples were collected from untreated and DSS-treated animals and Dounce homogenized in 1× PBS containing 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF. Triton-X (Fisher Scientific, BP151) was then added to a final concentration of 1 %. Samples were frozen at −70 °C, thawed, and centrifuged at 10,000×g for 5 min at 4 °C. The protein content of the lysate was quantified by use of Bradford reagent and 200 μg was incubated with the array membrane overnight at 4 °C. The membrane was then washed in 1× TBST and developed in accordance with the manufacturer’s recommended procedure. The signals from two independent experiments (four spots in total for each cytokine) were quantified by use of Image J software.
Statistical Analysis
Leukocytes, neutrophils, and macrophages were quantified from 10 high-powered fields of view per animal (n = 3 mice per genotype per time point) and p values were assessed by use of the Mann–Whitney U test. In MPO assays, flow cytometry, and TRITC-dextran experiments, p values were determined by use of Student’s t test (n = 3–6 mice per genotype per experiment, as described).
Results
DSS Causes a Greater Influx of Leukocytes into the Colonic Mucosal Layers of Myc 3′ WRE−/− Mice in Comparison with WT Mice
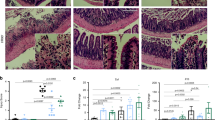
To study colonic regeneration in response to injury, Myc 3′ WRE−/− and WT mice were fed 2.5 % DSS in their drinking water for five days and then returned to normal drinking water for four days (Fig. 1a) [10, 27]. We collected colonic tissues before DSS treatment (PT), after DSS treatment (DSS), and two or four days during the recovery period (RD 2 and RD 4, respectively). The tissues were sectioned, stained with hematoxylin and eosin, and the leukocytes were tallied in the mucosal layer. Before the treatment, there were slightly more resident leukocytes within the colons of Myc 3′ WRE−/− animals (Fig. 1b, c). DSS treatment caused a more dramatic influx of leukocytes into Myc 3′ WRE−/− mucosal layers. As we had previously reported, more accelerated regeneration of the colonic architecture in knockout mice was evident at RD 2 [22].
DSS causes a greater influx of leukocytes into the colonic mucosa of Myc 3′ WRE−/− mice in comparison with WT mice. a Schematic diagram of the DSS treatment/recovery protocol. Mice were given 2.5 % DSS in their drinking water for five days and then returned to normal drinking water. Colonic tissue was collected and sectioned before treatment (pretreatment, PT), after DSS exposure (DSS), and after two (RD 2) or four (RD 4) days of returning them to normal drinking water. b H&E-stained sections of colonic tissue prepared after the indicated treatments. Each panel is a representative image from n = 3 mice examined per genotype and enlargements of the boxed regions are placed to the right of each image. Yellow arrows identify representative leukocytes in each panel. c Quantification of leukocytes in 10 fields of the colonic mucosa. Error is standard error of the mean (*p < 0.05 and **p < 0.0005)
Next, we examined leukocytes in the submucosal layer and found that although levels in the colonic submucosa were negligible in pretreated animals, DSS treatment caused a marked increase in infiltrating leukocytes in both groups of mice. In fact, there were 2.5-fold more leukocytes in damaged Myc 3′ WRE−/− versus WT colons (Fig. 2a, b). Together, these results indicate that injury induces a more robust immune response within the colons of Myc 3′ WRE−/− mice and implicate the Myc 3′ WRE as a critical mediator of colonic inflammation.
