Abstract
Background
Intestinal pacing (IP) has been previously shown to delay gastric emptying and reduce food intake in animals. The aims of this study were to investigate the effect and mechanism of IP on nutrient absorption in healthy volunteers.
Methods
Twelve healthy volunteers (six men, six women) were involved in a two-session (one session without IP and one with IP) study. At the beginning of each session, a nasal-duodenal feeding tube, with two ring electrodes (used for IP) on the tip of the tube, was incubated into the duodenum under endoscopy. After a complete recovery from the incubation, the duodenum was infused via the feeding tube with 150 ml 30% intralipid + 25 g D-xylose within 30 min, and the stool was collected for 24 h for the analysis of fecal lipid during which a controlled meal was taken. Then 100 ml 1mCi99Tc-labeled non-absorbable solution was infused within 3 min. The subject was asked to lie under a γ camera for at least 1 h for the measurement of small bowel transit. The movement of isotopes was monitored by γ camera at an interval of 10 s. The first appearance of isotopes in the cecum was considered as small intestinal transit time. The order of the two sessions was randomized and 1 week apart. In the IP session, intestinal pacing was performed via the pair of the ring electrodes for 2 h initiated at the beginning of infusion with a pacing frequency of 13 pulses/min, pulse width of 300 ms and amplitude of 5 mA.
Results
(1) IP significantly reduced lipid and D-xylose absorption. The fecal lipid was 6.6 ± 4.6 g without IP and almost doubled with IP (11.1 ± 6.5 g, P = 0.047). Similarly, the D-xylose in urine was 3.46 ± 2.22 g with IP, which was significantly lower than that without IP (6.63 ± 5.06 g, p = 0.049). (2) IP accelerated intestinal transit. The transit time was 39 ± 17 min in the control session and reduced to 28 ± 10 min in the IP session (p < 0.03). (3) Diarrhea was reported in one subject without IP but in six subjects with IP (p < 0.05).
Conclusions
The increased fecal lipid and induction of diarrhea with intestinal pacing suggest that intestinal pacing is capable of inducing malabsorption. This effect maybe contributed to the acceleration of intestinal transit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is the most prevalent public health problem which affects more than 1.7 billions of global population and causes 2.5 millions deaths every year [1, 2]. Currently, this disease is still lack of satisfactory therapeutics although various treatment options are available, such as diet, exercises, pharmacotherapy, and surgery. There is an urgent need to develop safer and more effective methods to treat patients with morbid obesity, as concluded by a panel of expert at a National Institutes of Health consensus conference [3].
Previous studies have suggested that gastric electrical stimulation maybe a potential therapeutic method on the treatment of obesity. Preliminary clinical studies by Cigaina et al. [4] showed that gastric electrical stimulation induced weight loss in morbidly obese patients. Shikora et al. [5] reported a multicenter, randomized, double-blinded clinical trial in which the safety and efficacy of a transcend implantable gastric stimulation system for weight loss was evaluated. In these methods, electrical stimulation was performed using serosal electrodes implanted on the gastric serosa.
Preliminary human studies in our laboratory have shown that electrical stimulation of the small intestine delays gastric emptying and reduces water intake in healthy volunteers [6]. Our animal studies have also shown that intestinal electrical stimulation (IES) delayed gastric emptying [7], induced gastric relaxation [8], reduced food intake in dogs [8], and significantly decreased lipid absorption in rats [9]. All these preliminary studies suggested that IES may have a therapeutic potential for obesity. But no previous study has ever investigated the effect of IES on nutrient absorption in human and its possible mechanisms.
Therefore, the aims of this clinical study were to investigate the possible effect of the novel nonsurgical method, IES by using intraluminal electrodes, on nutrient absorption, dyspeptic and malabsorption symptoms, and its possible mechanisms.
Methods
Subjects
Twelve healthy volunteers (6 men and 6 women, mean age 22, range 22–24 years) were involved in this study. The body mass index (BMI) was 20.53 ± 2.17 kg/m2. All the subjects were free of any symptoms, had no history of gastrointestinal disease or surgery, and had not taken any medications during the past 2 weeks. Furthermore, biochemistry measurement of liver function, renal function, fasting blood glucose, and ultrasound examinations were performed to exclude the possible disease, which might impair the absorption function. Written consent was obtained from every participant, and the study protocol was approved by the ethical review board of the institution of Tongji Medical College.
