Abstract
Apoptosis is the predominant mechanism of liver cell death in autoimmune hepatitis, and interventions that can modulate this activity are emerging. The aim of this review was to describe the apoptotic mechanisms, possible aberrations, and opportunities for intervention in autoimmune hepatitis. Studies cited in PubMed from 1972 to 2014 for autoimmune hepatitis, apoptosis in liver disease, apoptosis mechanisms, and apoptosis treatment were examined. Apoptosis is overactive in autoimmune hepatitis, and the principal pathway of cell death is receptor mediated. Surface death receptors are activated by extrinsic factors including liver-infiltrating cytotoxic T cells and the cytokine milieu. The executioner caspases 3 and 7 cleave nuclear deoxyribonucleic acid, and the release of apoptotic bodies can stimulate inflammatory, immune, and fibrotic responses. Changes in mitochondrial membrane permeability can be initiated by caspase 8, and an intrinsic pathway of apoptosis can complement the extrinsic pathway. Defects in the apoptosis of activated effector cells can prolong their survival and sustain the immune response. Caspase inhibitors have been used in diverse experimental and human diseases to retard apoptosis. Oligonucleotides that inhibit the signaling of toll-like receptors can limit the presentation of auto-antigens, and inhibitors of apoptosis that extend the survival of effector cells can be blocked by antisense oligonucleotides. Mechanisms that enhance the clearance of apoptotic bodies and affect key signaling pathways are also feasible. Interventions that influence the survival of liver and effector cells by altering their apoptosis are candidates for study in autoimmune hepatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Autoimmune hepatitis is a chronic inflammation of the liver that is characterized by autoantibodies, hypergammaglobulinemia, and histological findings of interface hepatitis and portal lymphocytic infiltration, commonly in association with plasma cells [1]. Apoptosis is the predominant pathway of liver cell death in areas of interface hepatitis [2], and it may occur in conjunction with features of necrosis as a manifestation of immune intolerance to self-antigens [3–5]. Chromatin condensation, membrane blebs, cell shrinkage, and cellular fragmentation into vesicles (apoptotic bodies) are the morphological manifestations of apoptosis [4, 6], and apoptotic hepatocytes are recognized as condensed acidophilic cells (previously called, “Councilman bodies”) by light microscopy [6–8].
The reasons for a persistent immune response in autoimmune hepatitis are unclear, but they may relate in part to the generation of neo-antigens during tissue injury [9–13] and failure to eliminate dying hepatocytes or activated effector cells from the microenvironment [14–17]. Accelerated apoptosis may account for the severity of liver injury [4, 18, 19]; the products of liver cell death (apoptotic bodies) may promote the immune response and stimulate hepatic fibrosis [10, 20, 21]; defective apoptosis may perpetuate liver-infiltrating effector cells [22]. Dysregulation of the apoptotic pathways may be a critical factor in the occurrence and severity of autoimmune hepatitis [23, 24], and aberrant signals within these pathways are feasible therapeutic targets [25–27].
Apoptosis or programmed cell death is a highly regulated normal physiological mechanism by which damaged cells are eliminated without generating inflammatory activity [4, 19, 24–29]. The process is a genetically controlled method of counterbalancing normal mitotic activity and stabilizing tissue mass. It is also a mechanism of restoring immune homeostasis by limiting the survival of activated effector cells [30]. Diseases, such as cancer, have been associated with an inhibition of apoptosis and increased cell survival, whereas diseases, such as acute severe (fulminant) hepatitis and neurodegenerative diseases, have been associated with overactive apoptosis and increased cell death and tissue loss [4, 19, 26]. The nature of the cell populations undergoing apoptosis is critical in understanding the net effect of programmed cell death on the clinical phenotype of complex immune-mediated diseases, such as autoimmune hepatitis. Overactive apoptosis of hepatocytes may influence the severity of the histological manifestations and the vigor of the immune response, whereas underactive apoptosis of liver-infiltrating effector cells may perpetuate the disease.
The key disturbances in the apoptotic pathways in autoimmune hepatitis have not been fully defined, but the interaction of cell surface receptors with ligands that directly induce cell death (extrinsic pathway) is probably the predominant mechanism [23, 24]. Mitochondrial dysfunction secondary to oxidative stress (intrinsic pathway) or a combination of both the intrinsic and extrinsic pathways in an amplification loop may also be involved [24]. In this review, the mechanisms of apoptosis are described, the key molecular components of the apoptotic pathways are identified, the aberrations that may promote and perpetuate autoimmune liver disease are examined, and opportunities for therapeutic intervention are considered.
Methods
The English abstracts cited in PubMed from 1972 to 2014 for autoimmune hepatitis, apoptosis in liver disease, apoptosis mechanisms, and apoptosis treatment were reviewed. Abstracts judged pertinent to the review were identified; key aspects were noted; full-length articles were selected from the abstracts judged germane to the review. A secondary bibliography was developed from the references cited in the selected full-length articles and additional PubMed searches were performed to expand the concepts developed in these articles. Secondary PubMed searches focused mainly on individual apoptotic mechanisms, pertinent molecular interactions, signaling pathways, and the role of apoptotic bodies in liver disease. The discovery process involving abstract review and the acquisition of full-length articles was repeated, and a tertiary bibliography was developed after reviewing these selected articles. The tertiary references were then expanded by additional PubMed searches. The number of abstracts cited by PubMed and reviewed for pertinence to this topic during the primary, secondary, and tertiary searches exceeded 3,000. Those judged most pertinent to the topic exceeded 300, and the number of full-length articles reviewed exceeded 60.
Mechanisms of Apoptosis
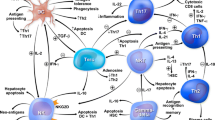
Apoptosis can occur because of mitochondrial dysfunction or the activation of death receptors on the cell surface [24, 27]. The mitochondrial or intrinsic pathway of apoptosis is initiated by mitochondrial damage and the subsequent release of cytochrome c [31] (Fig. 1). Changes in the permeability of the mitochondrial outer membrane (MOM) occur mainly as a result of oxidative stress [24, 32], and these membrane changes are influenced by the B cell lymphoma 2 (Bcl-2) family of proteins [27, 33]. Pro-apoptotic members of the Bcl-2 family (Bax and Bak) undergo conformational changes when stimulated by cellular distress signals, destabilize the lipid bilayer of the MOM, create membrane pores or channels, and facilitate entry of cytochrome c into the cytoplasm [27, 31, 34]. Anti-apoptotic members of the Bcl-2 family [Bcl-2, Bcl-XL, and myeloid cell leukemia sequence-1 (MCL-1)] can counteract the permeabilization of the MOM, and they prevent the efflux of cytochrome c [27, 34, 35]. If the pro-apoptotic effects of Bax and Bak outweigh the anti-apoptotic effects of Bcl-2, Bcl-XL, and MCL-1, the released cytochrome c can form a macromolecular complex (apoptosome) in the cytoplasm [27]. This complex can in turn convert procaspase 9 to caspase 9 and initiate the death sequence [27, 36, 37]. Caspases 3 and 7 are activated by caspase 9, and these “executioner” caspases then cleave chromosomal deoxyribonucleic acid (DNA) and induce apoptosis [27, 37].
