Abstract
In this work we describe the adaptation of channel catfish ovary (CCO) cell line to commercially available Ultra Culture serum-free medium by gradual reduction of serum concentration from 10 to 0 %. With this approach we obtained CCO cells fully adapted to serum-free conditions in 32 days. Growth, nutritional and morphological characteristics of these cells remained unchanged when compared to the control group kept in the presence of serum. Additionally, three commercially available protein hydrolysates were tested for the effects on growth performance of the newly serum-free adapted CCO cells. Supplementation with wheat gluten hydrolysate resulted in growth similar to serum free medium solely, while yeast and soy hydrolysates showed inhibitory effects on the cell growth. Taken together, the successful adaptation of CCO cells to serum-free conditions indicates their potential to be used in cytotoxicity assays when serum omission is demanded or for developing serum free bioprocesses using CCO cells. However, a more extended study on nutrient supplementation is still required to further boost the cell growth in a serum free culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell lines obtained from fish have been developed and utilized since the early 1960s and currently, over 280 different fish cell lines are established around the world (Lakra et al. 2011). Fish cells were initially developed for the use as a diagnostic and research tool, particularly for diagnosis and research of viral infections in fish (Wolf and Quimbly 1962). However, in more recent years they have been intensively used in the ecotoxicity assays of chemicals and environmental samples (Bols et al. 2005) showing to be equally sensitive as mammalian cells when basal toxicity is determined (Castaño and Gómez-Lechón 2005).
Fish cells grow well in most basal mammalian culture media supplemented with 5–10 % (v/v) animal serum (usually fetal bovine serum-FBS). Serum supports cell proliferation and growth by providing nutrients and growth factors but its use in animal cell processes has many disadvantages including animal suffering, possible contamination, composition variation, high ratio of proteins which affects downstream processing, price etc. (van der Valk et al. 2010). Also, the tendency in industrial cell culture based bioprocesses is the use of serum-free or protein-free media due to biological safety reasons (Keenan et al. 2006; Merten 2006). In order to overcome the disadvantages of using serum, during the last decades much effort has been made to define a formulation of low-serum and serum-free media (SFM) leading to a substantial increase in their commercial supply (Brunner et al. 2010). However, no universal SFM applicable to all cell lines is available indicating that virtually custom made SFM media for a particular cell line has to be developed in order to support maximal cell growth (Kim and Lee 2009). Furthermore, cells need to be adapted to serum-free conditions which is time consuming and not necessarily a successful process. Although adaptation to SFM is often referred in the literature, data on cell growth during this process or total time taken to reach full adaptability are scarce. Early attempts to grow fish cell cultures in SFM are reported by Shea and Berry (1983) who showed capability of 5 different fish cell lines to grow and support viral replication in the SFM at rates equivalent to serum-grown cells. Also, it was reported that fish cell lines most frequently used in ecotoxicology, RTG-2 and PLHC-1 cells, were successfully adapted to SFM conditions (Kohlpoth and Rusche 1997; Ackermann and Fent 1998) showing similar or better growth characteristics when compared to regular culture medium. Omission of FBS from culture medium in toxicological screenings is highly desirable, not only for ethical and economic considerations, but also for its possible influence on toxicant availability during cytotoxicity assays with fish cells showing that culture environment can have profound impact on the responsiveness of cultured cells (Schirmer 2006). Some authors pointed out that the major limitation in the more extensive application of fish cell systems is their insufficient characterization, both with respect to cellular and functional properties as well as to their nutritional requirements (Castaño et al. 2003; Bols et al. 2005). However, in our earlier studies we determined certain nutritional and growth characteristics of channel catfish ovary (CCO) cells by investigating their glucose and glutamine consumption (Slivac et al. 2008), and lactate and ammonia production (Slivac et al. 2010). Current EU legislation (REACH 2006), which promotes the development of alternative methods for the assessment of hazardous substances, as well as recommendations on optimization and implementation of chemically defined cell culture media as a part of the good cell culture practice in the field of toxicology provoked us to perform this study.
Therefore, we aimed to adapt CCO cells in commercially available SFM by gradual serum reduction approach. Cell growth characteristics, glucose and lactate concentrations and morphological changes were determined during the whole adaptation process. After successful adaptation process, influence of three different non-animal protein hydrolysates was tested in serum-free CCO cultures in order to determine whether these hydrolysates can be used for stimulation of CCO proliferation.