Experimental Protocol
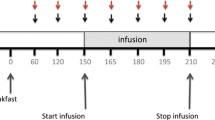
This study was composed of two sessions with two steps in each session. The two steps were performed in two consecutive days in each session and the duration between each session was 1 week. All the subjects were fasted for 12 h prior to the initiation of each session. A regular nasal-jejunal feeding tube with a diameter of 0.4 cm (Flexiflo Flocator, Ross Product Division, Abbott Laboratories, Columbus, OH) attached with three ring electrodes was intubated via the nose under gastric endoscopy. To avoid the possible effect of anesthesia on gastrointestinal motility and sensation, no sedation was taken during the operation of endoscopy. The end tip of the feeding tube was placed in the duodenum about 15 cm beyond the pylorus. The three ring electrodes at the end tip of the tube were arranged with an interval of 5 cm and the two distal electrodes were used for electrical stimulation and electrodes. The location of the electrodes was checked by abdominal X-ray to confirm the right position in duodenum before the initiation of every step. Otherwise, replacement of tube was performed via endoscopy. All steps were performed in a silent room with temperature of 28°C.
Measurement of Small Bowel Absorption
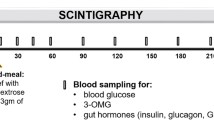
In stimulation session first step, after a 1 h recovery time from the intubation of feeding tube, a set of duodenal electrical stimulation with parameter of frequency 13 cpm, pulse width of 300 ms and amplitude of 5 mA generated by a universal stimulator (WPI, Sarasota, Florida) was applied via the electrodes for 1.5 h. At the initiation of stimulation, the subject received an infusion of 250 ml test liquid meal in 30 min, which was consisted of 150 ml 30% intralipid (triacylglycerol emulsion, Kabi Pharmacia, Milton Keynes, UK), 25 g D-xylose (Sigma) + 100 ml physical saline, followed by an infusion of 480 ml 0.9% physical saline in 1 h. Infusion rate were 8 ml/min for both test liquid meal and physical saline, which was controlled by an infusion pump (Terumo Corp, TE-171, Jpn). To avoid the possible effect of movement on small bowel transit, subject was required to keep supine position for 5 hours from the beginning of the procedure. A standard meal with total vegetable oil of 80 g was taken and nothing was taken after then except water till the initiation of the 2nd step.
Measurement of Small Bowel Transit
The second step was undergone 24 h after the initiation of the first step. A same set of electrical stimulation as step 1 was delivered via the electrodes for 1 h or γcamera scan showed that isotopes arrived into cecum. Simultaneously, 100 ml 1mCi99Tc labeled nonabsorbable solution (25 mM NaCl, 20 mM NaHCO3, 40 mM Na2SO4, 10 mM KCl, 5 g/L PEG, 80 mM Mannitol) was infused into small intestine via the feeding tube in 3 min followed by physical saline infused at speed of 8 ml/l till the end of step 2 [10]. Gamma camera imaging was started at the initiation of infusion in order to monitor the movement of radiolabel along the small intestine. Scans were taken every 10 seconds for 1 h or till the isotopes arrived into cecum.
The procedure of control session was the same as stimulation session except a sham stimulation (electrode wire was connected with stimulator, no stimulation was delivered) was applied during the infusion.
Symptoms Score
Symptoms of dyspepsia, which includes fullness, appetite, abdominal pain, bloating, nausea and vomiting, were scored by the subjects themselves at the initiation and end of infusion, respectively. These symptoms were assessed based on their severity and/or frequency (0: never; 1: seldom; 2: often; 3: continues or cannot study). Symptom of malabsorption, which was represented by the frequency of diarrhea (0: none; 1: one time; 2: two times; 3: more than three times). Diarrhea was defined by the Bristol criteria and type 6 or 7 was considered as diarrhea.
Measurement of Fecal Lipid
The excreted stool was collected during the 24 h from the initiation of procedure for the analysis of fecal lipid. The feces were weighed, and stored at −20°C till the time for fecal lipid analysis. The amount of lipid excreted in the feces was quantified by the method of Van de Kamer et al. [11].
Measurement of Urinary D-xylose
Urine was collected for the analysis of D-xylose during the 5 h after the test meal was infused, and aliquots subsequently stored at −20°C until process. Urinary D-xylose was measured by standard colorimetry [12].