Intrinsic (mitochondrial) and extrinsic (receptor-mediated) pathways of hepatocyte apoptosis. The stimulation of cell surface death receptor Fas by the ligand, FasL, triggers the extrinsic apoptotic pathway. The death domain of the stimulated death receptor interacts with adaptor proteins, such as the Fas-associated death domain (FADD), and a death-inducing signaling complex (DISC) is formed. DISC activates caspases 8 and 10 and initiates a cascade that activates the executioner caspases 3 and 7. The FLICE (FADD-like interleukin-1β converting enzyme)-like inhibitory protein (FLIP) binds to caspases 8 and 10, and it inhibits the formation of DISC. Caspases 3 and 7 enter the cell nucleus and cleave deoxyribonucleic acid (DNA) resulting in cell death and fragmentation of the cell into apoptotic bodies. The X-linked inhibitor of apoptosis protein (XIAP) can bind to caspases 3 and 7 and prevent nuclear fragmentation. The intrinsic pathway of apoptosis is activated by reactive oxygen species (ROS) which stimulate the pro-apoptotic B cell lymphoma (Bcl) proteins, Bak and Bax, to increase permeability of the outer mitochondrial membrane and release cytochrome c into the cytoplasm. Membrane permeabilization is countered by Bcl-2. Apoptotic protease activating factor-1 (APAF-1) can bind with cytochrome c and deoxyadenosine triphosphate (dATP) to form an apoptosome which in turn activates caspase 9. XIAP can bind to caspase 9 and inhibit the subsequent activation sequence. The tumor up-regulated CARD (caspase activation and recruitment domain)-containing antagonist of caspase 9 (TUCAN) can bind to procaspase 9 and inhibit its interaction with APAF-1. The intrinsic and extrinsic pathways each activate the executioner caspases 3 and 7. Caspase 8 can stimulate both pathways by activating pro-apoptotic proteins with homology to the Bcl family [Bid, Bim, and Puma (p53 up-regulated modulator of apoptosis)]. Processing of the apoptotic bodies by macrophages or lymphocytes determines the consequences of hepatocyte death
The death receptor or extrinsic pathway of apoptosis is activated by death receptors that have intracellular death domains (DD) [27, 38]. Fas, tumor necrosis factor receptor-1 (TNF-R1), TNF-related apoptosis-inducing ligand receptor-1 (TRAIL-R1), and receptor-2 (TRAIL-R2) are death receptors that activate the extrinsic apoptotic pathway, and they each have ligands that can activate the pathway [38, 39]. The Fas death receptor (CD95/APO-1) is mediated by the Fas ligand (FasL/CD95L) [24, 40–42] (Fig. 1). The ligation of Fas (CD95/APO-1) with Fas ligand (FasL/CD95L) triggers interaction with the death domain of adaptor proteins within the cytoplasm such as the Fas-associated death domain (FADD) [27, 38, 43]. A death-inducing signal complex (DISC) is formed within the target cell, and the DISC facilitates the activation of caspases 8 and 10 [44]. Caspase 8 can directly activate caspases 3 and 7 [27, 44, 45], and cell death occurs as the activated caspases cleave cell proteins (Fig. 1). Caspase 8 can also activate Bid, another pro-apoptotic member of the Bcl-2 family [33–35], and truncated Bid can translocate to the MOM, activate Bax and Bak, increase permeability of the MOM, facilitate the release of cytochrome c into the cytoplasm, and activate the intrinsic pathway of apoptosis to complement the extrinsic pathway [27, 33–35, 44, 46]. In this fashion, both the intrinsic and extrinsic pathways can be involved in the apoptotic process [38]. Other members of the subfamily of pro-apoptotic proteins characterized by Bid are Bim and Puma [35].
Caspase 10 has high sequence homology and genetic linkage to caspase 8 [47]. Caspases 8 and 10 can share functions, including cleavage of Bid and activation of caspases 3 and 7, but their functions are not totally redundant. Both caspases can function independently of each other [48–50], and caspase 10 does not substitute for caspase 8 in caspase 8-deficient cell lines [51]. Furthermore, caspase 10 is more resistant to inhibitors than caspase 8 [49], and each proteolytic enzyme has different substrate specificities [48, 52, 53]. Caspase 10 has three full-length isoforms and a truncated isoform, and these isoforms may have varying levels of expression, degradation, and function, including pro-, anti-, or no apoptotic activity in tumor cell lines [49]. The complexities in expression and function of these “initiator caspases” contribute to the diversity of their functions despite similarities in structure and other actions.
The extrinsic pathway of apoptosis is also mediated by the ligation of the cell surface receptor for tumor necrosis factor-α (TNF-R1) with TNF-α and by the release of perforin and granzyme B from activated cytotoxic T cells [24]. The ligation of TNF-R1 on the surface of activated cytotoxic T cells with TNF-α in liver cells activates death receptors on the hepatocytes and cleaves procaspase 8 into its active form. Downstream caspases are activated, and liver cell apoptosis occurs [24]. Activated cytotoxic T lymphocytes release perforin, which allow channels to form within the hepatocyte membrane, and granzyme B, which enters the hepatocyte and cleaves proteins necessary for cell survival [24].
Apoptosis is the likely initial response of hepatocytes and biliary cells to injury [54, 55], and this response through receptor-mediated and mitochondrial pathways can release neo-antigens, activate liver-infiltrating cytotoxic lymphocytes, promote cytokine and chemokine production, attract inflammatory cells, activate hepatic stellate cells, and increase hepatic fibrosis [54–57]. Tissue injury that began by mechanisms that trigger apoptosis may be extended by mechanisms that cause necrosis [58, 59]. Both apoptosis and necrosis involve DNA fragmentation, and the final consequence of liver injury may reflect apoptotic and necrotic pathways which overlap or constitute a continuum of tissue damage [3, 4]. The coexistence of apoptosis and necrosis and the variable and uncertain predominance of one process over the other in a particular disease may limit the effectiveness of anti-apoptotic interventions.
Regulators of Apoptosis
The occurrence and vigor of apoptosis are influenced in apart by the avidity of the Fas receptor (CD95/APO-1) for the FasL (CD95L), the generation of signals that indicate cell distress, the balance between the pro- and anti-apoptotic actions of the Bcl-2 proteins, the stability and function of the apoptosome within the cytoplasm, and the activation and inhibition of the caspases. Interactions between these factors can shape the clinical phenotype of the disease [27].
Fas and FasL
The apoptotic pathway dependent on the Fas/FasL interaction can be influenced by genetic polymorphisms, interactions within the Bcl-2 family of proteins, levels of soluble CD95 that compete for FasL (CD95L) or block the membrane-bound receptor, and the cytokine milieu that evolves during tissue damage [19, 60–62].
The human Fas gene is located on chromosome 10q24.1, and 20 polymorphisms have been described over a span of 26 kilobases [63, 64] (Table 1). Four polymorphisms have been associated with the occurrence of autoimmune hepatitis in Japan [63], and in white North American and northern European patients with autoimmune hepatitis, a polymorphism involving the substitution of adenosine for guanine at position -670 (tumor necrosis factor receptor superfamily-6; TNFRSF6) has been associated with an increased frequency of cirrhosis at presentation [65]. The basis for the relationship between a single Fas gene polymorphism and progression to cirrhosis is unclear, but the association suggests that a genetic predisposition which intensifies the apoptosis of hepatocytes or delays the demise of activated effector cells may influence the severity and outcome of autoimmune hepatitis in an individual- or ethnic-specific fashion [65].
CD95 and apoptosis antigen-1 (APO-1) are alternative designations for the Fas receptor, and activated CD4+ and CD8+ lymphocytes expressing CD95 are increased in the peripheral blood of patients with autoimmune hepatitis and patients with chronic hepatitis C and autoimmune features [22] (Table 1). The increased population of effector cells bearing the death receptor, CD95/APO-1, has been associated with a failure of these cells to down-regulate the expression of the anti-apoptotic protein, Bcl-2 [22]. This functional failure may in turn increase the expression of Bcl-2, reciprocally inhibit the apoptosis of activated lymphocytes, and increase the number and persistence of these effector cells within the liver [22, 66]. This hypothesis presumes that the Fas/FasL interaction is intact and that the effect of the ligation on apoptosis is blunted by Bcl-2.