DSS causes a greater influx of leukocytes into the colonic submucosa of Myc 3′ WRE−/− mice in comparison with WT mice. a H&E-stained sections of colonic tissue prepared before treatment and after five days of DSS treatment. Each panel is a representative image from n = 3 mice examined per genotype and enlargements of the boxed regions are placed to the right of each image. Yellow arrows identify representative leukocytes in each image. b Quantification of leukocytes in 10 fields of the colonic submucosa. Error is standard error of the mean (*p < 0.0005)
Because we observed a slight, but significant increase in the number of leukocytes within the mucosa of knockout animals before treatment (Fig. 1b, c), we hypothesized that Myc 3′ WRE−/− intestines may have a reduced barrier capacity. To test this hypothesis, untreated animals were gavaged with TRITC-dextran and, 4 h later, blood was collected via cardiac puncture. Low levels of TRITC-dextran will pass into the bloodstream if the intestinal barrier is intact, and these levels will increase if the barrier function of the intestinal epithelium is disrupted. This analysis found higher levels of TRITC-dextran in the bloodstream of Myc 3′ WRE−/− mice, suggesting that deletion of the Myc 3′ WRE compromises the barrier function of the intestine (Fig. 3).
The intestinal barrier is slightly compromised in the intestines of Myc 3′ WRE−/− mice. Mice were gavaged with TRITC-dextran and fluorescence was measured in the blood 4 h later. Values detected are normalized to WT levels and n = 6 mice were examined for each genotype. Error is standard error of the mean (*p < 0.005)
DSS Causes Greater Infiltration of Neutrophils into the Colons of Myc 3′ WRE−/− Mice in Comparison with WT
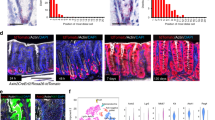
When the colonic epithelium is damaged, neutrophils migrate from the bloodstream to the site of injury and phagocytose invading bacteria. To determine whether neutrophils are among the leukocytes recruited in response to DSS-induced colonic injury, we stained colonic sections with antibodies against the neutrophil surface antigen, Ly6/G. Myc 3′ WRE−/− colons contained fivefold more neutrophils after DSS treatment and this enhanced infiltration characterized both the colonic mucosal and submucosal layers (Fig. 4a–d). Interestingly, even though there were more neutrophils recruited to damaged Myc 3′ WRE knockout colons, the Myc 3′ WRE does not seem to have a major effect in resolving the inflammatory response as neutrophil levels decreased at the same rate in the colons of both groups of mice during the recovery phases of the protocol (Fig. 4b).
DSS causes greater infiltration of neutrophils into the colons of Myc 3′ WRE−/− mice in comparison with WT. a Colonic sections were prepared from Myc 3′ WRE−/− and WT mice at the indicated time during the DSS protocol and neutrophils were identified by staining sections with antibodies against Ly6/G. Representative images are shown and arrows identify Ly6/G+ leukocytes. b Quantification of Ly6/G+ cells from 10 fields for each time point per mouse. c Quantification of Ly6/G+ cells in 10 fields of colonic mucosal and d submucosal layers before and after DSS treatment. In b–d, n = 3 mice were examined at each time point for each genotype. Error is standard error of the mean and *p < 0.05, **p < 0.005, ***p < 0.0005
We next determined whether the infiltrating neutrophils were functional by measuring myeloperoxidase (MPO) activity. MPO is an enzyme expressed in activated neutrophils that facilitates the degradation of phagocytosed bacteria and bacterial products [26]. We did not detect MPO activity in colonic lysates prepared from untreated mice, which is consistent with the low levels of resident neutrophils in undamaged colons (Fig. 5a). DSS treatment caused a substantial increase in MPO activity within the colons of both Myc 3′ WRE−/− and WT mice (Fig. 5a). Importantly, in comparison with WT, knockout colons contained a 2.5-fold increase in DSS-induced MPO activity (Fig. 5a).