Statistics
All values were expressed as mean ± STD. The data were compared using Student’s t test. The symptom of diarrhea was analyzed by Chi-square test. p < 0.05 was considered significant.
Results
Effect of IP on Nutrient Absorption
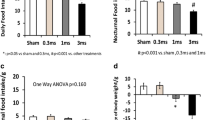
IP decreased lipid and D-xylose absorption. As shown in Fig. 1, in stimulation session, the volume of D-xylose in the collected urine during the 5 h after the test meal was infused were significantly lower than those in control session (p < 0.05). Similarly, the volume of lipid in stimulation session was significantly higher than those in control session, which meant that more lipids were excreted and less were absorbed (p < 0.05; Fig. 2).
Effect of IP on Malabsorption and Dyspepsia Symptoms
IP induced more symptoms of malabsorption and dyspepsia. In stimulation session first step, the mean dyspepsia symptoms score during the test meal was infused was 8.5 ± 5.7, which was significantly higher than that in its baseline (1.3 ± 1.5) and accordingly period in control session (4.1 ± 3.0; p < 0.003; Fig. 3). This difference were contributed to all the symptoms, including fullness, appetite, abdominal pain, bloating, nausea, and vomiting (p < 0.05; see Fig. 4). Whereas, in the stimulation session second step, which non-absorbable liquid was infused, IP did not induce more malabsorption symptoms when comparing to its baseline and that in control session (p > 0.05). Furthermore, as the typical malabsorption symptoms-diarrhea was concerned, six subjects presented symptoms of diarrhea in the IP session and only one subject had diarrhea in the control session (p < 0.05, Chi-square test; Table 1).
IP significantly increase dyspepsia symptoms score during nutrition infusion. In the stimulation session first step, the symptoms score after the nutrition was infused was significantly higher than that at baseline and accordingly period in control session (p < 0.003 vs control or baseline). In control session first step, the symptoms score after the nutrition infusion was significantly higher than those at baseline and during nonabsorbable liquid infusion step (second step; p < 0.05)
Effect of IP on Small Bowel Transit
IP significantly accelerated the small bowel transit. The mean time of arrival of isotopes into the cecum in IP session was 28 ± 10 min, which was significantly lower than that in control session (p < 0.05). But the relationship between small bowel transit and D-xylose absorption was not significant (p > 0.05; Fig. 5)
Discussion
In the present study, we demonstrated that IES significantly decreased the volume of D-xylose in the urine: increased the volume of fecal lipid. Furthermore, our data suggested that IES induced dyspeptic and malabsorption symptoms when nutrient was infused. The small bowel transit was also accelerated with IES.
An intraluminal stimulation method was used in this IP study and certain dose of intralipid plus D-xylose were used as the marker for investigating intestinal absorption ability. Our previous canine study investigated the effect of intestinal electrical stimulation on intestinal slow wave using the same intraluminal ring electrodes and showed that intestinal slow waves recorded by the ring electrodes were exactly the same as those recorded by the serosal electrodes [13]. In our previous human study, the regular duodenal slow waves were also recorded by the same electrodes, which proved the reliability of the contact between the ring electrodes and intestinal mucosa and lead to the possibility of using intraluminal probe for electrical stimulation in the small intestine [6]. D-xylose is the common marker for the analysis of small intestinal absorption. It’s absorbed unchangingly in jejunum and excreted into the urine, which allowing it for the measurement [14]. According to our knowledge, the dose of D-xylose for analyzing intestinal absorption change in the range of 5 to 25 g [15]. In this study, to investigate the effect of IP on inducing malabsorption, we used the highest dose of 25 g. Similarly, the dose of lipid for intestinal infusion is in the range of 3 to 32 g [16, 17]. Also, it is reported that common dose of lipid only consume 58% of the small intestine absorption capacity [18]. In our preliminary study, the dose of 50 g lipid induced diarrhea even in control session although it was much less than the limit of human daily energy consumption. This maybe due to the infusion of intralipid in our study was directly into the small intestine instead of into stomach. Therefore, we chose the dose of in the present study and only one subject presented symptom of diarrhea in control session.