Apoptosis may also be modulated by the endogenous production of soluble CD95/APO-1 [60, 61, 67] (Table 1). Transcripts of the messenger ribonucleic acid (mRNA) of the Fas gene may lack a transmembrane domain due to the normal genetic mechanism of alternative splicing [61]. During this process, the Fas gene joins splice sites in various combinations and produces isoforms of the Fas receptor that are not membrane bound [61, 68]. The resultant soluble isoforms of CD95 can interfere with the ligation of membrane-bound Fas receptor with FasL and inhibit the apoptosis of activated effector cells [60, 61]. Increased levels of soluble CD95 have been described in diverse autoimmune diseases [69–71] and malignancies [72–74], suggesting that soluble CD95 may have clinical relevance by hampering the programmed cell death of critical cell populations. In immune-mediated diseases, the production of soluble CD95 may be a protective response triggered by cell injury and the accelerated transcription of Fas mRNA [69].
Distress Signals
Signals of cell distress that are not disease specific may also trigger apoptosis, and different gene products, such as p53, can modulate the process [19, 75, 76]. The soluble forms of the major histocompatibility complex (MHC) class I-related chains A and B (MIC A and MIC B) are membrane glycoproteins that are induced by cell stress [55]. These glycoproteins are cellular distress signals that can activate cytotoxic natural killer (NK) cells, natural killer T (NKT) cells, and cytotoxic CD8+ lymphocytes by ligating with their C2D natural killer group 2 member D (NKG2D) receptors. The NKG2D receptor is expressed mainly on NK cells, and the ligation of this receptor with the MIC A and MIC B ligands induces NK cell-mediated cytolysis. NK cells induce cell death by pathways involving the release of granzyme and perforin and ligation with membrane-bound death receptors [55, 77].
The serum levels of MIC A and MIC B are increased in patients with diverse liver diseases, including non-alcoholic fatty liver disease (NAFLD) [78], chronic liver disease (mainly, chronic hepatitis B and chronic hepatitis C) [79], and hepatocellular carcinoma [79] (Table 1). The serum levels of the MIC proteins have correlated with hepatocyte apoptosis and hepatic fibrosis in NAFLD [78], and these findings suggest that the NK cells can increase the severity of liver injury by responding to the stress-induced MIC A and MIC B ligands [55].
Neutralization of soluble MIC A and MIC B by monoclonal antibodies is a theoretical mechanism of attenuating NK cell activity and reducing liver damage, but the pathways regulating NK cell function, the disease specificity of the interaction, and the overall importance of this mechanism in reducing liver injury are uncertain [55]. The serum levels of MIC A and MIC B have been low or absent in patients with autoimmune hepatitis, primary biliary cirrhosis (PBC), and primary sclerosing cholangitis [80], and the role of the NGK2D ligands in autoimmune liver disease is speculative. Susceptibility to primary sclerosing cholangitis has been associated with the MICA *008 allele [81], and there may be genetic factors that enhance or inhibit the expression of the NGK2D ligands and modulate disease severity in certain diseases [82].
Bcl-2 Family
The Bcl-2 proteins regulate the intrinsic pathway of apoptosis by influencing the permeability of the MOM and affecting the release of cytochrome c into the cytoplasm [33] (Table 1). Each family member is characterized by one or more Bcl-2 homology (BH) domains, and they can be subdivided into proteins containing multiple Bcl-2 homology domains (BH1, BH2, and BH3) and proteins containing only one Bcl-2 homology domain (BH3 only) [33, 35]. Bax and Bak are multi-domain pro-apoptotic proteins that mediate permeability of the MOM, and Bcl-2, Bcl-XL, and MCL-1 are anti-apoptotic proteins that prevent mitochondrial release of cytochrome c [35]. A subfamily of proteins containing only one Bcl-2 homology domain (BH3 only) regulates the apoptotic activity of the multi-domain proteins [35]. Bid, Bim, and Puma (p53 up-regulated modulator of apoptosis) are proteins with only the BH3 domain, and they directly activate the pro-apoptotic Bax and Bak proteins [35]. The interactions between these Bcl-2 family members tightly regulate the intrinsic pathway of apoptosis, and imbalances in this axis can also affect the clinical phenotype [35].
Apoptosome Formation and Function
The apoptosome is a macromolecular complex that forms in the cytoplasm after the release of cytochrome c and activation of the intrinsic (mitochondrial) pathway of apoptosis [27, 83] (Table 1). The core component of the apoptosome is apoptotic protease activating factor-1 (APAF-1) which can bind cytochrome c and deoxyadenosine triphosphate (dATP) [83–85]. The oligomeric apoptosome contains three domains that include a caspase-associated recruitment domain (CARD), a nucleotide binding domain, and a C-terminal domain that binds cytochrome c [27, 37]. Procaspase-9 is bound to and activated by the apoptosome, and an activation cascade of other caspases follows [36, 37, 86] (Fig. 1). The formation and stability of the apoptosome can be affected by heat-shock proteins that bind to APAF-1 and prevent its oligomerization [87–89], intracellular nucleotides that bind to cytochrome c and inhibit its binding to APAF-1 [90], and concentrations of calcium and potassium within the cell that impair apoptosomal function [27].
Caspase Activation
Caspases are produced as zymogens, and they constitute a family of proteolytic enzymes (cysteine-aspartic acid proteases) whose activities can cascade in sequence and result in the cleavage of chromosomal DNA and the digestion of cytoskeletal and nuclear components [27, 45, 91] (Table 1). Caspases 8, 9, and 10 (“initiator caspases”) can trigger the apoptotic sequence by activating caspases 3, 6, and 7. Caspases 3, 6, and 7 (“executioner caspases”) can then cleave the intracellular substrates and induce apoptosis [27, 45]. The activation of caspases is involved in both the intrinsic and extrinsic apoptotic pathways [24, 27, 45] (Fig. 1).
Caspases can be inhibited directly or indirectly by the inhibitors of apoptosis proteins (IAPs) [27, 92] (Table 1). The x-linked inhibitor of apoptosis protein (XIAP) is a direct-acting caspase inhibitor [92, 93], and it binds to caspases 3, 7, and 9. A tumor up-regulated CARD [caspase activation and recruitment domain]-containing antagonist of caspase 9 (TUCAN) can bind to procaspase 9, inhibit its interaction with APAF-1, and indirectly prevent conversion to the active protease [94, 95] (Fig. 1). Caspases can also be strengthened or derepressed. The putative HLA-DR-associated protein (PHAPI) can promote apoptosis by increasing the activity of caspase-3 [83]. Other proteins (Smac/DIABLO and Omi/HtrA2) can be released into the cytoplasm by damaged mitochondria and promote apoptosis by inhibiting the IAPs [83, 96]. The membrane-bound heat-shock-inducible protein, HtrA2, can efflux into the cytoplasm during tissue injury and prevent the suppression of caspase 3 by XIAP [96].
The survival of activated T lymphocytes is influenced by the IAPs, and they can prolong the immune response and associate with disease activity [97, 98] (Table 1). IAP-1, IAP-2, Bcl-XL, and XIAP are up-regulated in activated T lymphocytes from patients with multiple sclerosis, and the level of IAP expression correlates inversely with T cell susceptibility to apoptosis and directly with disease activity [97–99]. The cellular caspase-inhibitory protein, FLIP [FADD-like interleukin-1β converting enzyme (FLICE)-like inhibitory protein], is another potent inhibitor of T cell susceptibility to receptor-mediated apoptosis [99–103]. FLIP suppresses apoptosis triggered by TNF-α, FasL, and TRAIL. FLIP binds to caspases 8, 10, and TRAIL, and it inhibits the formation of a death-inducing signaling complex (DISC) [102] (Fig. 1). The suppression or inactivation of these IAPs by small interfering ribonucleic acids (siRNA) has been an active area of investigation [97, 98, 101, 102]. Decreased T cell responsiveness to apoptotic triggers may also be due to other failures in the execution sequence that may be genetically controlled [104].
Apoptosis in Autoimmune Hepatitis
The apoptosis of hepatocytes is increased in autoimmune hepatitis [23] (Table 2). The principal pathway of liver cell loss is receptor mediated and triggered by liver-infiltrating cytotoxic lymphocytes [24]. Apoptosis occurs mainly in biliary epithelial cells, proliferating bile ductules, areas of confluent necrosis that are densely infiltrated with lymphocytes, and hepatocytes (especially those in rosette formation) [105]. The consequences of overactive apoptosis (fragmented DNA and disrupted cell membranes) can then initiate molecular and cellular responses that translate into the classical phenotype of autoimmune hepatitis [2].
