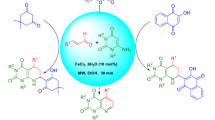
A microwave-assisted Biginelli-like three-component condensation using salicylic aldehyde derivatives, acetone, and 5-substituted 3-amino-1,2,4-triazoles instead of the urea component results in the formation of oxygen-bridged tetrahydrotriazolopyrimidine derivatives (11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5-c][1,3,5]benzoxadiazocines) in good yields and high purity. A plausible reaction mechanism for this transformation is discussed in details using literature and experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Biginelli multicomponent reaction discovered in 18911 has been intensively used as an efficient tool for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives.2 This approach is still topical, especially in the last decades its facile performance and wide scope have attracted a considerable attention of scientists working in the fields of process development3 and drug discovery.4 An intriguing and challenging task is also to broaden the horizons of classical multicomponent reactions (MCRs) by means of replacing the conventional building blocks with novel and often more complex starting molecules,5 which can be considered as synthetic equivalents, but due to their different nature the resulting modified Biginelli reaction can lead even beyond the classical Biginelli products.
This happens, in particular, when salicylic aldehydes are applied in Biginelli-like MCRs leading to the formation of oxygen-bridged benzoxadiazocines 16 (Fig. 1). Also, the use of heterocyclic building blocks instead of urea in such MCRs makes these reactions to be even more complicated.7,8 A literature overview of three-component Biginelli-like condensations using 3-amino-1,2,4-triazole as a 1,3-binucleophile showed that 4,7-dihydro-1,2,4-triazolo[1,5-a]-pyrimidines (2) are the most common products obtained under harsh reaction conditions,9 however, variations of the conditions and specific substituents in the starting materials can switch these reactions to the formation of other product types, which are mainly formed as intermediates or side reaction products in the mainstream to compound 2. A Biginelli-type condensation using both salicylic aldehydes and 3-amino-1,2,4-triazoles also has several reaction pathways depending on the third reaction component and the reaction conditions applied. In our preliminary communication, the formation of alternative structures has been described, e.g., compounds 310 and 410a formed in very good yields at 40°C in MeOH in the presence of HCl. Interestingly, the formation of products of type 3, but without the o-hydroxy group in the aromatic ring, proceeds in acetone taken as solvent, and it requires much higher reaction temperature (125°C for 45 min under microwave irradiation in the presence of TsOH), giving only moderate product yields.11 The oxygen-bridged products 5 were obtained by microwave heating of the reaction mixture at 150°C for 30 min in the presence of HCl.10a This work was followed by a report from Svĕtlík and coworkers12 describing a pseudo-four component MCR of methyl acetoacetate, 3-amino-1,2,4-triazole, and 2 equiv of salicylic aldehydes in refluxing EtOH–HCl (cat.) for 20 h that led to spiro compounds 6 as a major products and their diastereoisomers accompanied with 3-acetylcoumarin (derived from the reaction of salicylic aldehyde and methyl acetoacetate) as minor ones.
More recently, Magedov's group published one example of MCR starting practically with the same reagents in refluxing acetic acid in the presence of piperidine that resulted in the formation of heterocyclic system 7.13 In all these cases, several substituted salicylic aldehydes, a few α-carbonyl CH acids, and single unsubstituted 3-amino-1,2,4-triazole were applied as initial compounds giving quite a narrow chemical diversity of the final products.
Taking into account the preliminary character of our previous publication10a and the limited number of the described compounds 5, here we aimed to explore the possibility of introducing 5-substituted 3-amino-1,2,4-triazoles into the three-component microwave-assisted reaction with salicylic aldehydes and acetone to elucidate the scope and limitations of this modified Biginelli reaction.