DSS causes greater infiltration of activated neutrophils into the colons of Myc 3′ WRE−/− mice in comparison with WT. a Relative myeloperoxidase (MPO) activity that was measured in protein homogenates from colonic tissue (n = 5 mice examined per genotype per time point; n.d. none detected). b Colonic sections were stained with Hanker–Yates reagent which detects myeloperoxidase activity. Representative images are shown and arrows indicate Hanker–Yates+ cells (n = 3 mice examined per genotype per time point). c Quantification of Hanker–Yates+ cells in 10 independent fields at the indicated times. In a, c, error is standard error of mean and *p < 0.05, **p < 0.0005
To test whether the increased MPO activity reflected an increased number of functional neutrophils, we assessed numbers of MPO+ cells in colonic tissue by staining sections with Hanker–Yates solution. This analysis clearly indicated that DSS-induced damage caused a higher number of activated neutrophils to infiltrate into Myc 3′ WRE−/− colons (Fig. 5b). Indeed, quantification of MPO+ cells revealed 2.5-fold more functional neutrophils that were recruited to Myc 3′ WRE−/− than to WT colons after damage (Fig. 5c). Taken together, these data indicate that DSS damage elicits a more dramatic influx of functional neutrophils into the mucosa and submucosa of Myc 3′ WRE−/− colons.
Because MYC is involved in neutrophil development, a simple explanation for our observations is that there are higher levels of circulating neutrophils in Myc 3′ WRE−/− mice. To assess this possibility, we used flow cytometry to analyze neutrophil levels in the blood of untreated animals. We observed no significant difference in the numbers of circulating neutrophils within the two groups of mice, and, if anything, there is a slight decrease in their numbers in Myc 3′ WRE−/− animals (Fig. 6a, b). Thus, the increased numbers of infiltrating neutrophils in Myc 3′ WRE−/− colons is not because of an overall increase in the number of neutrophils within these animals.
There is no difference in circulating neutrophils between Myc 3′ WRE−/− and WT mice. a Representative scatter plots of blood leukocytes labeled with anti-Ly6/G specific antibodies. b Average levels of circulating neutrophils reported as percentage of Ly6/G+ cells detected in purified white blood cells. Error is standard error of the mean (n = 5 mice examined per genotype). ns not significant
DSS Causes Higher Expression of Known Neutrophil Chemoattractants in the Colons of Myc 3′ WRE−/− Mice in Comparison with WT Mice
When tissue is damaged, epithelial cells and resident leukocytes are stimulated to secrete cytokines and chemokines to elicit both innate and adaptive immune responses to neutralize invading pathogens. To assess cytokine levels in the colons of Myc 3′ WRE−/− and WT mice, we prepared colonic lysates and probed cytokine arrays. Before treatment with DSS, we did not detect differences in cytokine levels in the colons of knockout versus WT mice (Fig. 7a, left panels). In contrast, DSS-induced damage led to elevated expression of several cytokines in the colons of Myc 3′ WRE−/− mice relative to WT (Fig. 7a, right panels). In particular, three known neutrophil chemoattractants, C5/C5a, CXCL2, and CXCL1 [28, 29], were expressed at higher levels in Myc 3′ WRE−/− colons after DSS treatment (Fig. 7b, c). These results suggest that the increased neutrophil infiltration of knockout colons after DSS-induced injury results from increased production of known neutrophil chemoattractants.
DSS causes higher expression of known neutrophil chemoattractants in the colons of Myc 3′ WRE−/− mice in comparison with WT mice. a Cytokine/chemokine arrays were probed with colonic lysates prepared from Myc 3′ WRE−/− and WT mice that were untreated or treated with DSS for five days. Signals on the blots that correspond to neutrophil chemoattractants are circled. Shown are representative blots from two independent experiments. b, c Densitometric quantification of b weakly and c highly expressed cytokines and chemokines from two independent experiments after DSS treatment. Shown is the average signal detected with error as the standard error of the mean. n.d. not detected
Because several cytokines, including CXCL2, CXCL1, and IL-6, are secreted by macrophages, we used immunohistochemistry with anti-F4/80 antibodies to analyze macrophages within the colons Myc 3′ WRE−/− and WT mice subjected to the DSS damage/recovery protocol. This analysis found no difference in the numbers of resident macrophages in pre-treated mice and DSS induced an increase in macrophages in the colons of both groups of mice (Fig. 8a, b). In fact, there were significantly more macrophages in damaged Myc 3′ WRE−/− colons, which is especially evident in the submucosa (Fig. 8b–d).
