Abstract
P38 mitogen-activated protein kinases (p38 MAPK) and tumor necrosis factor-α (TNF-α) play important roles in oxidative stress-induced apoptosis in cardiac myocytes. However, the regulation and functional role of cross-talk between p38 MAPK and TNF-α pathways have not yet been fully characterized in cardiac myocytes. In this study, we found that inhibition of p38 MAPK with SB-203580 (SB) reduced H2O2-stimulated secretion of TNF-α, whereas pre-activation of p38 MAPK with sodium arsenite (SA) enhanced H2O2-stimulated secretion of TNF-α. In addition, pretreatment of cells with TNF-α increased basal and H2O2-stimulated p38 MAPK and apoptosis of cardiac myocytes, and p38 MAPK-associated apoptosis of cardiac myocytes induced by TNF-α was blocked by inhibition of p38 MAPK with SB. Finally, H2O2-induced apoptosis was attenuated by the inhibitors of p38 MAPK or reactive oxygen species (ROS), whereas it was enhanced by p38 MAPK agonist SA. These results suggest that H2O2-induced secretion of TNF-α increases apoptosis of cardiac myocytes through ROS-dependent activation of p38 MAPK. This may represent a novel mechanism that TNF-α partly interplays with p38 MAPK pathways during oxidative stress-modulated apoptosis in cardiac myocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many studies have shown that p38 protein, one of mitogen-activated protein kinases (MAPKs), play important roles in cell survival and apoptosis (Bragado et al. 2007; Ozkan et al. 2006). Moreover, apoptosis of cardiomyocytes was enhanced by activation of p38 MAPK induced by various stimulants such as H2O2, AngII or mechanical stretch, and ischemia (Rossa et al. 2005). However, the mechanisms have not yet been fully investigated. H2O2-induced reactive oxygen species (ROS) in cells are thought to serve as second messengers to control many physiological and pathological responses in the cardiovascular system, such as cardiac hypertrophy, atherosclerosis, and apoptosis of cardiac myocytes (Lee et al. 2002). Elevated ROS can regulate the activity of MAPKs pathways that may be involved in apoptosis (Lee et al. 2002). Moreover, activation of p38 MAPK play an important role in H2O2-induced secretion of tumor necrosis factor-α (TNF-α) (Lee et al. 2002). Therefore, the regulation between the effects of p38 and TNF-α is important in determining whether a cell will survive or undergo apoptosis. Elevation of ROS results in potentially cytotoxic oxidative stress and increases expression of TNF-α which leads to apoptosis as a final event (Kim et al. 2010). Yet the precise mechanism underlying the regulation of the balance of these opposing signaling events between these MAPK and TNF-α pathways remains poorly understood.
Mounting evidence suggests a cross-interplay between the p38 MAPK pathway and TNF-α pathway in a variety of eukaryotic cells (Cain et al. 1999; Peng et al. 2003). Recently, p38 MAPK-mediated inhibition of TNF-α expression was reported during simulated ischemia (Meldrum et al. 2001). Furthermore, it was reported in renal tubular cell activation of p38 MAPK enhances TNF-α secretion and induced apoptosis (Meldrum et al. 2001). These observations led us to hypothesize that TNF-α and p38 MAPK are mutual activated through oxidative stress-induced pathways in cardiac cells. Cross-talk between p38 MAPK and TNF-α pathways, which have important effects in cell apoptosis, would represent a novel mechanism for the regulation of oxidative stress-induced apoptosis.
Recent studies demonstrate that activation of p38 MAPK by sodium arsenite (SA) increases the expression of TNF-α via a serinethreonine phosphatase in endothelial cells (Chen et al. 2009). It was also reported that TNF-α, through the Toll-like receptors, regulates activation of the JNK pathway in cardiomyocytes (Yeh et al. 2008). In addition, it was previously reported (Kulisz et al. 2002) that p38 MAPK is modulated through a ROS pathway in adult cardiac cells. Therefore, we hypothesized that TNF-α activates p38 MAPK signaling pathway through a ROS-mediated mechanism in cardiac myocytes during oxidative stress.