The release of apoptotic bodies from the dying hepatocytes activates Kupffer cells and quiescent hepatic stellate cells [20, 56, 106–108] (Table 2). The Kupffer cells release chemokines, cytokines, and reactive oxygen species which further stimulate the hepatic stellate cells as well as promote the apoptosis of hepatocytes. The activated hepatic stellate cells transform into myofibroblasts, and the myofibroblasts enhance the apoptosis of hepatocytes by contributing to an amplification loop that involves the release of reactive oxygen species and increased oxidative stress on the liver [56, 108]. The free apoptotic material in turn stimulates an adaptive immune response as evidenced clinically by the development of autoantibodies and the emergence of liver-infiltrating cytotoxic T cells [17, 109].
Animal studies have strengthened the association between hepatocyte apoptosis and hepatic fibrosis [38]. Bile duct ligated mice who are Fas-deficient have less apoptosis, lower serum ALT levels, and less evidence of hepatic stellate cell activation after 3 days than wild-type mice, and they have less evidence of hepatic stellate activation and hepatic collagen deposition after 3 weeks [110]. Pharmacological inhibition of caspase activity in this same bile duct ligation model has been associated with reduced hepatocyte apoptosis, histological findings of liver injury, markers of liver inflammation, hepatic stellate cell activation, and features of fibrogenesis in both 3- and 10-day ligated animals compared to saline-treated animals [111]. Mice deficient in the anti-apoptotic protein, Bcl-XL, have also manifested spontaneous and protracted apoptosis of hepatocytes with progressive liver fibrosis in the absence of hepatic inflammation [112].
The surface expression of the Fas receptor (CD95) is increased in the lymphocyte subsets of patients with autoimmune hepatitis, and liver tissue examinations have disclosed increased levels of FasL in patients with autoimmune hepatitis compared to tissue samples from patients with PBC [23] (Table 2). Furthermore, the progenitor cells in long-term cultures of bone marrow from patients with autoimmune hepatitis have demonstrated increased apoptotic markers compared to samples from normal individuals. The Fas-positive cells have corresponded to the apoptotic cells, and the production of TNF-α and IFN-γ in cell culture has also been increased [113].
A key factor that can modulate the immune response in autoimmune hepatitis is the efficiency by which the apoptotic cells are cleared from the microenvironment [14–17] (Table 2). Studies specific to autoimmune hepatitis have not been performed to assess this process, but the body of investigational evidence supports the importance of these clearance mechanisms in regulating immune tolerance to self-antigens [16, 114]. The apoptotic cells must be eliminated without generating an inflammatory, fibrotic, or immune response.
Apoptosis is characterized by surface membrane distortion and cell fragmentation, and the loss of membrane symmetry during apoptosis can stimulate oxidation of phospholipids in the surface membrane of the dying hepatocytes [115] (Table 2). The surface expression of phosphatidylserine can trigger a sensing system that activates macrophages [116], and a family of surface receptors [TAM (Tyro3, Axl, Mer) receptor tyrosine kinases] on macrophages and dendritic cells can interact with the dying cells to promote their phagocytosis [117, 118]. Liver X receptors (LXR) are lipid-dependent regulators of inflammatory gene expression [119], and they are activated by excess lipoprotein-derived cholesterol in macrophages [17, 120]. The LXR can induce the production of the TAM receptor, Mer, and help repress the inflammatory and immune responses [17, 119].
Kupffer cells can also participate in the clearance of apoptotic cells [29], and non-professional phagocytic cells, such as biliary epithelial cells, can contribute to this process [121]. Human biliary epithelial cells have receptors for phosphatidylserine, and their phagocytic property may be triggered by the expression of phosphotidylserine on apoptotic cells [121]. The nucleotides, adenosine triphosphate (ATP) and uridine triphosphate (UTP), are also released by apoptotic cells, and these chemical mediators can attract macrophages and hepatic stellate cells that express purinergic receptors, especially P2Y2 [38, 122, 123]. These nucleotides can promote clearance of apoptotic bodies by professional phagocytic cells, and they can also recruit and activate hepatic stellate cells [123]. Fractalkine, adenosine, and lysophosphatidylcholine are other chemoattractants released by apoptotic cells that can influence clearance of cellular fragments [38, 124].
The ingestion of the apoptotic cells by macrophages releases anti-inflammatory cytokines (IL-10 and TGF-β), suppresses the production of pro-inflammatory cytokines, and limits the immune response to the apoptotic material [16, 114, 125, 126] (Fig. 1). Deficiencies in the clearance of apoptotic bodies because of overactive apoptosis or intrinsic defects in macrophage signaling and recruitment may be important drivers in perpetuating and accentuating the inflammatory and immune response in autoimmune hepatitis. Clarification of these deficiencies may identify opportunities for corrective interventions.
Feasible Apoptosis-Directed Interventions in Autoimmune Hepatitis
The complexities of the apoptotic pathways suggest multiple sites which might be targeted in autoimmune hepatitis [27, 62, 127]. These sites are involved in homeostatic mechanisms that protect against infection, malignancy, and autoreactivity, and interventions that target these sites may affect diverse cell populations that could weaken normal innate and adaptive immune responses [4]. The lack of cell type specificity may be one reason that therapies designed to directly modulate apoptotic activity have been slow to evolve in diseases outside of malignancy [27, 128–130].
In autoimmune hepatitis, site-specific interventions that shorten the survival of autoreactive effector cells or reduce the apoptosis of hepatocytes are desirable therapeutic objectives, especially as adjunctive treatments in selected problematic patients. Such therapies based on modulation of the apoptotic pathways are only theoretical considerations in autoimmune hepatitis and far from clinical application. The extrinsic or receptor-mediated pathway of apoptosis is the principal pathogenic pathway of autoimmune hepatitis and the appropriate focus of future investigational activity [23].
Caspase Inhibitors
Agents that inhibit caspase activity have been studied most commonly in experimental and human disease, and they are candidates for study in autoimmune hepatitis [27, 38, 131] (Table 3). Caspase inhibitors reduce apoptosis, and they are logical interventions to consider in diseases characterized by an overactive apoptotic state [27]. Broad spectrum irreversible caspase inhibitors have reduced apoptosis in murine models of acute liver injury [132, 133], bile duct ligation [111], NAFLD [134, 135], acute liver failure after massive hepatectomy [136], and organ ischemia [137]. Caspase inhibitors have also been used in preliminary studies of patients with diverse liver test abnormalities [138] and patients with chronic hepatitis C [131, 139, 140], and they have protected organs from ischemia/reperfusion injury in liver transplantation [141]. These experiences support the candidacy of caspase inhibition as a treatment consideration in autoimmune hepatitis.
Toll-Like Receptor Antagonism
Nuclear fragments that result from apoptosis may act as neo-antigens [13], and the toll-like receptors, TLR7 and TLR9, of the innate immune system can in turn recognize these antigens [142] (Table 3). Dendritic cells and autoreactive B cells are then activated, and the immune response can be triggered, strengthened, or perpetuated [142]. Systemic lupus erythematosus is a prototypic immune-mediated disease that is characterized by B cell hyperactivity, and toll-like receptors have been implicated in its pathogenesis. Accordingly, oligonucleotide inhibitors of TLR7 and TLR9 signaling have been appropriate therapeutic considerations, and they have shown promise in murine models of this disease [38, 143]. Autoimmune hepatitis is also associated with increased B cell activity, but it is distinguished mainly by liver-infiltrating cytotoxic T cells and excessive apoptosis of hepatocytes [23]. The rationale for considering this intervention in autoimmune hepatitis is tenuous.
