Abstract
In a retrospective cohort, we investigated the presence of the JAK2V617F mutation in chronic non-cirrhotic portal vein thrombosis (PVT) patients, irrespective of the presence or absence of myeloproliferative diseases (MPDs). We identified 25 patients in whom thrombophilia workup was completed. The diagnoses of MPDs were made according to the World Health Organization (WHO) criteria. JAK2V617F mutation analysis was performed by allele-specific polymerase chain reaction (PCR). There were 9 male and 16 female patients. Prior to JAK2V617F analysis, there were one or more thrombophilic risk factors in 19 patients (76%). The JAK2V617F mutation analysis revealed the presence of this mutation (all in the heterozygote state) in six patients (24%; two male, four female). Five of the six cases with prior clinical diagnosis of MPDs were found to have wild-type JAK2. We found that the addition of JAK2V617F analysis into the thrombophilia workup in patients with chronic PVT contributes to a 4% increase in the diagnosis of thrombophilic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Portal vein thrombosis (PVT) in non-cirrhotic patients is an established risk factor for both morbidity and mortality. In some patients, there may be a thrombophilic condition, resulting in “primary” thrombosis. The clinical importance of an underlying thrombophilic condition is based on the observation indicating an increment of the overall mortality by four times in the presence of thrombophilia [1]. Studies investigating the etiology of thrombosis in the hepatic vasculature showed that predisposing thrombophilic conditions were deficiencies of protein C, protein S, and antithrombin, factor V Leiden and prothrombin 20210A mutations, myeloproliferative diseases (MPDs), and other less common disorders, including Behçet’s disease [2–6].
Currently, “idiopathic” hepatic/portal venous thrombosis patients (i.e., those without any thrombophilic or mechanical risk factors) constitute about 7–22% of all cases [7, 8]. In a study by Denninger et al. [7], an underlying prothrombotic condition was identified in 72% of PVT patients. There were one or more prothrombotic conditions in 26 out of 36 patients studied and primary myeloproliferative disorders were the leading cause, with a prevalence of 30% in this study. Other studies by Valla et al. [9–11] and De Stefano et al. [12] reported similar findings, suggesting that myeloproliferative disorders (overt or occult) rank first among thrombophilic conditions leading to PVT. Valla et al. [9] proposed that overt or latent MPDs might be responsible for thrombophilic milieu in 48% of the patients with PVT.
PVT may lead to the masking of MPD features due to gastrointestinal bleeding (occult or variceal) and hemodilution secondary to portal hypertension and splenomegaly (which is usually attributed solely to PVT by clinicians). In addition, Chait et al. [13] found that the serum erythropoietin level might be low in PVT patients, making this test unreliable for the diagnosis of polycythemia vera (PV) in 68% of cases. Endogenous erythroid colony (EEC) formation and bone marrow biopsy were suggested to have diagnostic value for MPDs in the setting of PVT, masking the usual features of the hematological disorders. However, these tests are not considered between the major diagnostic criteria of PV in the World Health Organization (WHO) [14] and the Polycythemia Vera Study Group (PVSG) [15] classifications. Moreover, EEC formation is not standardized, not widely available, not technically practical, and only diagnostic in 30–80% of all cases [16, 17]. De Stefano et al. [12] found that the classical criteria and EEC formation can diagnose only 23–49% of cases of MPDs in patients with PVT. Therefore, gastroenterologists need more objective clues in order to suspect MPDs.
Recently, the discovery of the Janus Kinase 2 V617F (JAK2V617F) mutation [18–21] has gained great interest in the setting of splanchnic venous thrombosis. In this retrospective cohort study, we aimed to investigate the presence of the JAK2V617F mutation in patients with chronic non-cirrhotic PVT, irrespective of the presence or absence of MPDs.
Methods
Patients
The study was conducted at the Hacettepe University Hospital, Turkey. The Institutional Ethical Review Board of the Hacettepe University Medical School approved the study protocol before any laboratory observations and retrospective data analyses were performed.
