Abstract
To determine the prevalence of the V617F Janus Kinase 2 (JAK2) mutation in patients with thrombosis without other biological signs of underlying myeloproliferative neoplasm (MPN) and identify associated risk factors for thrombosis. Over a 10-year period, data were collected from patients with thrombotic events and who had also been screened for the V617F JAK2 mutation. Patients with signs of underlying MPN, such as haematocrit levels ≥ 50% and/or platelet counts ≥ 450 × 109/L and/or splanchnic thrombosis were excluded from the study. Of 340 patients fulfilling inclusion criteria, JAK2 mutation was found in 9 (2.65%), the allele burden being at least 2% in 4 (1.1%). Upon follow-up, MPN was diagnosed in the latter 4. Univariate analysis of the whole cohort showed that age (54 ± 15 vs. 64 ± 13, p = 0.027), platelet count (317 ± 111 vs. 255 ± 75, p = 0.017), C-reactive protein level > 5 mg/L (OR 7.29, p = 0.014), and splenomegaly (OR 54.5, p = 0.0002) were significantly associated with JAK2 mutation. There was also a trend for an increased risk of cerebral venous thrombosis (OR 6.54, p = 0.064). Logistic regression confirmed a significant association between splenomegaly and JAK2 mutation (OR 43.15 [95%CI, 3.05–610.95], p = 0.0054). The V617F JAK2 mutation is rarely found in patients with thrombotic events without overt MPN. Splenomegaly, however, is a statistically and clinically relevant indicator of a potential JAK2 mutation in patients with non-splanchnic thrombotic events. Such patients should require further assessment and a close follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Systematic testing for JAK2 mutation in unprovoked thrombosis is controversial
-
JAK2 testing in patients without overt biological signs of myeloproliferative disease is addressed
-
The prevalence of JAK2 mutations is less than 3% in such cases
-
Splenomegaly is associated with JAK2 mutation in non-splanchnic thrombotic events
Introduction
Thrombosis is a major cause of morbidity in patients with myeloproliferative neoplasms (MPN) harbouring a mutation in the Janus kinase 2 gene (JAK2) leading to a self-activated V617F JAK2 mutant protein [1,2,3,4]. The latter is the most frequent one in Philadelphia chromosome-negative MPN, present in 95% of polycythemia vera (PV) cases and up to 65% cases of essential thrombocythemia (ET) and primary myelofibrosis. It is part of the WHO diagnostic criteria for MPN [5, 6] and has been shown to be an independent predictor of thrombotic events [5, 7]. Its incidence indeed ranges from 17 to 41% in individuals presenting with splanchnic vein thrombosis [8,9,10]. This has led to recommending testing for JAK2 mutation as an indicator of potential latent MPN in patients with splanchnic vein thrombosis [11, 12]. However, the incidence of the JAK2 mutation in patients presenting with thrombosis at other vascular sites has been reported to be significantly lower (i.e. less than 11%) [4].
Increased risks for hematological and non-hematological cancers have been associated with the JAK2 mutation [13]. More specifically, it is believed that individuals with a mutational burden below 2% may suffer from a latent form of MPN [14].
All in all, although little is known of the implications of JAK2 testing and the significance of the mutation in thrombotic patients with normal complete blood counts (CBC), there is a controversial and liberal attitude for JAK2 mutation testing in patients with unprovoked thrombotic events.
Based on these premises, the study reported here aimed at determining the prevalence of JAK2 mutations in subjects presenting with thrombotic events and normal CBC. Secondary objectives were to specify the clinical and biological profile of such patients and ultimately offer a better insight into the relevance of JAK2 testing in this population.
Materials and methods
Study population
This retrospective study spanning ten years (January 2006 to December 2016) analysed data from patients tested at Nice University Hospital (France) for JAK2 mutation from electronic medical files (Fig. 1). Selection criteria were: patients aged 18 years and older with thrombophilia workup for an arterial (AT) or venous (VT) thrombotic event, documented by imaging techniques or histologically proven. Patients with splanchnic thromboses were excluded as well as those with CBC suggestive of MPN (i.e. haematocrit over 49% and/or platelets over 450*109/L) since testing for JAK2 mutation in such circumstances is anyhow warranted [15].