Findings from the present study demonstrated that TNF-α does exert active effects on p38 MAPK signaling in cardiomyocytes. Our results showed that H2O2 could activate ROS, p38 MAPK and enhance the secretion of TNF-α. In addition, the activation of p38 MAPK evoked by elevated ROS induced the increased secretion of TNF-α and the resultant apoptosis of myocytes. Oxidative stress-induced apoptosis in cardiomyocytes was also decreased through inhibition of p38 MAPK with SB-203580 (SB). However, the apoptosis of cardiac cells increased once addition of TNF-α in the culture medium. We conclude that the cross-talk between p38 MAPK and TNF-α through ROS-mediated mechanism may provide a novel pathway for the regulation of apoptosis in cardiac myocytes.
Methods
Isolation of adult rat ventricular myocytes
All animals were handled in accordance with the guidelines of the Animal Care and Use Committee at the University of Wuhan. Cardiomyocytes of neonatal rat (RCM) were enzymatically isolated from 1-day-old Sprague–Dawley rats as reported previously (Liu and Hofmann 2003; Mitsuhashi et al. 2003; Tomomi et al. 2007).
Western blot analysis of p38 MAPKs
Activations p38 MAPK were examined by Western blotting with antibodies that specifically recognize the phosphorylated p38 MAPK (catalog no. sc-365550, Santa Crus Biotechnology; Santa Crus, CA). The loading amount of protein was 20 μg for each land. The immunoblots were visualized using enhanced chemiluminescence (New England Biolabs). Densitometry was performed on scanned images using NIH Image 1.6 software, and values were normalized for corresponding controls in each experiment.
ROS determination
ROS generation in the cardiomyocytes was quantified before and after the time of H2O2 stimulation as it was indicated, with 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2-DCFDA) staining method. This assay is based on the principle that the nonpolar, nonionic H2-DCFDA crosses cell membranes and is enzymatically hydrolyzed into nonfluorescent H2-DCF by intracellular esterases. In the presence of ROS, H2-DCF is rapidly oxidized to become highly fluorescent DCF. Cells were incubated at 37 °C for 30 min with 5 μM carboxy-H2-DCFDA dissolved in the culture medium. 5 × 105 cells were resuspended in phosphate-buffered saline (PBS, pH 7.4) and sent to flow cytometry analysis (Epics Altra, BECKMAN, USA). The percent of fluorescence-positive cells as a measure of ROS generation was recorded on a spectrofluorometer using excitation and emission filters of 488 and 530 nm, respectively. For ROS inhibition assay, 1 mmol/L NAC or 5 μmol/L DPI were added to the cultures 2 h before H2O2 treatment.
Determination of cell apoptosis by cell death-detection ELISA
RCM were pretreated with 5 μmol/L SB, 1 mmol/L DPI or vehicle for 1 h followed by treatment of the cells with 100 μmol/L H2O2 for 6 h. After treatment of RCM the supernatants were collected for detecting the concentration of TNF-α according to the kit’s manual (kit no. ab46070, Abcam Company, UK). Cell extracts were then prepared according to the manufacturer’s protocol, and histone-associated DNA fragments in the cytosolic fraction of the cell lysates were measured spectrophotometrically at 405 nm (kit no. 1774425, Boehringer-Mannheim, Indianapolis, IN, USA).
Statistical analysis
Data from at least 4 independent experiments are expressed as mean ± SD; multiple-group comparison was performed by one-way analysis of variance followed by least-significant difference procedure for comparison of means. Comparison between two groups under identical conditions was performed by the two-tailed Student’s t test. A value of P < 0.05 was considered statistically significant.
Results
H2O2-stimulated ROS elevation, p38 MAPK activation and TNF-α secretion in cardiomyocytes
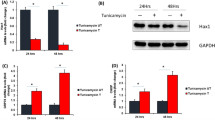
The effects of oxidative stress on the MAPK signaling pathway were determined in RCM. Time courses of p38 MAPK, TNF-α, and ROS activation in cardiomyocytes are shown in Fig. 1. A rapid p38 MAPK phosphorylation was observed after treatment with stimulation of H2O2. A maximum response was observed after H2O2 exposure at 20 min and remained elevated for at least 80 min (Fig. 1b). RCM was stimulated by H2O2 with a maximum response observed at 10 min remaining at a high level even after 1 h (Fig. 1a). However, the level of TNF-α reached a maximum 4 h after the treatment of RCM with H2O2 (Fig. 1c). These observations suggested that H2O2 induced ROS elevation, p38 MAPK activation and an increase of TNF-α secretion in cardiomyocytes.