The effect of intestinal electrical stimulation on small bowel transit has been explored previously [19–24]. The majority of the studies were designed to delay intestinal transit using backward stimulation so that the absorption would be increased and the diseases, such as dumping syndrome or short bowel syndrome, might be resolved. Acceleration of intestinal transit in a canine model of ileal brake was also reported with forward intestinal stimulation [25]. To the best of our knowledge, all of these previous studies were performed in animals. In this preliminary clinical study, we found that forward IP accelerated small bowel transit in healthy humans and the results were consistent with previous canine studies.
The mechanism by which small bowel transit was accelerated with IP is not understood. Intestinal electrical stimulation, using the long pulses similar to this current study, has shown to be capable of entraining intestinal slow waves and normalizing dysrhythmia [13, 26, 27]; its effect dose not seem to be mediated via central cholinergic vagal pathway [28]. Our recent canine study has suggested that intestinal stimulation with long pulses decrease the intraluminal pressure in small intestine and it is partly mediated by a nitric oxide pathway [29]. This maybe the cause for the acceleration of small bowel transit with long pulse stimulation since the resistance in small intestine is reduced when the intraluminal pressure is decreased. Further studies are needed to investigate possible mechanisms involved with intestinal electrical stimulation.
Huge amount of studies have confirmed that the absorption of D-xylose and lipid is associated with the surface of the small intestine which the nutrient contacted and in the condition of reduced small bowel surface, such as short-bowel syndrome, the absorption of D-xylose is significantly impaired [12]. In the present study, we demonstrated that IP significantly decreased the volume of D-xylose in the urine and increased the volume of fecal lipid, which meant that IP reduced both lipid and D-xylose absorption. This is consistent with previous studies in our lab, which also found that IP can significantly decrease lipid absorption in rats [9]. This may largely contribute to the effect of IP on accelerating the small bowel transit, which shortened the time for the nutrient to contact the intestinal mucosa. Unfortunately, the present study did not find the relationship between nutrition absorption and small bowel transit, which suggested that some other factors, such as ingestive enzyme secretion, which may also participated in the absorption [23].
In the present study, we noted that IP induced no symptoms during the nonabsorbable liquid was infused, nor in control session. Interestingly, the effect of IP on the symptoms was noted during the nutrition was infused. We suspect this may contribute to accelerated nutrient transit induced by IP. Our previous study has already demonstrated that IP significantly decreased water intake and gastric emptying, which suggested that IP may impair the gastric accommodation and have the potential to induce dyspeptic symptoms [6]. Cherbut have proven that rapid nutrition input can evoke the ileum brake reflex, which can also delay gastric emptying [30]. Also the infusion of lipid into the small intestine can impair the food intake and appetite [17]. This may be the cause which nutrient infusion induces more symptoms of dyspepsia. We noted that the symptoms of dyspepsia and malabsorption were mild to moderate, and none of all 12 subjects quit the study for intolerable symptoms, which suggests the symptoms are safe and may benefit for reducing the food intake.
Obesity is a popular medical problem with a lack of optimal therapies. The result of this study (decreased nutrition absorption, accelerated small bowel transit, induced dyspeptic and malabsorption) combining the result in our previous human study suggests that intestinal electrical stimulation may have a therapeutic potential for obesity. Although this was an acute study and the clinical meaning of the data is subjective to further verification with chronic studies, we anticipate the dyspeptic and malabsorption induced by IP would prolong the interval and reduce the energy intake of meals. The reduction of nutrient absorption is also helpful for the decrease of overweight. Furthermore, the result of IP induces no dyspeptic and malabsorption symptoms when energy-free meal is taken suggests that this method may not impair the subjects’ common life, which indicates that IP is very meaningful for its clinical application. Chronic study with IP should be performed to access the therapeutic potential of IP for obesity.
In conclusion, IP with a long pulse decreases absorption of nutrients, induces dyspeptic and malabsorption symptoms during intake, accelerates small bowel transit, and may have a therapeutic potential for obesity.
References
Professor Philip James, Chair of the London-based international obesity task force. Monte Carlo, March 17, 2003. Available at: www.itof.org/media. Accessed 1 Mar 2004.
World Health Report 2002. Available at: www.itof.org. Accessed 1 Mar 2004.
Gastrointestinal surgery for severe obesity. National Institutes of Health Consensus Development Conference Draft Statement. Obes Surg. 1991;1:157–65.
Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12:12S–6S.
Shikora SA, Bessier M, Fisher BL, Trigillo C, Mincure M, Greenstein R. Laparoscopic insertion of the implantable gastric stimulator (IGSTM): initial surgical experience. Obes Surg. 2000;10:Appendix 3-A.