Materials and methods
Cell line and culture conditions
The CCO cell line was obtained from ATCC (CRL2772) (Manassas, VA, USA). DME medium (Sigma, St. Louis, MO, USA) supplemented with 10 % FBS (Gibco, Grand Island, NY, USA) was used for initial expanding of CCO cells in T-flasks, while for the adaptation to serum-free conditions UltraCulture SFM (UC SFM) purchased from Lonza (Verviers, Belgium) and supplemented with 10–0 % FBS was used. The cell adaptation to serum-free conditions was performed in 75 cm2 T-flasks (Corning, Corning, NY, USA) by gradually decreasing FBS content (v/v) in UC SFM until the final step was preformed in SFM only. For the study of growth characteristics and metabolic parameters during adaptation, cells were seeded in 24 well-plates in one ml of UC SFM supplemented with 10–0 % FBS at initial concentration of 5 × 104 cells ml−1. The cells were cultivated at 30 °C in the atmosphere consisting of humidified air (95 %) and CO2 (5 %). Antibiotics were not used during the CCO cell cultivation and adaptation process.
Light microscopy was used to determine the morphological changes during the adaptation process. CCO cells (1 × 105 cells ml−1) were seeded in the wells and were stained with crystal-violet (Sigma-Aldrich, Munich, Germany) dye. Morphological images of cells were taken using an inverted microscope (Carl Zeiss, Göttingen, Germany) and Dino-Eye Microscope Eyepiece Camera (AnMo Electronics Co., Taipei, Taiwan).
Cell freezing and thawing
After succesful adaptation to serum free conditions, CCO cells were frozen in UC SFM. Cells from exponential growth phase were concentrated to 1 × 107 cells ml−1 by centrifugation (5 min/100×g) and resuspended in 45 % of fresh and conditioned UC SFM, respectively, and 10 % dimethyl sulfoxide (Sigma). One ml of the final cell suspension was added in cryotubes and stored at −80 °C.
Cell thawing was performed 1 month after freezing by warming the cryotube at 30 °C and its content was used to inoculate a 25 cm2 T-flask containing 10 ml UC SFM. The cells were allowed to adhere to the T-flask for 2 h when the medium was discarged and 10 ml of fresh UC SFM was added. Cells were cultured and passaged twice a week.
Analytical methods
During cultivation and adaptation of CCO cells, samples are taken daily for cell counting by hemacytometer using Trypan-blue dye exclusion method. Concentrations of glucose and lactate were quantified by enzymatic assays. Glucose concentration was determined by Glucose-PAP enzymatic assay kit (Herbos, Sisak, Croatia), while lactate concentration was determined by Lactate UV-test (Boehringer Mannheim/RBiopharm, Mannheim, Germany).
Protein hydrolysates
Yeast (YH) and wheat gluten hydrolysates (GH) were purchased from Sigma while UF 50x Soy hydrolysate (SH) was purchased from SAFC Biosciences (Andover, UK). Stock solutions (20 % w/v) of YH and GH were prepared in deionized distilled water and filtered through 0.2 μm filter (Millipore Co., Billerica, MA, USA). To determine the effect of hydrolysate solution, adapted CCO cells were seeded in triplicate in 24-well plates at a initial cell concentration of 5 x 104 cells ml−1. Control CCO cells were seeded in UC SFM only. YH and SH were added to the CCO cell culture medium to a final concentration of 4 g l−1, while the influence of GH was tested in a concentration range of 2–12 g l−1.
Results and discussion
Adaptation of CCO cells in serum-free medium
The desire to eliminate as many animal derived raw-materials as possible from cell culture has led to the development of procedures which completely remove the need for serum. Several approaches for adaptation to serum-free conditions are available and typical adaptation process involve progressive adaptation to lower serum concentrations until serum-free conditions are reached (van der Valk et al. 2010).
The first part of our study was an attempt to adapt CCO cells to grow in UC SFM using gradual reduction of FBS content (10–0 %). During the whole process, serum content was reduced at each passage until 0 % FBS was reached (Fig. 1a). Growth curves of CCO cells obtained during the adaptation process showed that cells grew exponentially with no significant increase in duration of lag phase in all cultures and maximal cell number obtained after 96 h of cultivation. Comparing the growth of CCO cells in media with different serum contents, maximal cell number of 8.5 × 105 cells ml−1 was reached with 10 % FBS, while decrease in serum concentration resulted in slight decrease of cell number in all cultures compared to 10 % FBS. It was reported by Shen et al. (2006) that cell adaptation to serum-free conditions often results in a retarded growth profile and decreased viability for several passages. The process itself requires a carefully developed feeding protocol based on regular monitoring of cellular growth. This is in agreement with our research strategy and obtained results considering that at the end of adaptation process, which took 768 h or 32 days, CCO cells were able to grow in serum-free conditions. Influences of serum concentrations on growth kinetics and viability were reported during HeLa-S3 cell growth and adaptation to SFM conditions (El Enshasy et al. 2009) as well as in hybridoma cells during the adaptation to low serum and SFM (Ozturk and Palsson 1991).