Effects of H2O2 on the production of reactive oxygen species (ROS, a), p38 mitogen-activated protein kinase (p38 MAPK, b) and tumor necrosis factor-α (TNF-α, c) in cardiomyocytes of neonatal rat (RCM). The cells were treated with 100 μmol/L H2O2 for the times indicated, and the phosphorylation levels of p38 MAPK was determined by Western blot analysis, Densitometry values of specific bands were normalized to the untreated control. Expression of TNF-α was detected in culture medium by ELISA. ROS generation in cardiomyocytes was determined before and after H2O2 stimulation, with the 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2-DCFDA) staining method. Data are means ± SD of 3 independent experiments; *P < 0.05 compared with control (time 0)
H2O2 induced p38 MAPK activation through ROS
To determine whether H2O2 induced p38 MAPK activation via ROS in cardiac cells, we examined the effects of the antioxidant diphenyleneiodonium (DPI) on H2O2-induced p38 activation. DPI’s treatment decreased p38 MAPK phosphorylation in H2O2-stimulated cells but did not decrease p38 MAPK phosphorylation in non-H2O2-stimulated cells (Fig. 2). Similar effects were obtained when another antioxidant inhibitor N-acetylcysteine (NAC) was used (data not shown). This suggests that p38 MAPK was activated by elevated ROS when cardiomyocytes were pretreated with H2O2.
Inhibition of RAS depressed H2O2-induced p38 MAPK activation. Cardiomyocytes were pretreated with antioxidant diphenyleneiodonium (DPI) or vehicle for 2 h followed by treatment with 100 μmol/L H2O2 for 15 min. Representative Western blots (a) and cumulative data (b) are shown. Data are results of 4 independent experiments; *P < 0.05 compared with control; # P < 0.05 compared with H2O2-treated group
ROS-activated p38 MAPK increased the expression of TNF-α
To establish whether TNF-α secretion is modulated via activation of p38 MAPK, TNF-α was measured in the culture supernatant of cardiomyocytes pretreated with the p38 MAPK inhibitor SB and activator SA and subsequently stimulated with H2O2. SA is a strong activator of p38 MAPK in various cell types including cardiac myocytes. The ability of SA to activate p38 MAPK in cardiac myocytes was confirmed by Western blotting with phospho-p38 MAPK antibodies (Fig. 3a). SB is a strong inhibitor of p38 MAPK in various cell types including cardiac ventricular myocytes. In addition, the inhibitory activity of SB on p38 MAPK in cardiac myocytes was confirmed by Western blotting with phospho-p38 MAPK antibodies (Fig. 3a). The treatment with SB can significantly decrease the TNF-α secretion induced by H2O2, suggesting that activation of p38 MAPK exerts an active effect on H2O2-stimulated TNF-α expression. In addition, the analog SB-202474 inactive for p38 MAPK had no effect on TNF-α secretion (data not shown). Moreover, elevated TNF-α secretion induced by H2O2 was significantly enhanced by SA (Fig. 3), which also shows that p38 MAPK phosphorylation enhanced H2O2-stimulated TNF-α expression. In addition, the increase of TNF-α secretion was not observed in myocytes pretreated with p38 MAPK activator SA in the absence of H2O2. These results confirmed that H2O2-induced TNF-α secretion was modulated by activation of p38 MAPK.