The study subjects were chronic non-cirrhotic PVT cases who were diagnosed at our gastroenterology unit between January 2000 and January 2006. Chronic PVT was defined as partial or total occlusion of the portal system known for more than 6 months or as the presence of portal vein cavernous transformation. The primary diagnosis of PVT was made by Doppler ultrasound. Conventional portal venous angiography and non-invasive techniques, like magnetic resonance (MR) and computed tomography (CT)-based angiographies, were also performed (as needed) to confirm the diagnosis. All patients with acute or sub-acute PVT (arbitrarily defined as newly diagnosed PVT within 3 months), cirrhosis, or liver malignancy (hepatocellular carcinoma or liver metastasis) were excluded from the study.
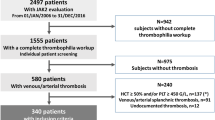
We screened 60 patients with chronic PVT diagnosis. The major determinants of eligibility were the availability of completed thrombophilia workup and the presence of DNA samples from previous studies in our center which were suitable for JAK2V617F mutation analysis. We identified 25 patients who had completed the thrombophilia workup and had DNA samples available for the JAK2V617F analysis. Thrombophilia screening tests included protein C, protein S, antithrombin, homocysteine, and fibrinogen levels, molecular genetic studies of methylenetetrahydrofolate reductase (MTHFR) mutation, factor V Leiden mutation and prothrombin 20210A mutation, anticardiolipin IgG and IgM antibodies, and lupus anticoagulant. The diagnosis of antiphospholipid syndrome had been made by previously described serologic and clinical criteria [22]. In our clinical practice, MPDs are considered if a patient with PVT has higher-than-expected blood counts and/or massive splenomegaly. In this retrospective cohort, MPDs had been considered in the same situations. In order to confirm the diagnosis of MPDs, bone marrow aspiration/biopsy, serum erythropoietin level, total red cell mass (TRCM), and t(9; 22) mutational analysis had been ordered depending on the clinical condition. The diagnoses of MPDs were made according to the WHO criteria. Spontaneous erythroid colony formation to screen latent MPDs had not been tested. None of the patients had arterial or venous thrombosis elsewhere.
Blood Tests
Blood tests for thrombophilia screening had been studied as previously described [3, 4, 23–25].
JAK2V617F Mutation Analysis
The genomic DNA extracted from the peripheral blood of the study samples were studied. The stored DNA samples were tested for the JAK2V617F mutation by allele-specific polymerase chain reaction (PCR), and the gel detection method (NuSieve 3:1 agarose/TBE gel) is used after amplification (InVivoScribe Technologies, San Diego, CA).
Statistical Analysis
The group means and ratios were compared by Mann-Whitney’s U-test and the chi-square test (Fisher’s exact test), respectively. Statistical significance is considered at P-values lower than 0.05. The SPSS (Statistical Package for Social Sciences Inc., Chicago, IL) software package was used to carry out the statistical analyses.
Results
The data from clinical and laboratory investigations of the patients are shown in Table 1. There were nine male and 16 female patients, with a mean age of 44.9 years (range: 24–73 years).
Previous MPD Diagnosis
There were six patients with MPDs (three patients with PV, two patients with essential thrombocythemia, one patient with myelofibrosis) in the PVT cohort. All of them had been diagnosed with MPD upon investigation for PVT prior to JAK2V617F mutation analysis.
JAK2V617F Mutation Analysis
The JAK2V617F mutation analysis revealed the presence of this mutation (all in the heterozygote state) in six patients (24%; two male, four female). There was only one patient in this group whose MPD diagnosis (PV) was evident before the mutation analysis. The other five patients had no “suspicious” findings to consider MPD. We accepted these cases as “latent MPD” after finding the JAK2V617F mutation as positive. Five of the six cases with prior clinical diagnosis of MPD were found to have wild-type JAK2.
The blood counts were not significantly different in 11 patients with (overt or latent) MPDs compared to 14 patients without MPDs.