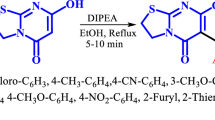
For the present study, several 5-substituted 3-amino-1,2,4-triazoles 8a–f were used as 1,3-binucleophiles in three-component interaction with salicylic aldehyde or its derivatives 9a–e and acetone (10) (Scheme 1). Application of the reaction conditions previously described for unsubstituted 3-amino-1,2,4-triazole10a provided expectedly the novel derivatives of methano[1,2,4]triazolo[1,5-c][1,s,5]-benzoxadiazocine 11a–w obtained in moderate to good yields (31–62%, Table 1). The products, whose purity was ascertained by NMR,14 precipitated from the reaction mixture at room temperature as colorless crystalline substances and were easily collected by filtration. The application of higher temperature or longer reaction times did not result in any significant increase of the reaction yields. Moderate product yields (compound 11d,i) were obtained only when 4-methoxysalicylaldehyde (9d) was used as a starting material in reactions with 5-alkyl-substituted 3-amino-1,2,4-triazoles 8a,b and acetone (10). The limitation of this reaction was also found in the case when the same aldehyde 9d reacted with pyridyl-derived 3-amino-1,2,4-triazoles 8d–f, where only in the case of compound 8e (R1 = 3-Py) formation of the expected oxygen-bridged product 11y was observed. However this product was isolated in a mixture with the corresponding Schiff base 12b, while in the cases of starting compounds 8d,f only Schiff bases 12a,c were obtained as the final products (Scheme 2). In all other cases such Schiff bases could be obtained under mild conditions and transformed into the desired oxygen-bridged products 11a–w by the reaction with acetone. Contrary, in the cases of azomethines 12a,c this reaction did not proceed even at elevated temperature (170°C) during 1 h.

Scheme 1

Scheme 2
Such influence of the substituents on the reactivity can be rationalized taking into account a set of different data about this type of heterocyclization collected from the literature to date and our assumptions about a possible reaction mechanism. In contrast to one of the previously suggested mechanisms for this reaction type,15 the formation of Schiff base 12 is the most probable first stage of the whole cascade. This Schiff base begins to form already after mixing all reagents at room temperature and can be readily isolated; it often accompanies the product's formation in such MCRs10 and also can serve as a starting material in the reaction with acetone giving the same oxygen-bridged product with a comparable yield. Structure 12 also corresponds to the iminium intermediate in the widely accepted mechanism for the classical Biginelli reaction.3,16b
Nucleophilic attack of the electron-rich enol carbon atom on the electron-deficit azomethine probably proceeds via a transition state with considerable charge separation, and its structure should be close to intermediate 14 (Scheme 3). The substituent's influence represented in Scheme 2 can be easily rationalized already on this stage taking into account the following factors: a) the presence of an electron-donating substituent (OMe) conjugated with the azomethine carbon atom in compounds 12a–c should increase the electron density on the center of the nucleophilic attack decreasing the reactivity; b) the electron-withdrawing pyridine rings decrease the electron density on the triazole endocyclic amino group, consequently decreasing its reactivity in the transformation 15 → 3, especially in the case of compounds 12a,c; c) the direct conjugation between the donor and acceptor groups, especially in the case of compounds 12a,c, thermodynamically stabilizes intermediates 12, obviously increasing the activation barrier of this reaction proceeding with a break of the conjugation. Consequently, in our experiments azomethine 12b shows decreased reactivity, while compounds 12a,c do not react with acetone at all.

Scheme 3
On the other hand, the o-hydroxy group also has the electron-donating properties and the intramolecular hydrogen bond between this group and azomethine nitrogen atom can also stabilize azomethine molecules, therefore, it could be expected that the reactivity of salicylic aldehydes in such transformation has to be lowered. But in fact, it is enhanced. As mentioned above, the parent salicylic aldehyde reacts more easily with acetone and 3-amino-1,2,4-triazole than aromatic aldehydes without the o-hydroxy group.11 To explain this phenomenon we have to suggest that the transition state of the reaction 15 → 3 is close to intermediate 14 and has a significant charge separation, thus the intramolecular hydrogen bond would be considerably enhanced and would reduce the energy of the activation complex to a much greater extent than the energy of the ground state, thereby increasing the reactivity of salicylic aldehydes.