Clinical data and thrombophilia screening
Clinical and biological features of the subjects were extracted from digital medical files. Iron deficiency as well as plasma inflammatory markers were collected whenever available. Results of bone marrow aspirates (cytology) and histological analyses of bone marrow biopsies were only recorded in patients with JAK2 mutations. A family history of thrombophilia and cardiovascular risk factors—such as age, hypertension, diabetes mellitus, dyslipidaemia, and smoking—were also recorded. Clinical changes, modifications in CBC at follow-up and outcome were documented over the ten-year period.
The provoked or unprovoked nature of AT or VT and their recurrence—as well as the location of the clot—were specified. Provoked VT with major triggering circumstances were defined as plaster cast immobilisation and/or fracture of a lower limb and/or surgery under general anaesthesia lasting more than 30 min and/or bedrest for more than 3 days, occurring in the 3 previous months and/or active cancer in the 2 preceding years [16, 17]. Moderate or minor triggering circumstances were pregnancy or puerperium, estrogen treatment or hormonal replacement therapy in the year preceding the VT or travelling for a journey of more than 6 h [16]. AT or VT occurring under antithrombotic treatment were also recorded.
Testing for JAK2 mutation is systematic as part of our institutional screening practices for patients with cerebral vein thrombosis (CVT) in search of thrombophilia. This includes investigation for antiphospholipid antibodies (anti-β2-glycoprotein-I and anti-cardiolipin), lupus anticoagulant, protein C and S activity, antithrombin level and factor V Leiden or prothrombin G20210A mutations. All patients provided written informed consent for molecular analyses. However, JAK2 testing was only performed in case of unprovoked thrombotic events if the thrombophilic workup (specified above) was negative, and as a “second-line” approach.
JAK2 V617F mutation
After DNA extraction from blood samples (Qiagen, France), JAK2 V617F mutation was investigated. Quantification was only performed upon JAK2 mutation detection. A 2% cut-off was used to classify patients with a significant allelic burden. Real-time fluorescent quantitative PCR using allele-specific primers and a TaqMan-MGB probe for dual-inhibiting amplification of wild-type alleles were performed for the detection of as low as 0.05% mutant JAK2V617F cells [18] (“Appendix A” section).
Statistical analyses
Data are reported as mean ± standard deviation (SD). For small samples (i.e. n < 20), continuous variables are expressed as median values with their interquartile range (IQR), extreme values being specified when appropriate. The chi-square test was used to study statistical relationships between qualitative variables and the risk of thrombosis in JAK2 mutated patients. Significant variables were then tested in a multivariate model (logistic regression using backward analysis) with odd ratios and confidence intervals (CI). Differences between groups were analysed using the Mann–Whitney test. McNemar’s test with Yates’ continuity correction was used to compare sensitivity and specificity between groups. P values of less than 0.05 were considered statistically significant using two-sided tests. Statistical analyses were performed with GraphPad® Prism 6.2 and MS Excel 2010. Logistic regression was performed using MedCalc Statistical Software (Ostend, Belgium).
Ethics
Informed patient consent was collected as part of routine clinical practice and data were anonymized when recorded for the purpose of this study. Data are stored in the University Hospital’s electronic file repository under the identification number #277, according to Commission nationale de l'informatique et des libertés (CNIL) guidelines. The retrospective nature and format of the study did not require approval from an institutional review board.
Results
Patient characteristics
Over the 10-year period, 2497 patients with JAK2 evaluation were recovered, of whom 1555 also had been investigated for thrombophilia (Fig. 1). Ultimately, of the 580 cases of thrombotic events with thrombophilia screening, 340 were finally included in the study. Demographics and clinical data are shown in Table 1. None of the patients had been splenectomised and none had peripheral (or blood) cytological signs of asplenia. Splenomegaly was identified, either clinically or radiologically, in five patients. Of the latter, only two presented with cytopenia. Thirty-one of the 335 remaining patients were also found to have cytopenia: platelet count < 150*109/L (n = 8), haemoglobin level < 120 g/L (n = 21), and leukopenia (i.e. < 4*109/L) (n = 6).