Effects of p38 MAPK activation or inhibition with sodium arsenite (SA) or SB-203580 (SB) on H2O2-induced TNF-α generation. RCM were pretreated with vehicle, 5 μmol/L SB for 1 h followed by treatment with 100 μM SA for 15 min followed by 100 μmol/L H2O2 for an additional 15 min. Representative Western blots (a) and cumulative data (b) are shown. Data are results of 3 independent experiments and are expressed relative to untreated cells; *P < 0.05 as compared with control, # P < 0.05 compared H2O2-treated group
ROS-dependent p38 MAPK activation in H2O2-induced apoptosis in cardiomyocytes
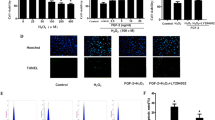
We then tested the possibility whether p38 MAPK activation or inhibition with SA or SB can affect H2O2-induced apoptosis in cardiomyocytes (Fig. 4). SA increased H2O2-stimulated apoptosis but did not increase basal apoptosis in non-H2O2-stimulated cells. This active effect of SA was reversed upon pretreatment of myocytes with the antioxidants DPI or NAC before SA exposure. The treatment with SB, an inhibitor of p38 MAPK, remarkably decreased H2O2-stimulated apoptosis. However, the treatment with SB significantly reduced TNF-α-stimulated apoptosis. Furthermore, this inhibitory effect of SB was augmented upon pretreatment of myocytes with the antioxidants DPI or NAC before SB exposure to the cells. These results suggest that p38 MAPK-induced apoptosis was dependent on the level of ROS generated in the H2O2-stimulated cardiomyocytes.
p38 MAPK affected H2O2-induced DNA fragmentation in cardiomyocytes of neonatal rat (RCM). RCM were pretreated with 5 μmol/L SB-203580 (SB, p38 MAPK inhibitor) or vehicle for 1 h before incubation with 100 μmol/L H2O2 or 100 μmol/L sodium arsenite (SA) for 6 h. For inhibition of ROS, 1 mmol/L NAC or 5 μmol/L DPI were added 2 h before H2O2 treatment. Then cells were lysed, and cytoplasmic histone-associated DNA fragments were determined by ELISA. Data are from 4 independent experiments and are expressed relative to control values; *P < 0.05 compared with control, # P < 0.05 compared with H2O2-treated group. & P < 0.05
TNF-α induced apoptosis of cardiomyocytes through ROS-mediated p38 MAPK
To further investigate the effect of secreted TNF-α modulated by ROS on cardiomyocytes apoptosis, we determined the effect of TNF-α treatment on p38 MAPK activity in cardiomyocytes. As shown in Fig. 5, adding 50 ng/mL recombinant TNF-α to cultured cardiomyocytes induced a significant activation of p38 MAPK, which lasted for about 60 min (data no shown). SA increased TNF-α-stimulated apoptosis but did not increase basal apoptosis in non-TNF-α-stimulated cells. This active effect of SA on cell apoptosis was depressed upon pretreatment of myocytes with the antioxidants DPI or NAC before SA exposure. The treatment with TNF-α remarkably induced cardiac apoptosis. However, the treatment with SB significantly reduced TNF-α stimulated the apoptotic responses. Furthermore, this inhibitory effect of SB was increased upon pretreatment of myocytes with the antioxidants DPI or NAC before SB exposure to the cells. Collecting all results, the data suggest that p38 MAPK-induced apoptosis was dependent on the level of ROS generated in the TNF-α-stimulated cardiomyocytes. Together with the above observation regarding p38-mediated TNF-α expression, they suggest that a potential positive feedback loop between p38 MAP activation and TNF-α modulated by H2O2.
TNF-α induced apoptosis in cardiomyocytes of neonatal rat (RCM) through activation of p38 MAPK. RCM were pretreated with 5 μmol/L SB-203580 (SB, p38 MAPK inhibitor) or vehicle for 1 h before incubation with 50 ng/mL TNF-α for 30 min or 100 μmol/L sodium arsenite (SA) for 6 h. For inhibition of ROS, 1 mmol/L NAC or 5 μmol/L were added 2 h before H2O2 treatment. Then cells were lysed, and cytoplasmic histone-associated DNA fragments were determined by ELISA. Data are from 4 independent experiments and are expressed relative to control values; *P < 0.05 compared with control, # P < 0.05 compared with only TNF-α treated group, & P < 0.05
Discussion
MAPKs play important roles in signaling pathways activated by mitogenic stimuli, mechanical stretch and inflammatory agents (Chen et al. 2003; Hansen and Jorgensen 2007; Kimura et al. 2006). The stress-activated protein kinase member p38 is activated by elevated ROS generated in response to angiotensin II or by hypoxia (Benhar et al. 2001; Di Lisa et al. 2011; Liu and Chang 2009). Phosphorylation of p38 MAPK also occurs in the presence of H2O2 (Hsieh and Papaconstantinou 2002), although the mechanism underlying this response is still to be investigated. It was reported that hypoxia induced an augmentation in mitochondrial generation of ROS in cardiomyocytes and in other cell lines (Kimura et al. 2006; Repicky et al. 2009). Therefore, this study investigated whether H2O2 induced activation of p38 MAPK by a way dependent or independent of ROS in cardiomyocytes. In the present study, we demonstrated that H2O2-induced activation of p38 MAPK was dependent of ROS in RCM.