The proposed role of acid catalyst in this reaction is to generate the acetone enol form. The initial protonation of one of the triazole pyridine-type nitrogen atoms could also increase the ability of azomethine carbon atom to undergo the nucleophilic attack.
Relative configuration of the stereogenic centers in tetrahydropyrimidines 3 remains unchanged in the products of similar heterocyclizations. In all the literature sources where the synthesis of such compounds with sufficient confirmation of the product structure is reported, only the isomers with the aryl or other bulky substituent located in the anti position to the hydroxy group are described,8d,10a,15,17,18 similarly as in the cases mentioned above (Fig. 1, compounds 3, 4). Thus, this is probably a stereospecific heterocyclization, and it can serve as a strict stereochemical characteristic of this reaction. This feature may be reasonably explained on the basis of the suggested reaction mechanism.
The reaction 15 → 3 (Scheme 3) could proceed via two rotamers, s-trans-14 and s-cis-14 giving two corresponding diastereomers. The widely accepted theory holds that the preferred approach of a nucleophile is in the plane bisecting the carbonyl group and orthogonal to the molecular plane, and that the Nu···C=O angle (α) is maintained constant at the value of Bürgi–Dunitz angle (107 ± 5°).19 Obviously, in rotamer s-trans-14 the approach of the triazole nitrogen atom at such angle would be associated with steric hindrances, while nothing prevents this attack at the favorable angle in rotamer s-cis-14, and simple rotation around C–CO σ-bond (s-trans-14 → s-cis-14) provides more favorable disposition for ring closure and the product is formed only as diastereomer 3.
The further closure of the oxygen bridge also should proceed according to Baldwin rules,20 and the mechanism for this cyclization type was initially suggested by Světlík21 in his first report devoted to such heterocyclization. Following his logic, we can suggest here that direct transformation 3 → 11 via 6/8-endo-tet process is hardly possible. The reason is that the original favored trajectory for tetrahedral cyclization (attack on sp3-atom) with an attack angle α of 180° is unrealizable in structure 3. Similarly, 6-endo/8-exo-trig nucleophilic addition (16 → 11) must be excluded due to unfavorable attack trajectory with a value of angle α much lower than Bürgi–Dunitz angle. Thus, to rationalize the final formation of benzoxadiazocine 11 we have to postulate the formation of protonated intermediate 17 (in fact, it is a carbocation) or terminal alkene 18, in which the final nucleophilic attack can proceed following the original favored trajectory. Thus, both the substituent influence and the stereochemical control of the reaction are in line with the suggested reaction mechanism.
In conclusion, the reaction studied is suitable for a wide range of starting salicylic aldehyde derivatives and 5-substituted 3-amino-1,2,4-triazoles, which allows obtaining an increased diversity of methanobenzoxadiazocines fused with 1,2,4-triazole ring and enlarges the scope of the Biginelli reaction. A plausible reaction mechanism rationalizing all known experimental data about such transformation is first proposed, which includes the formation of the Shiff bases between 3-amino-1,2,4-triazoles and salicylic aldehydes followed by the further reaction with enol form of acetone to form the tetrahydropyrimidine intermediates that give the final oxygen-bridged heterocycles via the above-discussed cascade of transformations.
Experimental
IR spectra were recorded in KBr pellets on a Shimadzu IR Prestige-21 Fourier spectrometer fitted with an attenuated total reflectance sampling accessory. 1H and 13C NMR spectra and APT were registered on a Varian Inova 400 NMR spectrometer (400 and 100 MHz, respectively) in DMSO-d 6 , internal standard TMS. 13C NMR spectra of compounds 11a,g,j,m,r,v,w were registered in the APT mode. Mass spectra were recorded with a Varian 1200L Triple Quadrupole GC/MS spectrometer (EI, 70 eV). Elemental analyses were performed on a vario MACRO cube CHNS elemental analyzer. Melting points were determined on a WRS-1B Digital Melting-point apparatus and were not corrected. Microwave experiments were carried out using a monomode Anton Paar Monowave 300 microwave reactor (2.45 GHz) in G10 microwave process vials. Reaction temperatures were monitored by an IR sensor. Microwave experiments were carried out in sealed microwave process vials (10 ml). After completion of the reaction, the vial was cooled to 50°C by air jet cooling. All reagents and solvents were purchased from commercial suppliers and used without further purification.