Glucose consumption and lactate production were measured during the whole adaptation process. In all cultures, glucose consumption was accompanied by a consequential lactate accumulation (Fig. 1b). Glucose was not the limiting substrate for CCO cell growth in all cultures, since its residual concentrations were in the range of 7.8–10.3 mM. At the same time produced lactate concentrations reached 2.1–2.5 mM in all cultures. It has been observed that increase in serum concentration from 2.5 to 10 % decreased glucose consumption in HL-60 cells (McDowell and Paputsakis 1998) while study of Ozturk and Palsson (1991) showed no effect of increasing serum concentrations on glucose utilization and lactate production in hybridoma cells. Our results showed relatively stable glucose consumption, being the highest at 10 % FBS, but after the cells were fully adapted to SFM obtained values were similar to 10 % FBS. Therefore, the adaptation process did not detectably modify cell physiology in terms of providing cells with supplemental nutrients to keep their growth rate unchanged.
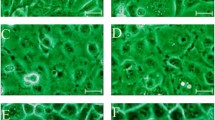
During the adaptation process, CCO cells were stained by crystal-violet dye and assessed under the light microscope (Fig. 2a-c). No visible changes in cell morphology or monolayer formation were observed in CCO cells cultured in reduced serum (Fig. 2b) or serum-free conditions (Fig. 2c) in comparison to control cells (Fig. 2a). These observations are consistent with unchanged growth characteristics and nutritional demands of SFM adapted CCO cells compared to cells grown in medium with serum.
Since the adaptation process is time-consuming and laborious work it is essential to protect obtained adapted cells by cryopreserving the cell line without losing cell growth characteristics in terms of cell viability and specific growth rate (Freshney 2005). Therefore, CCO cells were frozen at −80 °C, thawed after 30 days (cell viability was 96.7 %) and immediately inoculated in UC SFM. Following the 3rd culture passage the cells reached the same growth rate as before being preserved by freezing (Table 1). Such good result on cell viability after a cryopreservation is valuable in terms of cell line sustainability and definitely rounds up the whole adaptation process. These results agree with a cryopreservation study of Sf-9 insect cells during serum-free adaptation in stirred suspension cultures (Cruz et al. 1997).
Effects of different protein hydrolysates on CCO cell growth in serum-free conditions
In recent years, protein hydrolysates, especially of non-animal origin, became interesting as the supplements of SFM since they may serve as source of nutrients, adhering factors or growth factors. They stimulated growth and productivity of different cell lines such as CHO cells (Franěk et al. 2000; Sung et al. 2004; Fargess-Haddani et al. 2006; Kim and Lee 2009), insect cells (Wu and Lee 1998) as well as human THP-1 cells (Girón-Cale et al. 2008). To our knowledge, the effects of protein hydrolysate supplementation on the growth of fish cell lines in SFM conditions have not been reported yet.
To determine the effects of three commercially available hydrolysates prepared from yeast, soy and wheat gluten in regard to cell growth, CCO cells were cultivated in UC SFM supplemented with 4 g l−1 of each hydrolysate and cell density was monitored during 96 h (Fig. 3). Cells were also cultured in basal UC SFM as control. Maximal cell density was obtained in medium supplemented with GH and UC SFM only, while addition of SH and YH rather inhibited CCO cell growth at the tested concentration. Usually, SFM are fortified with low-cost hydrolysates which enhanced cell growth, productivity and extended culture longevity (Franěk and Katinger 2002). Sung et al. (2004) reported on yeast hydrolysate stimulating effects during the production of human thrombopoietin in CHO suspension cultures, while at the same time tested soy, wheat gluten and rice hydrolysates did not show dramatic effects on cell productivity. Moreover, wheat gluten and rice hydrolysates decreased CHO cell viability. In experiments performed by Chun et al. (2007) supporting capability of soy hydrolysate on growth, viability and longevity of CHO cells in chemically defined medium was achieved in comparison to other tested hydrolysates like wheat, rice and yeast hydrolysates. Considering above, it is obvious that supporting capacity of hydrolysate depends on its type and therefore has to be tested for each cell culture.