It was reported that elevated plasma levels of inflammatory cytokines were related to heart dysfunction (Chen et al. 2003; Meldrum et al. 2001; Peng et al. 2003). Previous studies have accumulated much evidence that the changes of inflammatory cytokines, such as some TNF-α and IL-6, plays an important role in the process of pathological heart remodeling. However, it has been undetermined whether cardiomyocytes themselves can secrete inflammatory cytokine and, if so, what signaling mechanisms result from this process. The present study provides direct in vitro evidence that p38 MAPK activation is sufficient to induce inflammatory cytokine TNF-α expression in cardiomyocytes and that p38 MAPK activity has a critical role in cytokine secretion from cardiomyocytes. Moreover, administration of the selective p38 MAPK inhibitor SB239068 reduces p38 MAPK activities in the RCM (Wang et al. 2011). These data demonstrated that p38-mediated TNF-α induction represents a potential signaling mechanism that contributes to myocardial remodeling.
TNF-α induction is observed in many different forms of stressed myocardium and may play a protective role in response to acute myocardial injury when the induction is self-limiting and transient as in innate immune responses (Kim et al. 2010; Kulisz et al. 2002; Yamamoto et al. 2006). However, long-term induction of TNF-α in failing hearts is believed to exert a detrimental effect by triggering a spectrum of pathological changes, including loss of contractility, apoptosis, contractile dysfunction, and ECM remodeling (Chen et al. 2009; Killock and Ivetic 2010; Kim et al. 2008; Li et al. 2009). The underlying regulatory mechanism that regulates the induction pattern of TNF-α in heart has not been clearly investigated. Interestingly, induction of p38 MAPK activity is also associated with a lot of cardiac pathologies caused by TNF-α (Ricote et al. 2006; Wang et al. 2010). More importantly, p38 MAPK activation has been greatly involved in the process of cardiac apoptosis and hypertrophy (Ranawat and Bansal 2009; Rossa et al. 2005). Our data showed that TNF-α was dramatically induced by activation of p38 MAPK, whereas TNF-α also induced sustained p38 MAPK activation. Therefore, our study show that p38 MAPK-mediated regulation of TNF-α secretion may have physiological significance because secreted and membrane-bound forms of TNF-α are shown to lead to apoptosis of cardiomyocytes.
Previous work has demonstrated interplay between TNF-α and ERK signaling pathways in some types of cells such as in human leukemia U937 cells (Liu and Chang 2009; Moon et al. 2010). Here our results suggested that p38 MAPK positively regulates H2O2-induced TNF-α secretion in RCM. TNF-α secretion was decreased by the p38 MAPK inhibitor SB, and increased by the p38 MAPK activator SA. Moreover, the strong p38 MAPK activator SA increased the DNA degradation in H2O2-treated cells. The inhibitory effect of SB on p38 phosphorylation exerts a tonic inhibition on the DNA-degradation pathway.
Conclusions
Our data suggest that an interaction between p38 MAPK and TNF-α pathway via ROS exists in RCM, and this novel pathway plays a role in the regulation of oxidative stress-induced apoptosis. We demonstrated in this study that in cardiac ventricular myocytes, (1) H2O2 led to activation of p38 MAPK; (2) inhibition of ROS decreased H2O2-stimulated activation of p38 MAPK; (3) preactivation of p38 MAPK increased H2O2-stimulated p38 MAPK phosphorylation via ROS and then led to increase in apoptosis in RCM. Thus the positive regulation loop of proapoptotic p38 MAPK and TNF-α via ROS may provide a functional link between the two signaling molecules and represent a novel pathway to modulate apoptosis during oxidative stress in RCM.