Synthesis of compounds 11a–w (General method). 3-Amino-1,2,4-triazole 8a–f (1.0 mmol), o-hydroxybenzaldehyde 9a–e (1.0 mmol), acetone (10) (0.22 ml, 3.0 mmol), and abs. EtOH (2 ml) were mixed in a microwave process vial, and then 4 N solution of HCl in dioxane (0.07 ml, 0.3 mmol) was added. The mixture was irradiated at 150°C for 30 min. The reaction mixture was cooled by an air flow and stirred for 24 h at room temperature for complete precipitation of the product. The precipitate was filtered off, washed with EtOH (1 ml) and Et2O (3×1 ml), and dried. Compounds 1a–w were obtained in a form of white solids.
2,5-Dimethyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11a). Yield 48%, mp 310–311°C. IR spectrum, ν, cm–1: 757, 1091, 1138, 1325, 1485, 1548, 1625, 2885, 2922, 3070, 3421. 1H NMR spectrum, δ, ppm: 7.76 (1H, s, NH); 7.33–7.26 (1H, m, H Ar); 7.20–7.12 (1H, m, H Ar); 6.98–6.90 (1H, m, H Ar); 6.82–6.76 (1H, m, H Ar); 4.52 (1H, s, 11-CH); 2.30 (2H, s, 13-CH2); 2.02 (3H, s, 2-CH3); 1.91 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 157.3 (C); 153.9 (C); 151.0 (C); 130.0 (CH); 129.4 (CH); 124.4 (C); 121.2 (CH); 116.7 (CH); 82.3 (C); 44.3 (CH); 32.3 (CH2); 24.0 (CH3); 14.2 (CH3). Mass spectrum, m/z: 242 [M]+. Found, %: C 64.34; H 5.54; N 23.29. C13H14N4O. Calculated, %: C 64.45; H 5.82; N 23.12.
7-Methoxy-2,5-dimethyl-11,12-dihydro-5,11-methano-[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11b). Yield 51%, mp 279–280°C. IR spectrum, ν, cm–1: 734, 922, 1055, 1078, 1183, 1265, 1328, 1452, 1482, 1582, 1626, 2838, 2923, 3076, 3420. 1H NMR spectrum, δ, ppm: 7.80 (1H, s, NH); 6.95–6.79 (3H, m, H Ar); 4.50 (1H, s, 11-CH); 3.70 (3H, s, OCH3); 2.32–2.22 (2H, m, 13-CH2); 2.02 (3H, s, 2-CH3); 1.95 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 157.6; 154.3; 148.4; 140.8; 125.2; 121.5; 121.3; 111.9; 82.5; 55.7; 44.7; 32.6; 24.4; 14.6. Mass spectrum, m/z: 272 [M]+. Found, %: C 61.64; H 5.46; N 20.96. C14H16N4O2. Calculated, %: C 61.75; H 5.92; N 20.57.
7-Ethoxy-2,5-dimethyl-11,12-dihydro-5,11-methano-[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11c). Yield 55%, mp 259–260°C. IR spectrum, ν, cm–1: 628, 850, 1031, 1088, 1129, 1269, 1332, 1504, 1552, 1625, 2944, 3070, 3141, 3226. 1H NMR spectrum, δ, ppm (J, Hz): 7.89 (1H, s, NH); 6.94–6.79 (3H, m, H Ar); 4.49 (1H, br. s, 11-CH); 3.95 (2H, q, J = 6.7, OCH2CH3); 2.31–2.21 (2H, m, 13-CH2); 2.01 (3H, s, 2-CH3); 1.94 (3H, s, 5-CH3); 1.30 (3H, t, J = 6.8, OCH2CH3). 13C NMR spectrum, δ, ppm: 157.6; 154.2; 147.6; 141.0; 125.3; 121.6; 121.3; 113.1; 82.5; 64.1; 44.7; 32.6; 24.4; 15.1; 14.6. Mass spectrum, m/z: 286 [M]+. Found, %: C 62.91; H 5.98; N 19.36. C15H18N4O2. Calculated, %: C 62.92; H 6.34; N 19.57.