Since addition of GH showed some supportive effect for CCO cell growth in comparison to SFM solely, different GH concentrations (2–12 g l−1) were tested during CCO cell growth in SFM conditions (Fig. 4). Maximal cell number of 6.9 × 105 cells ml−1 was obtained for 4 g l−1 GH, while at all other tested GH concentrations CCO cell number was in the range of 3.1–5.1 × 105 cells ml−1, which is a significant reduction in comparison to control cell number (6.4 × 105 cells ml−1). The highest tested GH concentrations (8 and 12 g l−1) reduced cell growth for almost 40 and 65 %, respectively, possibly by affecting the nutrient balance due to high amino acid or oligopeptide concentrations as reported by Chun et al. (2007) and Zhang et al. (1994). Sung et al. (2004) also reported that the supplementation with soy, wheat and rice hydrolysates over 5 g l−1 did not increase, even decrease maximal CHO cell concentration in SFM. Generally, addition of three tested protein hydrolysates in SFM did not show dramatic supporting effects on CCO cell growth compared to SFM only. Since the composition of UC SFM is undisclosed to the public as many other SFM (Kim and Lee 2009), we do not know if those hydrolysates are already present in UC SFM and therefore showed inhibitory effects on cell growth due to extreme protein concentration. On the other hand, it is likely that such formulation of UC SFM is well balanced and completely supports nutritional requirements of CCO cells in SFM conditions. However, we cannot exclude possible stimulating effects of other commercially or home-made protein hydrolysates on CCO cell growth for the further improvement and optimization of SFM.
To conclude, the present work shows that gradual reduction of FBS in UC SFM results in successful adaptation of CCO cells to serum-free conditions while growth, nutritional and morphological characteristics remained unchanged when compared to CCO cells cultured in the presence of serum. Growth stimulating effects of UC SFM supplemented with yeast, soy and wheat gluten hydrolysates were not achieved suggesting the presence of these substances (or their derivatives) in UC SFM. This furthermore opens a new prospect on investigation and formulation of new growth supporting substances. Nevertheless, serum-free adapted CCO cells firstly established by our team represent an important contribution when following demands for serum omission, in either cytotoxicity tests or potential bioprocesses for the production of channel catfish virus (CCV) which should be further investigated.
References
Ackermann G, Fent K (1998) The adaptation of the permanent fish cell lines PLHC-1 and RTG-2 to FCS-free media results in similar growth rates compared to FCS-containing conditions. Mar Environ Res 46:363–367
Bols N, Dayeh VR, Lee LEJ, Schirmer K (2005) Use of fish cell lines in the toxicology and ecotoxicologyof fish. Piscine cell lines in environmental toxicology. In: Mommsen TP, Moon TW (eds) Biochemistry and molecular biology of fishes, vol. 6. Environmental Toxicology, Elsevier BV, pp 43–84
Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthalter G (2010) Serum-free cell culture: the serum-free media interactive online database. ALTEX 27:1–10
Castaño A, Gómez-Lechón MJ (2005) Comparison of basal cytotoxicity data between mammalian and fish cell lines: a literature survey. Toxicol In Vitro 19:695–705
Castaño A, Bols N, Braunbeck T, Dierickx P, Halder M, Isomaa B, Kawahara, Lee LK, Mothersill C, Pärt P, Repetto G, Riego Sintes J, Rufli H, Smith R, Wood C, Segner H (2003) The use of fish cells in ecotoxicology: the report and recommendations of ECVAM Workshop 47. ATLA 31:317–351
Chun BH, Kim JH, Lee HJ, Chung NH (2007) Usability of size-excluded fractions of soy protein hydrolysates for growth and viability of Chinese hamster ovary cells in protein-free suspension culture. Bioresour Technol 98:1000–1005
Cruz PE, Moreira JL, Carrondo MJT (1997) Insect cell growth evaluation during serum-free adaptation in stirred suspension cultures. Biotechnol Tech 11:117–120
El Enshasy H, Abdeen A, Abdeen S, Elsayed EA, EL Demellawy M, EL Shereef AA (2009) Serum concentration effects on the kinetics and metabolism of HeLa-S3 cell growth and cell adaptability for successful proliferation in serum free medium. World Appl Sci J 6:608–615