References
Benhar M, Dalyot I, Engelberg D, Levitzki A (2001) Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol 21:6913–6926
Bragado P, Armesilla A, Silva A, Porras A (2007) Apoptosis by cisplatin requires p53 mediated p38 alpha MAPK activation through ROS generation. Apoptosis 12:1733–1742
Cain BS, Meldrum DR, Meng XZ, Dinarello CA, Shames BD, Banerjee A, Harken AH (1999) p38 MAPK inhibition decreases TNF-alpha production and enhances postischemic human myocardial function. J Surg Res 83:7–12
Chen Y, Ke QG, Yang YK, Rana JMS, Tang J, Morgan JP, Xiao YF (2003) Cardiomyocytes overexpressing TNF-alpha attract migration of embryonic stem cells via activation of p38 and c-Jun amino-terminal kinase. Faseb J 17:2231–2239
Chen XL, Dodd G, Kunsch C (2009) Sulforaphane inhibits TNF-alpha-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm Res 58:513–521
Di Lisa F, Kaludercic N, Paolocci N (2011) beta(2)-Adrenoceptors, NADPH oxidase, ROS and p38 MAPK: another ‘radical’ road to heart failure? Br J Pharmacol 162:1009–1011
Hansen TE, Jorgensen JB (2007) Cloning and characterisation of p38 MAP kinase from Atlantic salmon—a kinase important for regulating salmon TNF-2 and IL-1 beta expression. Mol Immunol 44:3137–3146
Hsieh CC, Papaconstantinou J (2002) The effect of aging on p38 signaling pathway activity in the mouse liver and in response to ROS generated by 3-nitropropionic acid. Mech Ageing Dev 123:1423–1435
Killock DJ, Ivetic A (2010) The cytoplasmic domains of TNF alpha-converting enzyme (TACE/ADAM17) and l-selectin are regulated differently by p38 MAPK and PKC to promote ectodomain shedding. Biochem J 428:293–304
Kim H, Hwang JS, Woo CH, Kim EY, Kim TH, Cho KJ, Seo JM, Lee SS, Kim JH (2008) TNF-alpha-induced up-regulation of intercellular adhesion molecule-1 is regulated by a Rac-ROS-dependent cascade in human airway epithelial cells. Exp Mol Med 40:167–175
Kim JJ, Lee SB, Park JK, Yoo YD (2010) TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X-L. Cell Death Differ 17:1420–1434
Kimura H, Shintani-Ishida K, Nakajima M, Liu S, Matsumoto K, Yoshida K (2006) Ischemic preconditioning or p38 MAP kinase inhibition attenuates myocardial TNF alpha production and mitochondria damage in brief myocardial ischemia. Life Sci 78:1901–1910
Kulisz A, Chen NF, Chandel NS, Shao ZH, Schumacker PT (2002) Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am J Physiol Lung Cell Mol Physiol 282:L1324–L1329
Lee MW, Park SC, Yang YG, Yim SO, Chae HS, Bach JH, Lee HJ, Kim KY, Lee WB, Kim SS (2002) The involvement of reactive oxygen species (ROS) and p38mitogen-activated protein (MAP) kinase in TRAIL/Apo2L-induced apoptosis. Febs Lett 512:313–318
Li XN, Rong YY, Zhang M, Wang XL, LeMaire SA, Coselli JS, Zhang Y, Shen YH (2009) Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun 381:660–665
Liu WH, Chang LS (2009) Arachidonic acid induces Fas and FasL upregulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-Fos pathway and activation of p38 MAPK/ATF-2 pathway. Toxicol Lett 191:140–148
Liu QH, Hofmann PA (2003) Modulation of protein phosphatase 2a by adenosine A(1) receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol Heart Circ Physiol 285:H97–H103
Meldrum KK, Meldrum DR, Hile KL, Yerkes EB, Ayala A, Cain MP, Rink RC, Casale AJ, Kaefer MA (2001) p38 MAPK mediates renal tubular cell TNF-alpha production and TNF-alpha-dependent apoptosis during simulated ischemia. Am J Physiol Cell Physiol 281:C563–C570