8-Methoxy-2,5-dimethyl-11,12-dihydro-5,11-methano-[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11d). Yield 35%, mp 263–264°C. IR spectrum, ν, cm–1: 734, 922, 1055, 1078, 1183, 1265, 1328, 1452, 1482, 1582, 1626, 2838, 2923, 3076, 3420. 1H NMR spectrum, δ, ppm (J, Hz): 7.81 (1H, br. s, NH); 7.21–7.13 (1H, m, H Ar); 6.58–6.48 (1H, m, H Ar); 6.41–6.33 (1H, m, H Ar); 4.46 (1H, d, J = 2.7, 11-CH); 3.67 (3H, s, OCH3); 2.32–2.22 (2H, m, 13-CH2); 2.01 (3H, s, 2-CH3); 1.92 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 160.1; 157.3; 154.0; 152.0; 130.4; 116.7; 108.2; 101.2; 82.3; 55.2; 43.9; 32.6; 23.9; 14.2. Mass spectrum, m/z: 272 [M]+. Found, %: C 61.34; H 5.54; N 20.29. C14H16N4O2. Calculated, %: C 61.75; H 5.92; N 20.58.
9-Bromo-2,5-dimethyl-11,12-dihydro-5,11-methano-[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11e). Yield 53%, mp 280–281°C. IR spectrum, ν, cm–1: 641, 762, 827, 868, 1090, 1138, 1326, 1473, 1484, 1547, 1619, 2938, 2990, 3065, 3420. 1H NMR spectrum, δ, ppm: 7.90–7.83 (1H, m, NH); 7.49–7.44 (1H, m, H Ar); 7.33–7.29 (1H, m, H Ar); 6.80–6.74 (1H, m, H Ar); 4.58–4.53 (1H, m, 11-CH); 2.35–2.25 (2H, m, 13-CH2); 2.00 (3H, s, 2-CH3); 1.91 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 157.8; 154.2; 150.7; 132.6; 132.3; 127.2; 119.5; 112.8; 83.0; 44.3; 32.2; 24.1; 14.5. Mass spectrum, m/z: 322 [M(81Br)]+, 320 [M(79Br)]+. Found, %: C 48.50; H 3.90; N 17.25. C13H13BrN4O. Calculated, %: C 48.62; H 4.08; N 17.44.
5-Methyl-2-pentyl-11,12-dihydro-5,11-methano[1,2,4]-triazolo[1,5- c ][1,3,5]benzoxadiazocine (11f). Yield 55%, mp 209–210°C. IR spectrum, ν, cm–1: 757, 1091, 1138, 1325, 1485, 1548, 1625, 2885, 2922, 3070, 3421. 1H NMR spectrum, δ, ppm (J, Hz): 7.88 (1H, s, NH); 7.32–7.25 (1H, m, H Ar); 7.20–7.13 (1H, m, H Ar); 6.98–6.91 (1H, m, H Ar); 6.85–6.78 (1H, m, H Ar); 4.52 (1H, d, J = 2.8, 11-CH); 2.34 (2H, t, J = 7.7, CH2(CH2)3CH3); 2.30 (2H, s, 13-CH2); 1.93 (3H, s, 5-CH3); 1.58–1.47 (2H, m) and 1.32–1.19 (4H, m, CH2(CH2)3CH3); 0.83 (3H, t, J = 7.7, (CH2)4CH3). 13C NMR spectrum, δ, ppm: 161.1; 153.9; 151.0; 129.9; 129.3; 124.4; 121.2; 116.7; 82.4; 44.3; 32.3; 31.1; 28.2; 27.2; 23.9; 21.9; 13.9. Mass spectrum, m/z: 298 [M]+. Found, %: C 67.92; H 6.90; N 18.53. C17H22N4O. Calculated, %: C 68.43; H 7.43; N 18.78.