XIAP Targeting
IAPs may protect effector T lymphocytes from apoptosis and thereby prolong the immune response by inhibiting procaspase 3 and 7 activation [144, 145] (Table 3). XIAP is the most potent and best characterized IAP, and increased levels of XIAP can inhibit both the intrinsic and extrinsic pathways of apoptosis [146–149]. Antisense oligonucleotides that bind to the XIAP mRNA promote degradation of this molecule, and this action might curtail autoimmune hepatitis [147, 150–152]. Elimination of disease-associated XIAP mRNA has improved experimental autoimmune encephalitis in mice, and it has theoretical advantages that might apply to autoimmune hepatitis [147].
Effector cells in immune-mediated disease models require only partial down-regulation of XIAP expression to promote their demise since sensitivity for apoptosis is increased [146, 147]. Macrophages are not eliminated by targeting XIAP since their survival is not apoptosis dependent, and protective T cells that are not disease-activated are unaffected by therapy [147]. Theoretically, treatment with antisense oligonucleotides could improve autoimmune hepatitis without abolishing XIAP activity or greatly compromising the integrity of the innate and adaptive immune systems. The major concern about this intervention is its uncertain immediate impact on an apoptotic process within the liver which is already overactive.
Promoters of Apoptotic Cell Clearance
Apoptotic bodies can enhance the inflammatory, immune, and fibrotic responses to tissue injury, and interventions that help clear them might ameliorate autoimmune hepatitis. Clearance mechanisms have not been evaluated in autoimmune hepatitis, but the burden of apoptotic bodies for elimination must be great in a disease characterized by overactive receptor-mediated cell death. Agonists of the LXR can promote phagocytosis of these bodies [17], and a histidine-rich glycoprotein (HRG) synthesized in the liver and released in the circulation can stimulate removal of these dying cells and modulate the adaptive immune system [153]. Treatments that up-regulate the expression of TAM receptor tyrosine kinases on macrophages and dendritic cells or modulate the production of LXR or HRG in autoimmune hepatitis are investigational opportunities that have yet to be explored (Table 3).
Distant Hypothetical Considerations
The identification of the key regulatory sites affecting disease severity in autoimmune hepatitis and the availability of agents that can precisely and safely target these sites will determine the direction of future investigations in the treatment of autoimmune hepatitis. Sites that can modulate the apoptotic pathways are abundant, but the critical sites and the optimal instruments by which to safely target them are unknown. Interventions that may mature into testable considerations in autoimmune hepatitis are those that affect mainly the extrinsic receptor-mediated apoptotic pathway. Antisense RNAs that bind to specific mRNAs and increase their degradation [150–152, 154, 155], monoclonal antibodies against Fas-bearing cells [156], peptides and peptide mimics [157], fusion molecules with TRAIL that are designed to reduce pro-inflammatory responses [158], agonists that enhance the expression of cell death ligands and inhibit T cell proliferation and cytokine production [159–162], and the genetic and molecular manipulation of signaling pathways that regulate cell fate [163, 164] are interventions that await assimilation into future investigational algorithms.
Obstacles to the Development of Apoptotic Interventions in Autoimmune Hepatitis
The principal obstacles to the development of apoptotic interventions in autoimmune hepatitis are the lack of an animal model that accurately reflects the overactive apoptotic state of the human disease, the possibility that interference with the apoptotic pathways may disrupt essential homeostatic mechanisms, and the paucity of human experiences with these interventions in non-malignant liver disease. Currently, the rationale for this therapy in liver disease is stronger than the human experience, and studies to date have mainly confirmed feasibility but not indicated uniform and compelling efficacy or established safety. There have been no animal or human experiences with apoptotic interventions in autoimmune hepatitis.
Two experimental animal models have emerged as possible resources for the development of site-specific interventions in autoimmune hepatitis. Both models are based on the use of human antigens implicated in the pathogenesis of autoimmune hepatitis, and they each generate characteristic histological changes and human autoantibodies. One model is based on the immunization of female mice with plasmids of cytomegalovirus containing the antigenic region of the human cytochrome mono-oxygenase, CYP2D6, and human formiminotransferase cyclodeaminase [165]. The other model is based on the infection of mice with an adenovirus expressing human CYP2D6 [166]. These models are resources that may be exploitable for the study of apoptotic mechanisms and therapy in autoimmune hepatitis.
Apoptotic pathways influence the development and effectiveness of the innate and adaptive immune systems to counter infection, autoreactive responses, and malignancy, and the maintenance of the pathways for programmed cell death in diverse cell populations is essential for a stable existence [4, 6, 24, 27, 39, 58]. Anti-apoptotic interventions have imprecise targets, and efforts to disrupt disease pathways may have collateral effects on essential homeostatic pathways that protect against infection and malignancy.
The human experiences with caspase inhibitors in diverse chronic liver diseases, especially chronic hepatitis C, have recorded no adverse reactions, albeit the exposures have been short (2 weeks) [131, 139]. Furthermore, concerns that anti-apoptotic therapy may promote malignancy have been eased by studies demonstrating the absence of spontaneous cancers in animals with genetic deletions of the death receptors [167, 168]. Animal studies have also indicated that the inhibition of apoptosis can reduce the formation of hepatic cancer in a transgenic murine model of hepatitis B virus infection possibly by diminishing cell turnover [131, 169]. The preliminary nature of the clinical experiences and the multiple unresolved safety issues, including possible increased susceptibility to certain infections (listeria monocytogenes) [170], warrant restriction of initial studies in autoimmune hepatitis to experimental cell lines and animal models.
Apoptotic therapies have been evaluated mainly in malignancy, and there have been no human studies in non-malignant liver diseases that have demonstrated a compelling advantage of this therapy over conventional management strategies. Of 105 patients with diverse chronic liver diseases enrolled in a multicenter, double-blind, placebo-controlled, dose-ranging study, all patients with chronic hepatitis C and those few with NAFLD who received a caspase inhibitor for 2 weeks experienced a significant improvement in serum aspartate (AST) and alanine (ALT) aminotransferase levels compared to placebo-treated patients [139]. These findings have justified the performance of longer studies to assess the possible benefits of caspase inhibition on liver inflammation and fibrosis [139]. A phase II clinical study of caspase inhibition has also been launched for acute alcoholic hepatitis [27].
Clinical studies of apoptotic therapy in non-malignant chronic liver diseases are just beginning, and the rationale for such therapy in immune-mediated liver disease, notably PBC [9, 171–175], is gathering strength. Apoptotic therapy in autoimmune hepatitis is in an early theoretical stage of development, but the rationale, instruments, and precedents are sufficient to encourage its consideration and active investigation.
Overview
Dysregulation of the normal apoptotic mechanisms that maintain organ integrity can contribute to the occurrence and severity of autoimmune hepatitis by promoting excessive hepatocyte loss and prolonging survival of autoreactive cells. Perturbations in the receptor-mediated (extrinsic) pathway of apoptosis predominate in autoimmune hepatitis, and these disturbances may relate to polymorphisms in the gene that encodes the Fas (CD95/APO-1) receptor, failures of IAPs to modulate the apoptosis of hepatocytes and effector cells, and defects in intercellular signaling pathways that support clearance (phagocytosis) of apoptotic bodies.
The challenges in developing therapies that modulate apoptosis in autoimmune hepatitis are to select the appropriate cell population to target and to design therapies that are restricted to that cell population. Activated liver-infiltrating lymphocytes are the key effector cells that promote hepatocyte apoptosis, and interventions that increase the apoptosis of these effector cells might diminish the excessive apoptosis of hepatocytes. They may also increase hepatocyte loss. Alternatively, interventions that reduce the apoptosis of hepatocytes might increase the survival of the effector cells and perpetuate the disease. These theoretical concerns must be addressed by appropriate investigations if interventions directed at the apoptotic pathways are to mature in autoimmune hepatitis.
Interventions that directly modulate apoptotic activity have not been studied in autoimmune hepatitis, but preliminary investigations in animal models and humans with ischemic, toxic, viral, and metabolic liver diseases suggest that caspase inhibition is a candidate for study. Preservation of the apoptotic pathways is critical in maintaining the homeostasis of the innate and adaptive immune systems, and any therapeutic consideration that influences these pathways must ensure the adequacy of the protective surveillance mechanisms.