DSS induces higher levels of macrophages within colonic tissue of Myc 3′ WRE−/− mice in comparison with WT mice. a Colonic sections, collected at the indicated times, were stained with anti-F4/80 antibodies to detect macrophages. Representative images are shown and arrows depict F4/80+ cells (n = 3 mice analyzed per genotype per time point). b–d Quantification of macrophages in b colonic tissue, c the colonic mucosal layer, and d the colonic submucosal layer. In each case, macrophages were tallied from 10 independent fields of view. Error is standard error of the mean and *p < 0.05, **p < 0.005
Discussion
The highly proliferative nature of colons enables their efficient repair and regeneration after acute injury. When colons are subjected to damage by DSS, an innate immune response initially results in deterioration of the colonic crypt architecture [10]. On removal of DSS, colonic stem cells divide and produce the highly proliferative transit amplifying cells [3]. As these migrate toward the lumen, TA cells differentiate into the functional cells of the colon. Remarkably, in as little as 3–4 days the repaired colons are virtually indistinguishable from pre-DSS treated colons [22]. The Wnt/β-catenin signaling pathway is important in stimulating cellular proliferation and regeneration after DSS-induced damage by activating expression of target genes including Myc. In the intestine, Wnt/β-catenin regulation of Myc expression is controlled by the Myc 3′ WRE. Although Myc 3′ WRE−/− mice contain 2.5-fold higher levels of colonic MYC [22], expression of another Wnt/β-catenin target gene, Axin2, was not affected (data not shown). This finding suggests that the Myc 3′ WRE does not affect global Wnt/β-catenin signaling in the intestine. Despite this, Myc 3′ WRE−/− mice recover more quickly from DSS-induced damage than WT littermates subjected to the same protocol [22]. During recovery, the heightened MYC expression in Myc 3′ WRE−/− mice led to a more rapid re-establishment of the colonic architecture by promoting colonocyte proliferation and restoring goblet cell populations. Here, we provide evidence that MYC also regulates neutrophil infiltration into colonic tissues during DSS-induced damage.
Our assessment of barrier function indicated that the intestinal epithelium was slightly compromised in Myc 3′ WRE−/− animals (Fig. 3). This may, in part, explain our observations that the colons of Myc 3′ WRE−/− mice had elevated levels of leukocytes prior to damage with DSS (Fig. 1c). Our data support the following model of how the elevated MYC expression in Myc 3′ WRE−/− colons leads to increased neutrophil infiltration during DSS-induced damage. As bacteria penetrate into the mucosal and submucosal layers, bacterial products, for example lipopolysaccharide (LPS), stimulate colonocytes and/or macrophages to express various cytokines and chemokines, including C5/C5a, CXCL1, and CXCL2 [30–32]. Neutrophils perceive these chemoattractants, and migrate from the blood stream into the colonic tissue. CXCL2 is a direct MYC target gene [33]. Therefore, the heightened MYC expression in Myc 3′ WRE−/− colonocytes may induce higher levels of CXCL2 expression. It is currently unknown whether C5/C5a, CXCL1, or any of the other factors that were differently expressed in DSS-treated WT and Myc 3′ WRE−/− mice in our array analysis are direct MYC target genes. The heightened expression of these targets may also reflect an indirect mechanism. More detailed characterization of target gene expression is required to distinguish between these two possibilities.