More than a third of patients were at least 60 years old and the male-to-female gender ratio was 0.89. Ninety-one patients presented with AT, 268 with VT, and 19 had mixed AT and VT. One hundred and nine patients (32.1%)—93 VT and 28 AT—presented with thrombophilia, 32 of whom with multiple thrombotic aetiologies. Antiphospholipid syndrome was diagnosed in 50 patients (14.7%).
Of the 64 patients with arterial thrombosis and one or more risk factors for cardio-vascular disease, 50 were smokers, 33 had high blood pressure, 10 had a body mass index ≥ 30 kg/m2 (Table 1).
Multiple causes of VT were identified in 31 of 109 patients presenting with constitutional and/or acquired thrombophilia. One patient had a homozygous prothrombin G20210A mutation.
At least one thrombotic event occurred in 73 patients who were on antithrombotic drugs prior to the thrombotic event leading to thrombophilia workup. Of note, 4/73 patients simultaneously had anticoagulants and antiplatelet drugs.
JAK2 V617F prevalence and mutational status
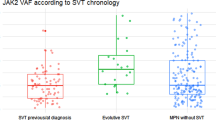
A JAK2 mutation was found in 9 patients with a normal CBC, thus yielding a prevalence of 2.65%. Only four patients (of which two had splenomegaly) had a significant JAK2 allele burden (≥ 2%). One patient presented with moderate thrombopenia (Table 2) whereas the others had no cytopenia.
The clinical and biological data of these 9 patients with JAK2 mutations are presented in Table 2. Their median age was 72 years old (IQR, 55.5–75). Central nervous system involvement was reported in 3 of the 5 cases of AT (i.e. stroke) and three of the four cases of VT (i.e. CVT). Two of the 3 patients with stroke were not known to have cardiovascular risk factors and none of them had a cardioembolic thrombosis. The four cases of JAK2-positive VT were unprovoked and without evidence of thrombophilia.
The prevalence of JAK2 mutation was, respectively, 7.7%, 5.9% and 1.8%, in patients receiving antiplatelet treatment (n = 39), anticoagulants (n = 34) or without antithrombotic treatment (n = 271) and respectively 10% (3/30) and 8.1% (3/37) for patients with ischemic stroke or CVT. Bone marrow biopsy pathology was performed in seven of the 9 patients with JAK2 mutation. Cytological features of bone marrow analysis were characteristic of ET in two cases, despite normal CBC, normal ferritin levels and no evidence for haemodilution. None of the patients with splenomegaly showed further signs of MPN within the timeframe of the study, although one patient was lost to follow-up after refusing bone marrow biopsy.
Factors associated with JAK2 mutant-induced thrombotic events
Cardiovascular risk factors as enumerated previously were not related to AT associated with JAK2 mutation (Table 3). Similarly, the most common causes of thrombophilia were not predictive of VT in JAK2 mutant patients. Neither the nature nor site of thrombosis were predictive of JAK2-associated thrombosis. In the univariate analysis, elevated C-reactive protein levels and splenomegaly remained associated with the JAK2 mutation. However, in multivariate analysis, only the association with splenomegaly was statistically significant.
Patients with the JAK2 mutation were younger (54 ± 15 years vs. 64 ± 13 years; p = 0.027), and had higher platelet levels (317 ± 111*109/L vs. 255 ± 75*109/L; p = 0.017). In multivariate analysis, age was not a significant predictor contrarily to platelet levels (p = 0.006), with an odds ratio of 1.012 (95%IC, 1.004–1.021).
Patient follow-up
Follow-up data were available for only 6 of the 9 patients with JAK2 mutation. The median time of follow-up was 12 months (IQR, 1–36). None of these patients presented with recurrent thrombosis during the follow-up period. Anomalies suggestive of MPN appeared on the CBC of the four of the 6 patients with a mutant allele burden greater than 2%: thrombocytosis (n = 3) and polycythemia (n = 1). These patients were subsequently treated with hydroxycarbamide.
Discussion
This study is one of the few to analyse the implications of screening for JAK2 V617F mutations in patients presenting with non-splanchnic thrombosis and without any biological signs of MPN. It also analysed potential thrombosis-associated risk factors.