Corticosteroid-based immunosuppressive regimens are effective in autoimmune hepatitis, and they alleviate the clinical (autoantibodies) and histological (interface hepatitis) manifestations of apoptosis probably by attenuating the immune-mediated response. Interventions that directly modulate apoptotic activity must be shown to have an additive or superior effect to current drug regimens, and they would likely emerge as adjunctive treatments in highly selected problematic patients.
Clinical studies of apoptosis have been facilitated by the emergence of enzyme-linked immunosorbent assays (ELISAs) that assess biomarkers of apoptosis and overall cell death [59]. Caspase 3 cleaves cytokeratin 18 at a specific site, and it generates a fragment of the cytoskeleton that contains the M30 epitope. The M30 epitope can be measured by an ELISA to assess apoptosis [176]. Overall liver cell injury can be evaluated by two ELISAs. The destruction of cytokeratin 18 during cell death releases diverse fragments that contain an M65 epitope, and this epitope can be measured by an ELISA to assess overall cell death [177]. The alpha subtype of glutathione-S-transferase (GST-α) is released from the cytosol of injured hepatocytes, and its short half-life allows another ELISA to assess active liver cell injury [178]. These assays for neo-antigens derived from liver cell injury have been used to assess the mechanisms of hepatocyte death and the severity of liver injury in diverse diseases [59, 177, 179], and their availability should improve the study of apoptosis in autoimmune hepatitis and facilitate the assessment of apoptotic interventions.
References
Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213.
Kerr JF, Cooksley WG, Searle J, et al. The nature of piecemeal necrosis in chronic active hepatitis. Lancet. 1979;2:827–828.
Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death. Involvement of serine but not cysteine proteases. Am J Pathol. 1997;151:1205–1213.
Afford S, Randhawa S. Apoptosis. Mol Pathol. 2000;53:55–63.
Czaja AJ. Autoimmune hepatitis. Part A: pathogenesis. Expert Rev Gastroenterol Hepatol. 2007;1:113–128.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257.
Kerr JF. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971;105:13–20.
Wyllie AH, Morris RG, Smith AL, Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984;142:67–77.
Berg CP, Stein GM, Keppeler H, et al. Apoptosis-associated antigens recognized by autoantibodies in patients with the autoimmune liver disease primary biliary cirrhosis. Apoptosis. 2008;13:63–75.
Zampieri S, Degen W, Ghiradello A, Doria A, van Venrooij WJ. Dephosphorylation of autoantigenic ribosomal P proteins during Fas-L induced apoptosis: a possible trigger for the development of the autoimmune response in patients with systemic lupus erythematosus. Ann Rheum Dis. 2001;60:72–76.
Greidinger EL, Foecking MF, Ranatunga S, Hoffman RW. Apoptotic U1-70 kd is antigenically distinct from the intact form of the U1-70-kd molecule. Arthritis Rheum. 2002;46:1264–1269.
Dieker JW, Fransen JH, van Bavel CC, et al. Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus. Arthritis Rheum. 2007;56:1921–1933.
Rosen A, Casciola-Rosen L. Autoantigens in systemic autoimmunity: critical partner in pathogenesis. J Intern Med. 2009;265:625–631.
Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788.
Grimsley C, Ravichandran KS. Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol. 2003;13:648–656.
Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250.
A-Gonzalez N, Bensinger SJ, Hong C, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312.
Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173–184.
Canbay A, Feldstein AE, Higuchi H, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198.
Zhan SS, Jiang JX, Wu J, et al. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–443.
Ogawa S, Sakaguchi K, Takaki A, et al. Increase in CD95 (Fas/APO-1)-positive CD4+ and CD8+ T cells in peripheral blood derived from patients with autoimmune hepatitis or chronic hepatitis C with autoimmune phenomena. J Gastroenterol Hepatol. 2000;15:69–75.
Fox CK, Furtwaengler A, Nepomuceno RR, Martinez OM, Krams SM. Apoptotic pathways in primary biliary cirrhosis and autoimmune hepatitis. Liver. 2001;21:272–279.
Bai J, Odin JA. Apoptosis and the liver: relation to autoimmunity and related conditions. Autoimmun Rev. 2003;2:36–42.
Patel T, Gores GJ. Apoptosis and hepatobiliary disease. Hepatology. 1995;21:1725–1741.
Thatte U, Dahanukar S. Apoptosis: clinical relevance and pharmacological manipulation. Drugs. 1997;54:511–532.
D’Amelio M, Tino E, Cecconi F. The apoptosome: emerging insights and new potential targets for drug design. Pharm Res. 2008;25:740–751.
O’Reilly LA, Strasser A. Apoptosis and autoimmune disease. Inflamm Res. 1999;48:5–21.
Neuman MG. Apoptosis in diseases of the liver. Crit Rev Clin Lab Sci. 2001;38:109–166.
Beland K, Lapierre P, Djilali-Saiah I, Alvarez F. Liver restores immune homeostasis after local inflammation despite the presence of autoreactive T cells. PLoS One. 2012;7:e48192.
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162.
Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006;1757:1297–1300.
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326.
Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163.
Ren D, Tu HC, Kim H, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393.
Ekert PG, Read SH, Silke J, et al. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol. 2004;165:835–842.
Rodriguez J, Lazebnik Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 1999;13:3179–3184.
Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402–410.
Faubion WA, Gores GJ. Death receptors in liver biology and pathobiology. Hepatology. 1999;29:1–4.
Desbarats J, Duke RC, Newell MK. Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ T cells. Nat Med. 1998;4:1377–1382.
Nagata S. Apoptosis mediated by the Fas system. Prog Mol Subcell Biol. 1996;16:87–103.
Wajant H. Death receptors. Essays Biochem. 2003;39:53–71.
Oberst A, Pop C, Tremblay AG, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010;285:16632–16642.
Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35.
Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232.
Ma S, Hockings C, Anwari K, et al. Assembly of the Bak apoptotic pore: a critical role for the Bak protein alpha6 helix in the multimerization of homodimers during apoptosis. J Biol Chem. 2013;288:26027–26038.
Grenet J, Teitz T, Wei T, Valentine V, Kidd VJ. Structure and chromosome localization of the human CASP8 gene. Gene. 1999;226:225–232.
Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA. 2001;98:13884–13888.
Muhlethaler-Mottet A, Flahaut M, Bourloud KB, et al. Individual caspase-10 isoforms play distinct and opposing roles in the initiation of death receptor-mediated tumour cell apoptosis. Cell Death Dis. 2011;2:e125.
Kischkel FC, Lawrence DA, Tinel A, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46646.
Sprick MR, Rieser E, Stahl H, et al. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. EMBO J. 2002;21:4520–4530.
Fischer U, Stroh C, Schulze-Osthoff K. Unique and overlapping substrate specificities of caspase-8 and caspase-10. Oncogene. 2006;25:152–159.
Wachmann K, Pop C, van Raam BJ, et al. Activation and specificity of human caspase-10. Biochemistry. 2010;49:8307–8315.
Kahraman A, Gerken G, Canbay A. Apoptosis in immune-mediated liver diseases. Dig Dis. 2010;28:144–149.
Kahraman A, Fingas CD, Syn WK, Gerken G, Canbay A. Role of stress-induced NKG2D ligands in liver diseases. Liver Int. 2012;32:370–382.
Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20:2515–2532.
Czaja AJ. Review article: Chemokines as orchestrators of autoimmune hepatitis and potential therapeutic targets. Aliment Pharmacol Ther. 2014;40:261–279.
Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278.
Bechmann LP, Jochum C, Kocabayoglu P, et al. Cytokeratin 18-based modification of the MELD score improves prediction of spontaneous survival after acute liver injury. J Hepatol. 2010;53:639–647.
Cheng J, Zhou T, Liu C, et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762.
Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154:2706–2713.