Thrombophilia Tests
There were one or more thrombophilic risk factors in 19 patients (76%) (Table 1) detected prior to JAK2V617F analysis. One JAK2V617F-positive case had a history of splenectomy due to severe thrombocytopenia (platelet count of less than 40,000/μl) before the diagnosis of PVT. Upon investigation of the medical records, we found that her leukocytosis and thrombocytosis had been attributed to splenectomy and no further MPD analysis had been carried out due to this presumption. Although PVT may be triggered by splenectomy in this case, JAK2V617F-positivity might be an underlying risk factor in this patient and, therefore, she was not excluded from the study.
Association of the JAK2V617F Mutation with Homozygous MTHFR C677T Polymorphism
In six JAK2V617F-positive patients, we coincidentally found that three subjects also had homozygote MTHFR C677T polymorphism. This genetic change was present in only in one out of 19 cases without the JAK2V617F mutation (p = 0.03)
Discussion
Recently, different JAK2V617F prevalence figures have been described in PVT. In a study by Primignani et al. [26], the prevalence was 35.6%. They also stated that the JAK2V617F analysis was highly predictive of MPD diagnosis when combined with positive findings in bone marrow. Another recent study by Colaizzo et al. [27] found that the prevalence of the JAK2V617F mutation was 17.2% and that none of the patients with the mutation were positive for any other inherited thrombophilic factors. De Stefano et al. [28] found a 42.9% prevalence of the JAK2V617F mutation in patients with idiopathic PVT. These differences in the prevalence of the JAK2V617F mutation might be related to the study populations and the patient selection bias problem.
We also evaluated the presence of the JAK2V617F mutation in a cohort of chronic PVT patients. JAK2V617F was present in 24% of the patients. Six patients had been diagnosed with MPDs before the genetic analysis, and only one of them was found to have JAK2V617F. If we eliminate this case and also removed one patient who had been considered to have postsplenectomy thrombocytosis, neither thrombocytosis (>600×103/μl) nor erythrocytosis (defined as hemoglobin >18.5 g/dl in men, >16.5 g/dl in women) were present in our JAK2V617F-positive patients. Considering the latent and classical cases together, a significant proportion (11/25, 44%) of the PVT cases had MPDs. This is consistent with previous clinical observations indicating that MPDs are the most frequent causes of PVT [7, 9, 12]. Our findings showed that six out of 11 patients with overt or latent MPDs had one additional inherited risk factor, and one patient had two additional risk factors. We found the JAK2V617F mutation in one out of six patients who had no other thrombophilic risk factors. It is obvious that the JAK2V617F mutational analysis may help clinicians in the diagnosis of masked or latent MPDs underlying hepatic/portal venous thromboses. However, the sensitivity of the test is probably not satisfactory. Additional clinical data, especially bone marrow examination, TRCM determination, and follow-up findings, are very important in establishing the diagnosis. The importance of bone marrow biopsy findings have been highlighted in the recent WHO classification of MPDs [14].
Generally, about 50% of patients with MPD have the JAK2V617F mutation. However, only one out of six cases with prior diagnosis of MPD was positive. This low sensitivity might be related with DNA sample deterioration over time or selection bias, which is very possible, due to the retrospective nature of this study.
Another coincidental and interesting result of our study, which has not been observed previously, is the possible association of the JAK2V617F mutation and the MTHFR C677T polymorphism. This original observation might be very important due to the fact that the MTHFR polymorphism is important in carcinogenesis and DNA repair. The MTHFR polymorphism and related DNA repair disorders have been recently suggested to play a possible role in cholangiocellular cancer [29], acute lymphoblastic leukemia [30], and colon cancer [31]. The MTHFR polymorphism might also be a risk factor for the occurrence of the clonal-acquired genetic mutation of the JAK2 gene.
In our study, we selected study patients by the presence of previous thrombophilia workup in order to present the complete thrombophilia risk factors. This process may have resulted in selection bias. Because of this reason, we also should acknowledge that the patient population we tested was, likely, not representative of the “general” chronic PVT patient population. A case–control study would have been most useful for trying to establish an association between the JAK2V617F mutation and chronic non-cirrhotic PVT.