Evidence was found that thrombotic events specifically related to JAK2 mutations, other than splanchnic thrombosis, are only exceptionally found in patients without CBC anomalies. The prevalence of JAK2 mutations is 2.65% in this series with, in most cases (5/9), allele burdens lower than 2%. Age-related clonal haematopoiesis of indeterminate potential or CHIP cannot be excluded but seems unlikely owing to the relatively young age of the patient-population (i.e. mean age of 54 years).
As for pathophysiological mechanisms, the JAK2 mutation has been shown to promote higher blood-cell counts. Thrombotic events thus could occur mainly through i) an increase of direct activation or endothelium adhesion of blood cells [19,20,21,22,23] or ii) an over-expression of the JAK2 mutation in endothelial cells [24,25,26]. It has also been shown that an increase in P-selectin levels through the activation of the JAK2/STAT3 pathway could lead to a prothrombotic state [27], mostly in response to an inflammatory process mediated by interleukin-9 [28]. It is also believed that hydroxycarbamide could reverse this prothrombotic state by stimulating the NO-cGMP pathway [27, 29, 30].
A previous study of 664 consecutive patients addressed a very similar issue and reported a JAK2 mutation in 6 (< 1.0%) patients [31]. The mutant allele burden was however extremely low and none of these patients developed either overt MPN nor recurrent thrombosis. Another study from Ianotto et al., exploring 372 patients with deep VT for JAK2 and calreticulin mutations, identified 10 JAK2-mutated patients, including 3 with MPN yielding a prevalence of 1.9% [32].
In the present series, latent ET was found in 2 of the 6 JAK2 mutated patients who had had bone marrow biopsies. It was also found that the ≥ 2% cut-off for allele burden was of clinical interest since such patients were more likely to present with splenomegaly and evolve towards MPN within the first year of follow-up. This, of course, does not exclude JAK2 allele burdens of < 2% from being implicated in thrombotic processes. In fact, presence of a JAK2 mutation has been described in up to 10% of healthy subjects [33]. The risk of thrombosis, however, has been shown to increase with the percentage of mutant allele burden and the type of MPN [31, 34].
The large series reported here confirms the low incidence of JAK2 mutation in thrombotic patients. However, it also indicates that a systemic inflammatory profile and splenomegaly should prompt JAK2 mutation analysis.
No prognostic value was found for other factors such as thrombosis in an atypical site, multiple thromboses, CVT, anticoagulation, anti-platelet treatment or cardiovascular risk factors. However, in the multivariate analysis, platelet levels were found to be predictive of JAK2 mutation [odds ratio 1.012 (95%IC, 1.004–1.021)]. This might be difficult to interpret in a daily clinical setting, especially in proinflammatory states such as thrombotic events. Of note, 6 of the 9 JAK2-mutated patients presented with cerebral involvement: respectively three strokes (10% of all strokes) and three CVT (8% of CVT). This was not statistically significant, and mirrors findings from previous studies [2, 8]. The prevalence rate of CVT of 8.1% observed in this cohort anyway seems higher than that from recent studies, where it ranges from 5.6 to 6.6% [35,36,37]. Despite limited data, it is suggested that testing for latent MPN should be performed in cases of CVT [38]. We believe, however, that this requires assessing and eliminating several confounding factors: (i) prior absence of constitutional/acquired thrombophilia, (ii) absence of a systemic inflammatory disease (iii) a CBC not suggestive of MPN and, of course, (iv) no palpable spleen or radiologic splenomegaly. Although it enrolled a large cohort of patients, this retrospective single-center initiative is insufficiently powered to detect MPN development because of the low number of patients with JAK2 mutations that were ultimately identified. Furthermore, the average follow-up for patients detected as carrying a JAK2 mutation is relatively short considering that subsequent diagnoses of MPN may occur as late as after 10 years, independently from the mutational burden. This implies that the association of JAK2 mutation and splenomegaly could be also be fortuitous (i.e. type I error), despite the latter being a well-known feature of MPN. As pointed out previously, it could also appear that a center-effect may have inflated the incidence of CVT when compared to data from other series. As expected from the retrospective design of this study, incidence rates could have further been increased by an unavoidable liberal attitude to JAK2 testing.