Fargess-Haddani B, Tessier B, Chenu S, Chevalot I, Harscoat C, Marc I, Goerge JL, Marc A (2006) Peptide fractions of rapeseed hydrolysates as an alternative to animal proteins in CHO cell culture media. Proc Biochem 41:2297–2304
Franěk F, Katinger H (2002) Specific effects of synthetic oligopeptides on cultured animal cells. Biotechnol Prog 18:155–158
Franěk F, Hohenwarter O, Katinger H (2000) Plant protein hydrolysates: preparation of defined peptide fractions promoting growth and production in animal cells cultures. Biotech Prog 16:688–692
Freshney RI (2005) Culture of animal cells: a manual of basic technique, 5th edn. Wiley, New Jersey
Girón-Cale J, Vioque J, Pedroche J, Alaiz M, Yust MM, Megías C, Millán F (2008) Chickpea protein hydrolysate as a substitute for serum in cell culture. Cytotechnology 57:263–272
Keenan J, Pearson D, Clynes M (2006) The role of recombinant proteins in the development of serum-free media. Cytotechnology 50:49–56
Kim S, Lee G (2009) Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl Microbiol Biotechnol 83:639–648
Kohlpoth M, Rusche M (1997) Cultivation of permanent fish cell line in serum-free media: special experiences with a cytotoxicity test for waste water samples. ALTEX 14:16–20
Lakra WS, Swaminathan TR, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37:1–20
McDowell CL, Paputsakis ET (1998) Serum increasec the CD13 receptor expression, reduces the transduction of fluid-mechanical forces and alters the metabolism of HL60 cells cultured in agitated bioreactors. Biotechnol Bioeng 60:259–268
Merten OW (2006) Introduction to animal cell culture technology: past, present and future. Cytotechnology 50:1–7
Ozturk SS, Palsson BO (1991) Physiological changes during the adaptation of hybridoma cells to low serum and serum-free media. Biotechnol Bioeng 37:35–46
Schirmer K (2006) Proposal to improve vertebrate cell cultures to establish them as substitutes for the regulatory testing of chemicals and effluents using fish. Toxicology 224:163–183
Shea TB, Berry ES (1983) A serum-free medium that supports the growth of piscine cell cultures. In Vitro 19:818–824
Shen AY, Van de Goor J, Zheng L, Reyes AE, Krummen LA (2006) Recombinant DNA technology and cell line development. In: Ozturk SS, Hu W-S (eds) Cell culture technology for pharmaceutical and cell-based therapies. CRC Press, Taylor and Francis Group, Boca Raton, pp 15–40
Slivac I, Gaurina Srček V, Radošević K, Porobić I, Bilić K, Fumić K, Kniewald Z (2008) Growth characteristics of channel catfish ovary cells-influence of glucose and glutamine. Cytotechnology 57:273–278
Slivac I, Blajić V, Radošević K, Kniewald Z, Gaurina Srček V (2010) Influence of different ammonium, lactate and glutamine concentrations on CCO cell growth. Cytotechnology 62:585–594
Sung YH, Lim SW, Chung JY, Lee GM (2004) Yeast hydrolysate as a low-cost additive to serum-free medium for the production of human thrombopoietin in suspension cultures of Chinese hamster ovary cells. Appl Microbiol Biotechnol 63:527–536
Van der Valk J, Brunner D, De Smet K, Fex Svenningsen Ä, Gstraunhaler G, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML (2010) Optimization of chemically defined cell culture media: replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro 24:1053–1063
Wolf K, Quimbly MC (1962) Established eurythermic line of fish cells in vitro. Science 135:1065–1066
Wu J, Lee KD (1998) Growth promotion by yeastolate and related components on insect cell. Biotechnol Tech 12:67–70
Zhang Y, Zhou Y, Yu J (1994) Effects of peptone on hybridoma growth and monoclonal antibody formation. Cytotechnology 16:147–150
Acknowledgments
Support from the Ministry of Science, Education and Sports, Republic of Croatia (Grant No. 058-0582184-2414) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radošević, K., Dukić, B., Andlar, M. et al. Adaptation and cultivation of permanent fish cell line CCO in serum-free medium and influence of protein hydrolysates on growth performance. Cytotechnology 68, 115–121 (2016). https://doi.org/10.1007/s10616-014-9760-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-014-9760-x