The addition of routine JAK2V617F analysis into thrombophilia workup might increase the percentage of thrombophilia risk factors in PVT cases. In this retrospective cohort, we found that the addition of JAK2V617F analysis in the routine screening of thrombophilia risk factors in chronic PVT patients increases the presence of these risk factors by 4%. In MPDs, a history of previous thromboembolic disorders defines a higher risk for developing new vascular events. In this case, cytoreductive therapy is advised [32, 33]. Currently, it is unknown whether this recommendation applies to latent MPDs observed by JAK2V617F mutational analysis. Further case-controlled studies which would determine the JAK2V617F mutation of unselected patients with PVT are needed to clarify the importance of this mutation for the management of PVT.
References
Condat B, Pessione F, Hillaire S, Denninger MH, Guillin MC, Poliquin M, Hadengue A, Erlinger S, Valla D (2001) Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 120:490–497
Sarin SK, Agarwal SR (2002) Extrahepatic portal vein obstruction. Semin Liver Dis 22:43–58
Gürgey A, Haznedaroglu IC, Egesel T, Büyükasik Y, Ozcebe OI, Sayinalp N, Dundar SV, Bayraktar Y (2001) Two common genetic thrombotic risk factors: factor V Leiden and prothrombin G20210A in adult Turkish patients with thrombosis. Am J Hematol 67:107–111
Egesel T, Büyükasik Y, Dundar SV, Gürgey A, Kirazli S, Bayraktar Y (2000) The role of natural anticoagulant deficiencies and factor V Leiden in the development of idiopathic portal vein thrombosis. J Clin Gastroenterol 30:66–71
Bayraktar Y, Balkanci F, Bayraktar M, Calguneri M (1997) Budd-Chiari syndrome: a common complication of Behçet’s disease. Am J Gastroenterol 92:858–862
Bayraktar Y, Balkanci F, Kansu E, Dundar S, Uzunalimoglu B, Kayhan B, Telatar H, Gurakar A, Van Thiel DH (1995) Cavernous transformation of the portal vein: a common manifestation of Behçet’s disease. Am J Gastroenterol 90:1476–1479
Denninger MH, Chait Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Brière J, Valla D (2000) Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology 31:587–591
Janssen HL, Wijnhoud A, Haagsma EB, van Uum SH, van Nieuwkerk CM, Adang RP, Chamuleau RA, van Hattum J, Vleggaar FP, Hansen BE, Rosendaal FR, van Hoek B (2001) Extrahepatic portal vein thrombosis: aetiology and determinants of survival. Gut 49:720–724
Valla D, Casadevall N, Huisse MG, Tulliez M, Grange JD, Muller O, Binda T, Varet B, Rueff B, Benhamou JP (1988) Etiology of portal vein thrombosis in adults. A prospective evaluation of primary myeloproliferative disorders. Gastroenterology 94:1063–1069
Valla D, Casadevall N, Lacombe C, Varet B, Goldwasser E, Franco D, Maillard JN, Pariente EA, Leporrier M, Rueff B, Muller O, Benhamou J-P (1985) Primary myeloproliferative disorder and hepatic vein thrombosis. A prospective study of erythroid colony formation in vitro in 20 patients with Budd-Chiari syndrome. Ann Intern Med 103:329–334
Valla DC, Condat B (2000) Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol 32:865–871
De Stefano V, Teofili L, Leone G, Michiels JJ (1997) Spontaneous erythroid colony formation as the clue to an underlying myeloproliferative disorder in patients with Budd-Chiari syndrome or portal vein thrombosis. Semin Thromb Hemost 23:411–418
Chait Y, Condat B, Cazals-Hatem D, Rufat P, Atmani S, Chaoui D, Guilmin F, Kiladjian JJ, Plessier A, Denninger MH, Casadevall N, Valla D, Brière JB (2005) Relevance of the criteria commonly used to diagnose myeloproliferative disorder in patients with splanchnic vein thrombosis. Br J Haematol 129:553–560
Vardiman JW, Harris NL, Brunning RD (2002) The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100:2292–2302