Our findings, however, besides being consistent with those of previous studies may impact day-to-day clinical (i.e. “real-life”) practice, notably by pointing out the importance of JAK2 testing in patients with splenomegaly as just discussed above. Based on data gathered from guidelines [39, 40], ours and previous studies, JAK2 testing needs to be considered in patients presenting with unprovoked VT and CVT especially when splenomegaly is also present.
Conclusion
The JAK2 mutation is only rarely associated with AT or VT events in patients without overt MPN. Splenomegaly is a clinically relevant marker for possible JAK2 mutation in patients with non-splanchnic thrombotic events. Other than that, screening for the JAK2 mutation should be subject to the prior exclusion of more common thrombophilic disorders.
References
Elliott MA, Tefferi A (2004) Pathogenesis and management of bleeding in essential thrombocythemia and polycythemia vera. Current Hematology Reports 3:344–351
Xavier SG, Gadelha T, Schaffel R, Britto L, Pimenta G, Ribeiro DD et al (2008) Low prevalence of the JAK2V617F in patients with ischemic stroke or cerebral venous thrombosis. Blood Coagul Fibrinolysis 19:468–469. https://doi.org/10.1097/MBC.0b013e328304e0a9
Xavier SG, Gadelha T, Pimenta G, Eugenio AM, Ribeiro DD, Gomes FM et al (2010) JAK2V617F mutation in patients with splanchnic vein thrombosis. Dig Dis Sci 55:1770–1777. https://doi.org/10.1007/s10620-009-0933-y
Xavier SG, Gadelha T, Rezende SM, Zalcberg IR, Spector N (2011) JAK2V617F mutation in patients with thrombosis: to screen or not to screen? Int J Lab Hematol 33:117–124. https://doi.org/10.1111/j.1751-553X.2010.01275.x
Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E et al (2012) Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 120:5128–5133. https://doi.org/10.1182/blood-2012-07-444067
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S et al (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. The Lancet 365:1054–1061. https://doi.org/10.1016/S0140-6736(05)71142-9
Barbui T, Vannucchi AM, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A et al (2015) Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer Journal 5:e369–e369. https://doi.org/10.1038/bcj.2015.94
De Stefano V, Fiorini A, Rossi E, Za T, Farina G, Chiusolo P et al (2007) Incidence of the JAK2 V617F mutation among patients with splanchnic or cerebral venous thrombosis and without overt chronic myeloproliferative disorders. J Thromb Haemost 5:708–714. https://doi.org/10.1111/j.1538-7836.2007.02424.x
Regina S, Herault O, D’Alteroche L, Binet C, Gruel Y (2007) JAK2 V617F is specifically associated with idiopathic splanchnic vein thrombosis. J Thromb Haemost 5:859–861. https://doi.org/10.1111/j.1538-7836.2007.02384.x
Colaizzo D, Amitrano L, Tiscia GL, Scenna G, Grandone E, Guardascione MA et al (2007) The JAK2 V617F mutation frequently occurs in patients with portal and mesenteric venous thrombosis. J Thromb Haemost 5:55–61. https://doi.org/10.1111/j.1538-7836.2006.02277.x
Smalberg JH, Arends LR, Valla DC, Kiladjian J-J, Janssen HLA, Leebeek FWG (2012) Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 120:4921–4928. https://doi.org/10.1182/blood-2011-09-376517
Dentali F, Squizzato A, Brivio L, Appio L, Campiotti L, Crowther M et al (2009) JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 113:5617–5623. https://doi.org/10.1182/blood-2008-12-196014
Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE (2011) The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica 96:450–453. https://doi.org/10.3324/haematol.2010.033191
Nielsen C, Bojesen SE, Nordestgaard BG, Kofoed KF, Birgens HS (2014) JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica 99:1448–1455. https://doi.org/10.3324/haematol.2014.107631
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391–2405. https://doi.org/10.1182/blood-2016-03-643544
Pernod G, Biron-Andreani C, Morange P-E, Boehlen F, Constans J, Couturaud F et al (2009) Recommendations on testing for thrombophilia in venous thromboembolic disease: a French consensus guideline. J Mal Vasc 34:156–203
Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing G-J, Kyrle PA et al (2016) Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost 14:1480–1483. https://doi.org/10.1111/jth.13336
Usseglio F, Beaufils N, Calleja A, Raynaud S, Gabert J (2017) Detection of CALR and MPL mutations in low allelic burden JAK2 V617F essential thrombocythemia. J Mol Diagn 19:92–98. https://doi.org/10.1016/j.jmoldx.2016.08.006
De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E et al (2009) Leukocytosis is a risk factor for recurrent arterial thrombosis in young patients with polycythemia vera and essential thrombocythemia. Am J Hematol 85(2):97–100.