We cannot formally exclude the possibility that the slightly increased levels of resident leukocytes in the colons of Myc 3′ WRE−/− mice may account, in part, for the enhanced immune response to acute damage caused by DSS (Fig. 1). However, the levels of neutrophils and resident macrophages in uninjured mucosal and submucosal colonic layers were largely equivalent in WT and Myc 3′ WRE−/− mice (Figs. 4, 8). It is possible that innate immune cells other than macrophages or neutrophils are involved in the response. Our attempts to analyze natural killer (NK) cell populations were unsuccessful (data not shown) leaving the identity of additional resident leukocytes unknown. In addition to heightened expression of known neutrophil chemoattactants in Myc 3′ WRE−/− colons after DSS-induced damage, several other chemokines and cytokines were also expressed to higher levels in the knockouts. Among these are IL-16, which modulates T cell activation [34, 35], and CCL2, which attracts monocytes [36]. In this acute model of DSS-induced damage, it is unlikely that adaptive immune cells are contributing to the inflammatory response over the time period of our protocol [37]. However, these results suggest that adaptive immune cells may also be important in the enhanced crypt regeneration of Myc 3′ WRE−/− mice after chronic damage and inflammation. We are currently analyzing the immune response and colonic regeneration in WT and Myc 3′ WRE−/− mice by use of a DSS treatment/recovery protocol that more faithfully models the chronic inflammation seen in UC patients.
The effect of neutrophils in the pathophysiology of UC is not fully resolved. On the one hand, blocking neutrophil recruitment limits damage to the colonic architecture [38–40]. On the other hand, neutrophil depletion also prevents effective clearance of bacteria from DSS-damaged colons [41, 42]. In the Myc 3′ WRE−/− colons, we did not observe increased tissue damage after DSS treatment compared with controls (Fig. 1; [22]). Therefore in this acute model of colitis, the enhanced recruitment of neutrophils is likely to contribute to the more rapid recovery of Myc 3′ WRE−/− colonic crypts after DSS is removed. However, UC patients contain elevated levels of MYC expression and persistent neutrophil infiltration within their colons [43–45]. Therefore, it is possible that in UC patients, increased MYC expression may contribute to disease pathogenesis. Several chemical inhibitors of MYC function or expression are now commercially available, but these have not been evaluated in mouse models of UC [46–49]. Because elevated MYC expression and colonic neutrophil infiltration are observed in Myc 3′ WRE−/− mice, these mice serve as an attractive model system for investigation of the involvement of MYC in chronic colitis and to test whether MYC inhibition is a therapeutically viable option.
References
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205.
Simons BD, Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res. 2011;317:2719–2724.
Davidson LA, Goldsby JS, Callaway ES, Shah MS, Barker N, Chapkin RS. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta. 1822;2012:1600–1607.
Fukui T, Takeda H, Shu HJ, et al. Investigation of Musashi-1 expressing cells in the murine model of dextran sodium sulfate-induced colitis. Dig Dis Sci. 2006;51:1260–1268.
Malvin NP, Seno H, Stappenbeck TS. Colonic epithelial response to injury requires Myd88 signaling in myeloid cells. Mucosal Immunol. 2012;5:194–206.
Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18:618–623.
Qin X. Etiology of inflammatory bowel disease: a unified hypothesis. World J Gastroenterol. 2012;18:1708–1722.
Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20.
Gersemann M, Wehkamp J, Stange EF. Innate immune dysfunction in inflammatory bowel disease. J Intern Med.. 2012;271:421–428.
Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc.. 2007;2:541–546.
Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev.. 2007;59:1073–1083.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713.
Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723.
He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512.
Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol. 2009;10:276–286.
Yochum GS, Cleland R, Goodman RH. A genome-wide screen for beta-catenin binding sites identifies a downstream enhancer element that controls c-Myc gene expression. Mol Cell Biol. 2008;28:7368–7379.
Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264.
Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–2766.
Yochum GS, McWeeney S, Rajaraman V, Cleland R, Peters S, Goodman RH. Serial analysis of chromatin occupancy identifies beta-catenin target genes in colorectal carcinoma cells. Proc Natl Acad Sci USA. 2007;104:3324–3329.