Eichhorst ST. Modulation of apoptosis as a target for liver disease. Expert Opin Ther Targets. 2005;9:83–99.
Hiraide A, Imazeki F, Yokosuka O, et al. Fas polymorphisms influence susceptibility to autoimmune hepatitis. Am J Gastroenterol. 2005;100:1322–1329.
Inazawa J, Itoh N, Abe T, Nagata S. Assignment of the human Fas antigen gene (Fas) to 10q24.1. Genomics. 1992;14:821–822.
Agarwal K, Czaja AJ, Donaldson PT. A functional Fas promoter polymorphism is associated with a severe phenotype in type 1 autoimmune hepatitis characterized by early development of cirrhosis. Tissue Antigens. 2007;69:227–235.
Ichiki Y, Aoki CA, Bowlus CL, et al. T cell immunity in autoimmune hepatitis. Autoimmun Rev. 2005;4:315–321.
Zipp F, Weller M, Calabresi PA, et al. Increased serum levels of soluble CD95 (APO-1/Fas) in relapsing-remitting multiple sclerosis. Ann Neurol. 1998;43:116–120.
Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol. 2005;6:386–398.
Jodo S, Kobayashi S, Kayagaki N, et al. Serum levels of soluble Fas/APO-1 (CD95) and its molecular structure in patients with systemic lupus erythematosus (SLE) and other autoimmune diseases. Clin Exp Immunol. 1997;107:89–95.
Fujihara T, Takeuchi T, Tsubota K, et al. Serum soluble Fas/APO-1 is increased in patients with primary Sjogren’s syndrome. Clin Rheumatol. 1998;17:496–499.
Jodo S, Kobayashi S, Nakajima Y, et al. Elevated serum levels of soluble Fas/APO-1 (CD95) in patients with hepatocellular carcinoma. Clin Exp Immunol. 1998;112:166–171.
Midis GP, Shen Y, Owen-Schaub LB. Elevated soluble Fas (sFas) levels in nonhematopoietic human malignancy. Cancer Res. 1996;56:3870–3874.
Ueno T, Toi M, Tominaga T. Circulating soluble Fas concentration in breast cancer patients. Clin Cancer Res. 1999;5:3529–3533.
Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased soluble CD95 (sFas/CD95) serum level correlates with poor prognosis in melanoma patients. Clin Cancer Res. 2001;7:1282–1286.
Wyllie AH, Arends MJ, Morris RG, Walker SW, Evan G. The apoptosis endonuclease and its regulation. Semin Immunol. 1992;4:389–397.
Wyllie AH. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993;67:205–208.
Ochi M, Ohdan H, Mitsuta H, et al. Liver NK cells expressing TRAIL are toxic against self hepatocytes in mice. Hepatology. 2004;39:1321–1331.
Kahraman A, Schlattjan M, Kocabayoglu P, et al. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology. 2010;51:92–102.
Kohga K, Takehara T, Tatsumi T, et al. Serum levels of soluble major histocompatibility complex (MHC) class I-related chain A in patients with chronic liver diseases and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci. 2008;99:1643–1649.
Holdenrieder S, Eichhorn P, Beuers U, et al. Soluble NKG2D ligands in hepatic autoimmune diseases and in benign diseases involved in marker metabolism. Anticancer Res. 2007;27:2041–2045.
Norris S, Kondeatis E, Collins R, et al. Mapping MHC-encoded susceptibility and resistance in primary sclerosing cholangitis: the role of MICA polymorphism. Gastroenterology. 2001;120:1475–1482.
Cox ST, Madrigal JA, Saudemont A. Diversity and characterization of polymorphic 5′ promoter haplotypes of MICA and MICB genes. Tissue Antigens. 2014. doi:10.1111/tan.12400.
Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 2004;23:2134–2145.
Miyazaki K, Yoshida H, Sasaki M, et al. Caspase-independent cell death and mitochondrial disruptions observed in the Apaf1-deficient cells. J Biochem. 2001;129:963–969.
Riedl SJ, Li W, Chao Y, Schwarzenbacher R, Shi Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature. 2005;434:926–933.
Pop C, Timmer J, Sperandio S, Salvesen GS. The apoptosome activates caspase-9 by dimerization. Mol Cell. 2006;22:269–275.
Pandey P, Saleh A, Nakazawa A, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322.
Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483.
Beere HM. Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest. 2005;115:2633–2639.
Chandra D, Bratton SB, Person MD, et al. Intracellular nucleotides act as critical prosurvival factors by binding to cytochrome C and inhibiting apoptosome. Cell. 2006;125:1333–1346.
Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781.
Schimmer AD, Dalili S, Batey RA, Riedl SJ. Targeting XIAP for the treatment of malignancy. Cell Death Differ. 2006;13:179–188.
Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994.
Pathan N, Marusawa H, Krajewska M, et al. TUCAN, an antiapoptotic caspase-associated recruitment domain family protein overexpressed in cancer. J Biol Chem. 2001;276:32220–32229.
Yamamoto M, Torigoe T, Kamiguchi K, et al. A novel isoform of TUCAN is overexpressed in human cancer tissues and suppresses both caspase-8- and caspase-9-mediated apoptosis. Cancer Res. 2005;65:8706–8714.
Verhagen AM, Silke J, Ekert PG, et al. HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem. 2002;277:445–454.
Sharief MK, Noori MA, Zoukos Y. Reduced expression of the inhibitor of apoptosis proteins in T cells from patients with multiple sclerosis following interferon-beta therapy. J Neuroimmunol. 2002;129:224–231.
Waiczies S, Weber A, Lunemann JD, et al. Elevated Bcl-X(L) levels correlate with T cell survival in multiple sclerosis. J Neuroimmunol. 2002;126:213–220.
Semra YK, Seidi OA, Sharief MK. Disease activity in multiple sclerosis correlates with T lymphocyte expression of the inhibitor of apoptosis proteins. J Neuroimmunol. 2002;122:159–166.
Semra YK, Seidi OA, Sharief MK. Overexpression of the apoptosis inhibitor FLIP in T cells correlates with disease activity in multiple sclerosis. J Neuroimmunol. 2001;113:268–274.
Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42:210–213.
Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol. 2012;34:176–184.
Piao X, Komazawa-Sakon S, Nishina T, et al. c-FLIP maintains tissue homeostasis by preventing apoptosis and programmed necrosis. Sci Signal. 2012;5:ra93.
Bona G, Defranco S, Chiocchetti A, et al. Defective function of Fas in T cells from paediatric patients with autoimmune thyroid diseases. Clin Exp Immunol. 2003;133:430–437.
Masuichi H, Seki S, Kitada T, et al. Significant role of apoptosis in type-1 autoimmune hepatitis. Osaka City Med J. 1999;45:61–79.
Canbay A, Taimr P, Torok N, et al. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663.
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669.
Czaja AJ. Review article: Prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther. 2014;39:385–406.
Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975.
Canbay A, Higuchi H, Bronk SF, et al. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330.
Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196.
Takehara T, Tatsumi T, Suzuki T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197.
Tsikrikoni A, Kyriakou DS, Rigopoulou EI, et al. Markers of cell activation and apoptosis in bone marrow mononuclear cells of patients with autoimmune hepatitis type 1 and primary biliary cirrhosis. J Hepatol. 2005;42:393–399.
Taylor PR, Martinez-Pomares L, Stacey M, et al. Macrophage receptors and immune recognition. Ann Rev Immunol. 2005;23:901–944.
Leitinger N. The role of phospholipid oxidation products in inflammatory and autoimmune diseases: evidence from animal models and in humans. Subcell Biochem. 2008;49:325–350.
Hanayama R, Tanaka M, Miwa K, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187.
Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974.
Lemke G, Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Ann NY Acad Sci. 2010;1209:23–29.
Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219.
Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–480.
Rong GH, Yang GX, Ando Y, et al. Human intrahepatic biliary epithelial cells engulf blebs from their apoptotic peers. Clin Exp Immunol. 2013;172:95–103.
Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286.
Dranoff JA, Ogawa M, Kruglov EA, et al. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424.
Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630.
Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476.
Sun EW, Shi YF. Apoptosis: the quiet death silences the immune system. Pharmacol Ther. 2001;92:135–145.
Ghavami S, Hashemi M, Kadkhoda K, et al. Apoptosis in liver diseases—detection and therapeutic applications. Med Sci Monit. 2005;11:RA337–RA345.
Sun H, Nikolovska-Coleska Z, Yang CY, et al. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J Med Chem. 2004;47:4147–4150.
Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745.
O’Brien S, Moore JO, Boyd TE, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–1120.
Masuoka HC, Guicciardi ME, Gores GJ. Caspase inhibitors for the treatment of hepatitis C. Clin Liver Dis. 2009;13:467–475.
Hoglen NC, Hirakawa BP, Fisher CD, et al. Characterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury. J Pharmacol Exp Ther. 2001;297:811–818.
Ueno Y, Ohmi T, Yamamoto M, et al. Orally-administered caspase inhibitor PF-03491390 is retained in the liver for prolonged periods with low systemic exposure, exerting a hepatoprotective effect against alpha-fas-induced liver injury in a mouse model. J Pharmacol Sci. 2007;105:201–205.
Witek RP, Stone WC, Karaca FG, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430.
Anstee QM, Concas D, Kudo H, et al. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53:542–550.
Yoshida N, Iwata H, Yamada T, et al. Improvement of the survival rate after rat massive hepatectomy due to the reduction of apoptosis by caspase inhibitor. J Gastroenterol Hepatol. 2007;22:2015–2021.
Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Curr Drug Targets Immune Endocr Metab Disord. 2005;5:269–287.
Valentino KL, Gutierrez M, Sanchez R, Winship MJ, Shapiro DA. First clinical trial of a novel caspase inhibitor: anti-apoptotic caspase inhibitor, IDN-6556, improves liver enzymes. Int J Clin Pharmacol Ther. 2003;41:441–449.
Pockros PJ, Schiff ER, Shiffman ML, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329.
Arends JE, Hoepelman AI, Nanlohy NM, et al. Low doses of the novel caspase-inhibitor GS-9450 leads to lower caspase-3 and -8 expression on peripheral CD4+ and CD8+ T-cells. Apoptosis. 2011;16:959–966.
Baskin-Bey ES, Washburn K, Feng S, et al. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–225.
Santiago-Raber ML, Baudino L, Izui S. Emerging roles of TLR7 and TLR9 in murine SLE. J Autoimmun. 2009;33:231–238.
Lenert PS. Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for toll-like receptors (TLR) 7 and 9. Mediat Inflamm. 2010;2010:986596.
LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259.
Wang K, Lin B. Inhibitor of apoptosis proteins (IAPs) as regulatory factors of hepatic apoptosis. Cell Signal. 2013;25:1970–1980.
Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261.
Zehntner SP, Bourbonniere L, Moore CS, et al. X-linked inhibitor of apoptosis regulates T cell effector function. J Immunol. 2007;179:7553–7560.
Jost PJ, Grabow S, Gray D, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039.
Mayer BA, Rehberg M, Erhardt A, et al. Inhibitor of apoptosis proteins as novel targets in inflammatory processes. Arterioscler Thromb Vasc Biol. 2011;31:2240–2250.
Agrawal S. Importance of nucleotide sequence and chemical modifications of antisense oligonucleotides. Biochim Biophys Acta. 1999;1489:53–68.
Hu Y, Cherton-Horvat G, Dragowska V, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9:2826–2836.
Chen J, Xiao XQ, Deng CM, Su XS, Li GY. Downregulation of xIAP expression by small interfering RNA inhibits cellular viability and increases chemosensitivity to methotrexate in human hepatoma cell line HepG2. J Chemother. 2006;18:525–531.
Gorgani NN, Theofilopoulos AN. Contribution of histidine-rich glycoprotein in clearance of immune complexes and apoptotic cells: implications for ameliorating autoimmune diseases. Autoimmunity. 2007;40:260–266.
Baker BF, Monia BP. Novel mechanisms for antisense-mediated regulation of gene expression. Biochim Biophys Acta. 1999;1489:3–18.
Cui D, Zhang S, Ma J, Han J, Jiang H. Short interfering RNA targeting NF-kappa B induces apoptosis of hepatic stellate cells and attenuates extracellular matrix production. Dig Liver Dis. 2010;42:813–817.
Yonehara S. Death receptor Fas and autoimmune disease: from the original generation to therapeutic application of agonistic anti-Fas monoclonal antibody. Cytokine Growth Factor Rev. 2002;13:393–402.
Orzaez M, Gortat A, Mondragon L, Perez-Paya E. Peptides and peptide mimics as modulators of apoptotic pathways. ChemMedChem. 2009;4:146–160.
Prinz-Hadad H, Mizrachi T, Irony-Tur-Sinai M, et al. Amelioration of autoimmune neuroinflammation by the fusion molecule Fn14.TRAIL. J Neuroinflamm. 2013;10:36.
Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034.
Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268.
Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245.
Dong H, Strome SE, Matteson EL, et al. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111:363–370.
Sabapathy K. Role of the JNK pathway in human diseases. Prog Mol Biol Transl Sci. 2012;106:145–169.
Minero VG, Khadjavi A, Costelli P, Baccino FM, Bonelli G. JNK activation is required for TNFalpha-induced apoptosis in human hepatocarcinoma cells. Int Immunopharmacol. 2013;17:92–98.
Lapierre P, Djilali-Saiah I, Vitozzi S, Alvarez F. A murine model of type 2 autoimmune hepatitis: xenoimmunization with human antigens. Hepatology. 2004;39:1066–1074.
Holdener M, Hintermann E, Bayer M, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–1422.
Adachi M, Suematsu S, Kondo T, et al. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300.
Yue HH, Diehl GE, Winoto A. Loss of TRAIL-R does not affect thymic or intestinal tumor development in p53 and adenomatous polyposis coli mutant mice. Cell Death Differ. 2005;12:94–97.
Nakamoto Y, Kaneko S, Fan H, et al. Prevention of hepatocellular carcinoma development associated with chronic hepatitis by anti-fas ligand antibody therapy. J Exp Med. 2002;196:1105–1111.
Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467.
Tinmouth J, Lee M, Wanless IR, et al. Apoptosis of biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. Liver. 2002;22:228–234.
Macdonald P, Palmer J, Kirby JA, Jones DE. Apoptosis as a mechanism for cell surface expression of the autoantigen pyruvate dehydrogenase complex. Clini Exp Immunol. 2004;136:559–567.
Lleo A, Selmi C, Invernizzi P, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879.
Lleo A, Bowlus CL, Yang GX, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998.
Rong G, Zhong R, Lleo A, et al. Epithelial cell specificity and apotope recognition by serum autoantibodies in primary biliary cirrhosis. Hepatology. 2011;54:196–203.
Greystoke A, Cummings J, Ward T, et al. Optimisation of circulating biomarkers of cell death for routine clinical use. Ann Oncol. 2008;19:990–995.
Bantel H, Ruck P, Gregor M, Schulze-Osthoff K. Detection of elevated caspase activation and early apoptosis in liver diseases. Eur J Cell Biol. 2001;80:230–239.
Hiley C, Fryer A, Bell J, Hume R, Strange RC. The human glutathione S-transferases. Immunohistochemical studies of the developmental expression of alpha- and pi-class isoenzymes in liver. Biochem J. 1988;254:255–259.
Denk G, Omary AJ, Reiter FP, et al. sICAM, M30, and M65 as serum markers of disease activity and prognosis in cholestatic liver diseases. Hepatol Res. 2014;. doi:10.1111/hepr.12304.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Czaja, A.J. Targeting Apoptosis in Autoimmune Hepatitis. Dig Dis Sci 59, 2890–2904 (2014). https://doi.org/10.1007/s10620-014-3284-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3284-2