De Grandis M, Cambot M, Wautier M-P, Cassinat B, Chomienne C, Colin Y et al (2013) JAK2V617F activates Lu/BCAM-mediated red cell adhesion in polycythemia vera through an EpoR-independent Rap1/Akt pathway. Blood 121:658–665. https://doi.org/10.1182/blood-2012-07-440487
Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R et al (2007) Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 109:2446–2452. https://doi.org/10.1182/blood-2006-08-042515
Barbui T, Carobbio A, Rambaldi A, Finazzi G (2009) Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood 114:759–763. https://doi.org/10.1182/blood-2009-02-206797
Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T (2005) Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol 33:523–530. https://doi.org/10.1016/j.exphem.2005.01.015
Teofili L, Martini M, Iachininoto MG, Capodimonti S, Nuzzolo ER, Torti L et al (2011) Endothelial progenitor cells are clonal and exhibit the JAK2V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood 117:2700–2707. https://doi.org/10.1182/blood-2010-07-297598
Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, Hoffman R (2009) The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 113:5246–5249. https://doi.org/10.1182/blood-2008-11-191544
Rosti V, Villani L, Riboni R, Poletto V, Bonetti E, Tozzi L et al (2013) Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 121:360–368. https://doi.org/10.1182/blood-2012-01-404889
Guy A, Gourdou-Latyszenok V, Le Lay N, Peghaire C, Kilani B, Dias JV et al (2019) Vascular endothelial cell expression of JAK2 V617F is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica 104:70–81. https://doi.org/10.3324/haematol.2018.195321
Feng Y, Yu M, Zhu F, Zhang S, Ding P, Wang M (2018) IL-9 promotes the development of deep venous thrombosis by facilitating platelet function. Thromb Haemost 118:1885–1894. https://doi.org/10.1055/s-0038-1673614
Cokic VP, Beleslin-Cokic BB, Noguchi CT, Schechter AN (2007) Hydroxyurea increases eNOS protein levels through inhibition of proteasome activity. Nitric Oxide 16:371–378. https://doi.org/10.1016/j.niox.2007.01.001
Cokic VP, Beleslin-Cokic BB, Tomic M, Stojilkovic SS, Noguchi CT, Schechter AN (2006) Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood 108:184–191. https://doi.org/10.1182/blood-2005-11-4454
Pardanani A, Lasho TL, Hussein K, Schwager SM, Finke CM, Pruthi RK et al (2008) JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 83:457–459. https://doi.org/10.4065/83.4.457
Ianotto J-C, Chauveau A, Mottier D, Ugo V, Berthou C, Lippert E et al (2017) JAK2V617F and calreticulin mutations in recurrent venous thromboembolism: results from the EDITH prospective cohort. Ann Hematol 96:383–386. https://doi.org/10.1007/s00277-016-2853-1
Sidon P, El Housni H, Dessars B, Heimann P (2006) The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia 20:1622. https://doi.org/10.1038/sj.leu.2404292
Borowczyk M, Wojtaszewska M, Lewandowski K, Gil L, Lewandowska M, Lehmann-Kopydłowska A et al (2015) The JAK2 V617F mutational status and allele burden may be related with the risk of venous thromboembolic events in patients with Philadelphia-negative myeloproliferative neoplasms. Thromb Res 135:272–280. https://doi.org/10.1016/j.thromres.2014.11.006
De T, Prabhakar P, Nagaraja D, Christopher R (2012) Janus kinase (JAK) 2 V617F mutation in Asian Indians with cerebral venous thrombosis and without overt myeloproliferative disorders. J Neurol Sci 323:178–182. https://doi.org/10.1016/j.jns.2012.09.012
Lamy M, Palazzo P, Agius P, Chomel JC, Ciron J, Berthomet A et al (2017) Should we screen for janus kinase 2 V617F mutation in cerebral venous thrombosis? Cerebrovasc Dis 44:97–104. https://doi.org/10.1159/000471891