7-Methoxy-5-methyl-2-pentyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11g). Yield 57%, mp 227–228°C. IR spectrum, ν, cm–1: 742, 918, 1071, 1266, 1486, 1546, 1611, 2858, 2945, 3063, 3208. 1H NMR spectrum, δ, ppm (J, Hz): 7.88 (1H, s, NH); 6.96–6.80 (3H, m, H Ar); 4.50 (1H, br. s, 11-CH); 3.70 (3H, s, OCH3); 2.33 (2H, t, J = 7.4, CH2(CH2)3CH3); 2.27 (2H, s, 13-CH2); 1.94 (3H, s, 5-CH3); 1.58–1.48 (2H, m) and 1.32–1.19 (4H, m, CH2(CH2)3CH3); 0.88–0.79 (3H, m, (CH2)4CH3). 13C NMR spectrum, δ, ppm: 161.0 (C); 153.8 (C); 148.0 (C); 140.4 (C); 124.9 (C); 121.2 (CH); 121.0 (CH); 111.6 (CH); 82.2 (C); 55.4 (CH3); 44.3 (CH); 32.3 (CH2); 31.2 (CH2); 28.2 (CH2); 27.2 (CH2); 24.0 (CH3); 21.9 (CH2); 14.0 (CH3). Mass spectrum, m/z: 328 [M]+. Found, %: C 66.19; H 7.65; N 17.20. C18H24N4O2. Calculated, %: C 65.83; H 7.37; N 17.06.
7-Ethoxy-5-methyl-2-pentyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11h). Yield 62%, mp 209–210°C. IR spectrum, ν, cm–1: 742, 924, 1049, 1068, 1195, 1262, 1472, 1544, 1625, 2885, 2938, 3065, 3237, 3420. 1H NMR spectrum, δ, ppm (J, Hz): 7.82 (1H, s, NH); 6.93–6.79 (3H, m, H Ar); 4.50 (1H, br. s, 11-CH); 4.05–3.90 (2H, m, OCH2CH3); 2.35 (2H, t, J = 7.0, CH2(CH2)3CH3); 2.27 (2H, s, 13-CH2); 1.94 (3H, s, 5-CH3); 1.58–1.49 (2H, m) and 1.34–1.21 (7H, m, CH2(CH2)3CH3, OCH2CH3); 0.88–0.79 (3H, m, (CH2)4CH3). 13C NMR spectrum, δ, ppm: 161.0; 153.7; 147.2; 140.7; 125.0; 121.3; 120.9; 112.9; 82.2; 63.8; 44.3; 32.2; 31.1; 28.2; 27.2; 24.1; 21.9; 14.7; 13.9. Mass spectrum, m/z: 342 [M]+. Found, %: C 66.36; H 7.25; N 15.95. C19H26N4O2. Calculated, %: C 66.64; H 7.65; N 16.36.
8-Methoxy-5-methyl-2-pentyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11i). Yield 31%, mp 169–170°C. IR spectrum, ν, cm–1: 850, 977, 1092, 1132, 1270, 1445, 1502, 1614, 1620, 2937, 3067, 3017. 1H NMR spectrum, δ, ppm (J, Hz): 7.79 (1H, br. s, NH); 7.22–7.12 (1H, m, H Ar); 6.57–6.48 (1H, m, H Ar); 6.40–6.34 (1H, m, H Ar); 4.46 (1H, d, J = 2.7, 11-CH); 3.67 (3H, s, OCH3); 2.33 (2H, t, J = 7.4, CH2(CH2)3CH3); 2.27 (2H, s, 13-CH2); 1.92 (3H, s, 5-CH3); 1.58–1.48 (2H, m) and 1.33–1.19 (4H, m, CH2(CH2)3CH3); 0.90–0.78 (3H, m, (CH2)4CH3). 13C NMR spectrum, δ, ppm: 162.0; 160.1; 153.9; 152.0; 130.5; 116.7; 108.2; 101.2; 82.4; 55.2; 43.9; 32.6; 31.1; 28.3; 27.2; 23.9; 21.9; 13.9. Mass spectrum, m/z: 328 [M]+. Found, %: C 65.91; H 7.88; N 17.36. C18H24N4O2. Calculated, %: C 65.83; H 7.37; N 17.06.