Mitsuhashi S, Shima H, Tanuma N, Matsuura N, Takehawa M, Urano T, Kataoka T, Ubukata M, Kikuchi K (2003) Usage of tautomycetin, a novel inhibitor of protein phosphatase 1 (PP1), reveals that PP1 is a positive regulator of Raf-1 in vivo. J Biol Chem 278:82–88
Moon D-O, Kim M-O, Lee J-D, Choi YH, Kim G-Y (2010) Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappa B activation and ROS generation in human leukemia U937 cells. Cancer Lett 288:183–191
Ozkan Y, Yardim-Akaydin S, Imren E, Torun M, Simsek B (2006) Increased plasma homocysteine and allantoin levels in coronary artery disease: possible link between homocysteine and uric acid oxidation. Acta Cardiol 61:432–439
Peng TQ, Lu XR, Lei M, Moe GW, Feng QP (2003) Inhibition of p38 MAPK decreases myocardial TNF-alpha expression and improves myocardial function and survival in endotoxemia. Cardiovasc Res 59:893–900
Ranawat P, Bansal MP (2009) Apoptosis induced by modulation in selenium status involves p38 MAPK and ROS: implications in spermatogenesis. Mol Cell Biochem 330:83–95
Repicky A, Jantova S, Cipak L (2009) Apoptosis induced by 2-acetyl-3-(6-methoxybenzothiazo)-2-yl-amino-acrylonitrile in human leukemia cells involves ROS-mitochondrial mediated death signaling and activation of p38 MAPK. Cancer Lett 277:55–63
Ricote M, Garcia-Tunon I, Fraile B, Fernandez C, Aller P, Paniagua R, Royuela M (2006) P38 MAPK protects against TNF-alpha-provoked apoptosis in LNCaP prostatic cancer cells. Apoptosis 11:1969–1975
Rossa C, Liu M, Patil C, Kirkwood KL (2005) MKK3/6–p38 MAPK negatively regulates murine MMP-13 gene expression induced by IL-1 beta and TNF-alpha in immortalized periodontal ligament fibroblasts. Matrix Biol 24:478–488
Tomomi O, Toshio N, Hiroshi W, Atsuhiko T, Katsuhisa M, Koji I, Toshinao T, Motohiro G, Yoko M, Noritaka Y, Hiroshi A, Akiyoshi U, Shin T, Issei K (2007) Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol 176:329–341
Wang T, Chiang ET, Moreno-Vinasco L, Lang GD, Pendyala S, Samet JM, Geyh AS, Breysse PN, Chillrud SN, Natarajan V, Garcia JGN (2010) Particulate matter disrupts human lung endothelial barrier integrity via ROS- and p38 MAPK-dependent pathways. Am J Respir Cell Mol Biol 42:442–449
Wang W-L, Hong J-R, Lin G-H, Liu W, Gong H-Y, Lu M-W, Lin C-C, Wu J-L (2011) Stage-specific expression of TNF alpha regulates bad/bid-mediated apoptosis and RIP1/ROS-mediated secondary necrosis in birnavirus-Infected fish cells. PLoS One 6:e16740
Yamamoto T, Yuyama K, Yamamoto H (2006) Low concentrations of nitric oxide (NO) induced cell death in PC12 cells through activation of p38 mitogen-activated protein kinase (p38 MAPK) but not via extracellular signal-regulated kinases (ERK1/2) or c-Jun N-terminal protein kinase (JNK). Neurosci Lett 392:170–173
Yeh CJ, Lin PY, Liao MH, Liu HJ, Lee JW, Chiu SJ, Hsu HY, Shih WL (2008) TNF-alpha mediates pseudorabies virus-induced apoptosis via the activation of p38 MAPK and JNK/SAPK signaling. Virology 381:55–66
Acknowledgments
We thank Dr Chen for his excellent technical assistance. This work was supported by the grant from Natural Science fund of Hubei Province (2009CDB244).
Conflict of interest
The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., Jiang, H., Wan, Y. et al. H2O2-induced secretion of tumor necrosis factor-α evokes apoptosis of cardiac myocytes through reactive oxygen species-dependent activation of p38 MAPK. Cytotechnology 64, 65–73 (2012). https://doi.org/10.1007/s10616-011-9392-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-011-9392-3