Passamonti SM, Biguzzi E, Cazzola M, Franchi F, Gianniello F, Bucciarelli P et al (2012) The JAK2 V617F mutation in patients with cerebral venous thrombosis. J Thromb Haemost 10:998–1003. https://doi.org/10.1111/j.1538-7836.2012.04719.x
Delluc A, Antic D, Lecumberri R, Ay C, Meyer G, Carrier M (2017) Occult cancer screening in patients with venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 15:2076–2079. https://doi.org/10.1111/jth.13791
Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M et al (2016) Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis 41:154–164. https://doi.org/10.1007/s11239-015-1316-1
Connors JM (2017) Thrombophilia testing and venous thrombosis. N Engl J Med 377:2297–2298. https://doi.org/10.1056/NEJMc1713797
Acknowledgements
All authors had full access to the study data and had final responsibility for the decision to submit for publication. We would like to thank Jacques Levraut for his help with the statistical analysis.
Author information
Authors and Affiliations
Contributions
ML, LL and NM designed the study. ML obtained the data. All authors analysed and interpreted the data. ML and NM wrote the report. All authors revised the report and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interests
The authors report no personal conflicts of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. According to current regulations, informed consent was systematically required prior to performing genetic tests. Due to the retrospective design of the study, informed consent from patients was not required for their inclusion.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A—DNA extraction and analysis
Appendix A—DNA extraction and analysis
The EZ1 Advanced XL instrument (Qiagen) was used to isolate genomic DNA from 0,2 mL of patient’s blood samples using the Advanced XL instrument (Qiagen) according to the manufacturer's recommendations. Quality and quantification were assessed using the NanoDrop 8000 spectrophotometer (Thermo Scientific) and samples were diluted to achieve a concentration of 5 ng/μL.
Quantification of the JAK2 V617F mutation was done through real-time fluorescent quantitative PCR with specific allele primers and TaqMan-MGB probe—the minimal detection rate for the mutation being 0.05%.
The Mx 3000P real-time PCR system (Agilent Technologies) testing duplicate samples was used. Genetic material was amplified with 25 ng of genomic DNA in 1 × TaqMan Universal PCR Master Mix (Life Technologies). Thermal cycling parameters were: 95 °C for 10 min, and 45 cycles at 95 °C for 30 s and 60 °C for 1 min.
The primers/probe sequences (with their concentrations) are presented in the following table:
Primer | Sequence | Concentration | Comments |
JAK2 V617F Forward primer | 5′-AGCTTTCTCACAAGCATTTGGTT-3′ | 600 nmol/L | |
JAK2 V617F Reverse primer | 5′-GTTTTACTTACTCTCGTCTCCACAAAA-3′ | 300 nmol/L | |
JAK2 V617F Probe | 5′-6FAM-AATTATGGAGTATGTTTCTG-MGBFNQ-3′ | 200 nmol/L | |
Albumin Forward primer | 5′-ATGCTGCACAGAATCCTTGGT-3′ | 300 nmol/L | Internal control |
Albumin Reverse primer | 5′-TCATCGACTTCCAGAGCTGAAA-3′ | 300 nmol/L | Internal control |
Albumin Probe | 5′-6VIC-AACAGGCGACCATGC-MGBBFNQ-3′ | 200 nmol/L | Internal control |
Plasmid calibrator standard curves were used to obtain JAK2 V617F and ALB copy numbers.
Rights and permissions
About this article
Cite this article
Levraut, M., Legros, L., Drappier, C. et al. Low prevalence of JAK2 V617F mutation in patients with thrombosis and normal blood counts: a retrospective impact study. J Thromb Thrombolysis 50, 995–1003 (2020). https://doi.org/10.1007/s11239-020-02100-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02100-z