Yochum GS, Sherrick CM, Macpartlin M, Goodman RH. A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5’ and 3′ Wnt responsive enhancers. Proc Natl Acad Sci USA. 2010;107:145–150.
Konsavage WM Jr, Jin G, Yochum GS. The myc 3′ wnt-responsive element regulates homeostasis and regeneration in the mouse intestinal tract. Mol Cell Biol. 2012;32:3891–3902.
Ashton GH, Morton JP, Myant K, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269.
Koch S, Nava P, Addis C, et al. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair Gastroenterology. 2011;141:259–268, 268 e251-258.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209.
Graff G, Gamache DA, Brady MT, Spellman JM, Yanni JM. Improved myeloperoxidase assay for quantitation of neutrophil influx in a rat model of endotoxin-induced uveitis. J Pharmacol Toxicol Methods. 1998;39:169–178.
Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267–278.
Bougarn S, Cunha P, Harmache A, Fromageau A, Gilbert FB, Rainard P. Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells. Clin Vaccine Immunol. 2010;17:1797–1809.
Reutershan J, Ley K. Bench-to-bedside review: acute respiratory distress syndrome: how neutrophils migrate into the lung. Crit Care. 2004;8:453–461.
De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180:4308–4315.
Godaly G, Bergsten G, Hang L, et al. Neutrophil recruitment, chemokine receptors, and resistance to mucosal infection. J Leukoc Biol. 2001;69:899–906.
Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol. 2002;16:241–246.
Fernandez PC, Frank SR, Wang L, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129.
Cruikshank W, Little F. lnterleukin-16: the ins and outs of regulating T-cell activation. Crit Rev Immunol. 2008;28:467–483.
Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. J Leukoc Biol.. 2000;67:757–766.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326.
Hall LJ, Faivre E, Quinlan A, Shanahan F, Nally K, Melgar S. Induction and activation of adaptive immune populations during acute and chronic phases of a murine model of experimental colitis. Dig Dis Sci. 2011;56:79–89.
Buanne P, Di Carlo E, Caputi L, et al. Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol. 2007;82:1239–1246.
Schicho R, Bashashati M, Bawa M, et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm Bowel Dis. 2011;17:1651–1664.
Sina C, Gavrilova O, Forster M, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183:7514–7522.
Butler M, Sanmugalingam D, Burton VJ, et al. Impairment of adenosine A3 receptor activity disrupts neutrophil migratory capacity and impacts innate immune function in vivo. Eur J Immunol. 2012;42:3358–3368.
Shea-Donohue T, Thomas K, Cody MJ, et al. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–124.
Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368.
Diamanti A, Colistro F, Basso MS, et al. Clinical role of calprotectin assay in determining histological relapses in children affected by inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14:1229–1235.
Farooq SM, Stillie R, Svensson M, Svanborg C, Strieter RM, Stadnyk AW. Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2009;329:123–129.
Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917.
Hammoudeh DI, Follis AV, Prochownik EV, Metallo SJ. Multiple independent binding sites for small-molecule inhibitors on the oncoprotein c-Myc. J Am Chem Soc.. 2009;131:7390–7401.
Rahl PB, Lin CY, Seila AC, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445.
Wang H, Hammoudeh DI, Follis AV, et al. Improved low molecular weight Myc-Max inhibitors. Mol Cancer Ther. 2007;6:2399–2408.
Acknowledgments
National Institutes of Health grant R01DK080805 to G.S.Y.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wesley M. Konsavage and Jennifer N. Roper: contributed equally to this work.
Rights and permissions
About this article
Cite this article
Konsavage, W.M., Roper, J.N., Ishmael, F.T. et al. The Myc 3′ Wnt Responsive Element Regulates Neutrophil Recruitment After Acute Colonic Injury in Mice. Dig Dis Sci 58, 2858–2867 (2013). https://doi.org/10.1007/s10620-013-2686-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2686-x