Shi L, Xiaohua H, Chen JDZ. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterology. 2005;100:792–6.
Zhao XT, Wang LJ, Xu XH, Chen JD. Electric stimulation of small intestine delays gastric emptying in the dog model [abstract]. Neurogastroenterol Motil. 2002;14:457.
Yin JY, Ouyang H, Chen JDZ. The effects of duodenal electrical stimulation (DES) on food intake, gastric tone and gastric myoelectrical activity on dogs [abstract]. Neurogastroenterol Motil. 2002;14:456.
Sun Y, Chen JDZ. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004;12:1235–42.
Glenn RD, Carol ASA, Stephen GM. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980;78:991–5.
Van de Kamer JH, Huinink HTB, Weijers HA. Rapid method for determination of fat in feces. J Biol Chem. 1949;177:349–55.
Craig RM, Atkinson AJ Jr. D-xylose testing: a review. Gastroenterology. 1988;95:223–31.
Lin XM, Hayes J, Peters LJ, Chen JDZ. Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Ann Biomed Eng. 2000;28:582–7.
Tomio U, Yuichiro H, Massaaki O, Takashi S. Oral absorption tests: absorption site of each substrate. Nutrition. 1998;14(1):7–10.
Craig R, Ehrenpreis E. D-xylose testing. Lippincott Williams & Wilkins, INC. 1999;29(2):143–50.
Hammer J, Hammer K, Kletter K. Lipids infused into the jejunum accelerate small intestinal transit but delay ileocolonic transit of solids and liquids. Gut. 1998;43:111–6.
Ian M, Elizabeth A, Gary A, Michael H. Effects of small-intestinal fat and carbohydrate infusions on appetite and food intake in obese and nonobese men. Am J Clin Nutr. 1999;69:6–12.
Weber E, Ehrlein HJ. Reserve capacities of the small intestine for absorption of energy. Am J Physiol. 1998;275(1):R300–307.
Kelly KA, Code CF. Duodenal-gastric reflux and slowed gastric emptying by electrical pacing of the canine duodenal pacesetter potentials. Gastroenterology. 1977;72:429–33.
Layzell T, Collin J. Retrograde electrical pacing of the small intestine—A new treatment for the short bowel syndrome? Br J Surg. 1981;68:711–3.
Cranley B, Kelly KA, Go VLW, Mcnichols LA. Enhancing the anti-dumping effect of Roux gastrojejunostomy with intestinal pacing. Ann Surg. 1983;198:516–24.
Miedema BW, Kelly KA. The Roux stasis syndrome: treatment by pacing and prevention by use of an “uncut” Roux limb. Arch Surg. 1992;127:295–300.
Reister SB, Schusdziarra V, Bollschweiler E, Holscher AH, Siewert JR. Effect of enteric pacing on intestinal motility and hormone secretion in dogs with short bowel. Gastroenterology. 1991;101:100–6.
Abo M, Liang J, Qian LW, Chen JDZ. Distention-induced myoelectrical dysrrhythmia and effect of intestinal pacing in dog. Dig Dis Sci. 2000;45:129–35.
Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003;48:251–6.
Qian LW, Lin XM, Chen JDZ. Normalization of atropine-induced postprandial dysrrhythmias with gastric pacing. Am J Physiol. 1999;276:G387–92.
Lin XM, Peters LJ, Hayes J, Chen JD. Entrainment of segmental small intestinal slow waves with electrical stimulation in dogs. Dig Dis Sci. 2000;45:652–6.
Chen JDZ, Qian LW, Ouyang H, Chen JD. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–9.
Zhao XT, Wang LJ, Douglas LB, Chen JD. Delayed liquid gastric emptying induced by electric stimulation of small intestine depends on nitric oxide pathway in conscious dogs[abstract]. Gastroenterology. 2003;124 Suppl 1:W1474.
Cherbut C, Aube AC, Blottiere HM, Galmiche JP. Effects of short-chain fatty acids on gastrointestinal motility. Scand J Gastroenterol Suppl. 1997;222:58–61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Qiao, X., Hou, X. et al. Effect of Intestinal Pacing on Small Bowel Transit and Nutrient Absorption in Healthy Volunteers. OBES SURG 19, 196–201 (2009). https://doi.org/10.1007/s11695-008-9533-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9533-8