Peterson P, Wasserman LR (1995) The natural history of polycythemia vera. In: Wasserman LR, Berk PD, Berlin NI (eds) Polycythemia vera and the myeloproliferative disorders. WB Saunders, Philadelphia, pp 14–21
Patel RK, Lea NC, Heneghan MA, Westwood NB, Milojkovic D, Thanigaikumar M, Yallop D, Arya R, Pagliuca A, Gäken J, Wendon J, Heaton ND, Mufti GJ (2006) Prevalence of the activating JAK2 tyrosine kinase mutation V617F in the Budd-Chiari syndrome. Gastroenterology 130:2031–2038
Lemoine F, Najman A, Baillou C, Stachowiak J, Boffa G, Aegerter P, Douay L, Laporte JP, Gorin NC, Duhamel G (1986) A prospective study of the value of bone marrow erythroid progenitor cultures in polycythemia. Blood 68:996–1002
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR; Cancer Genome Project (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D’Andrea A, Fröhling S, Döhner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, Meyerson M, Golub TR, Lee SJ, Gilliland DG (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7:387–397
James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434:1144–1148
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352:1779–1790
Levine JS, Branch W, Rausch J (2002) The antiphospholipid syndrome. N Engl J Med 346:752–763
Harmanci O, Ersoy O, Gürgey A, Büyükasik Y, Gedikoglu G, Balkanci F, Sivri B, Bayraktar Y (2007) The etiologic distribution of thrombophilic factors in chronic portal vein thrombosis. J Clin Gastroenterol 41:521–527
Harmanci O, Büyükasik Y, Kirazli S, Balkanci F, Bayraktar Y (2006) Does endothelium agree with the concept of idiopathic hepatic vessel thrombosis? World J Gastroenterol 28:1273–1277
Egesel T, Unsal I, Bayraktar Y (2002) Antiphospholipid antibodies and lipoprotein (a) as etiologic or contributory factors in patients with idiopathic cavernous transformation of portal vein. Turk J Gastroenterol 13:89–93
Primignani M, Barosi G, Bergamaschi G, Gianelli U, Fabris F, Reati R, Dell’Era A, Bucciarelli P, Mannucci PM (2006) Role of the JAK2 mutation in the diagnosis of chronic myeloproliferative disorders in splanchnic vein thrombosis. Hepatology 44:1528–1534
Colaizzo D, Amitrano L, Tiscia GL, Scenna G, Grandone E, Guardascione MA, Brancaccio V, Margaglione M (2007) The JAK2 V617F mutation frequently occurs in patients with portal and mesenteric venous thrombosis. J Thromb Haemost 5:55–61
De Stefano V, Fiorini A, Rossi E, Za T, Farina G, Chiusolo P, Sica S, Leone G (2007) Incidence of the JAK2 V617F mutation among patients with splanchnic or cerebral venous thrombosis and without overt chronic myeloproliferative disorders. J Thromb Haemost 5:708–714
Ko KH, Kim NK, Yim DJ, Hong SP, Park PW, Rim KS, Kim S, Hwang SG (2006) Polymorphisms of 5,10-methylenetetrahydrofolate reductase (MTHFR C677T) and thymidylate synthase enhancer region (TSER) as a risk factor of cholangiocarcinoma in a Korean population. Anticancer Res 26:4229–4233
Bolufer P, Barragan E, Collado M, Cervera J, López JA, Sanz MA (2006) Influence of genetic polymorphisms on the risk of developing leukemia and on disease progression. Leuk Res 30:1471–1491
Hubner RA, Lubbe S, Chandler I, Houlston RS (2007) MTHFR C677T has differential influence on risk of MSI and MSS colorectal cancer. Hum Mol Genet 16:1072–1077
Elliott MA, Tefferi A (2005) Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol 128:275–290
Barbui T (2007) The targets of therapy in polycythemia vera and thrombocythemia. Turk J Hematol 24(Suppl 1):54
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayraktar, Y., Harmanci, O., Büyükasik, Y. et al. JAK2V617F Mutation in Patients with Portal Vein Thrombosis. Dig Dis Sci 53, 2778–2783 (2008). https://doi.org/10.1007/s10620-008-0225-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0225-y