9-Bromo-5-methyl-2-pentyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11j). Yield 60%, mp 236–238°C. 1H NMR spectrum, δ, ppm: 7.83 (1H, s, NH); 7.49–7.43 (1H, m, H Ar); 7.34–7.26 (1H, m, H Ar); 6.82–6.74 (1H, m, H Ar); 4.55 (1H, br. s, 11-CH); 2.39–2.24 (4H, m, 13-CH2, CH2(CH2)3CH3); 1.91 (3H, s, 5-CH3); 1.57–1.42 (2H, m) and 1.29–1.14 (4H, m, CH2(CH2)3CH3); 0.86–0.75 (3H, m, (CH2)4CH3). 13C NMR spectrum, δ, ppm: 161.6 (C); 154.1 (C); 150.7 (C); 132.6 (CH); 132.3 (CH); 127.3 (C); 119.5 (CH); 112.8 (C); 83.1 (C); 44.4 (CH); 32.2 (CH2); 31.5 (CH2); 28.6 (CH2); 27.6 (CH2); 24.1 (CH3); 22.3 (CH2); 14.3 (CH3). Found, %: C 54.32; H 5.26; N 14.50. C17H21BrN4O. Calculated, %: C 54.12; H 5.61; N 14.85.
5-Methyl-2-phenyl-11,12-dihydro-5,11-methano[1,2,4]-triazolo[1,5- c ][1,3,5]benzoxadiazocine (11k). Yield 52%, mp 295–297°C. IR spectrum, ν, cm–1: 686, 735, 1085, 1147, 1344, 1444, 1621, 2873, 2959, 3039, 3429. 1H NMR spectrum, δ, ppm: 8.24–8.19 (1H, m, NH); 7.91–7.87 (2H, m, H Ar); 7.43–7.30 (4H, m, H Ar); 7.20–7.14 (1H, m, H Ar); 6.99–6.93 (1H, m, H Ar); 6.87–6.82 (1H, m, H Ar); 4.63–4.59 (1H, m, 11-CH); 2.39 (2H, s, 13-CH2); 2.04 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 158.6; 154.9; 151.3; 131.8; 130.3; 129.8; 129.3; 128.9 (2C); 126.0 (2C); 124.6; 121.8; 117.1; 83.3; 44.7; 32.6; 24.3. Found, %: C 71.21; H 5.12; N 18.24. C18H16N4O. Calculated, %: C 71.04; H 5.30; N 18.41.
7-Methoxy-5-methyl-2-phenyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11l). Yield 53%, mp 320–321°C. IR spectrum, ν, cm–1: 650, 691, 732, 1075, 1267, 1344, 1439, 1485, 1614, 2931, 2961, 3112, 3207. 1H NMR spectrum, δ, ppm: 8.23–8.17 (1H, m, NH); 7.90–7.85 (2H, m, H Ar); 7.61–7.56 (3H, m, H Ar); 6.92–6.82 (3H, m, H Ar); 4.63–4.59 (1H, m, 11-CH); 3.70 (3H, s, OCH3); 2.43–2.32 (2H, m, 13-CH2); 2.04 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 158.5; 154.8; 148.4; 140.6; 131.8; 129.3; 128.9 (2C); 126.0 (2C); 125.1; 121.5; 121.4; 112.0; 83.3; 55.7; 44.7; 32.6; 24.3. Mass spectrum, m/z: 334 [M]+. Found, %: C 68.21; H 5.18; N 16.54. C19H18N4O2. Calculated, %: C 68.25; H 5.43; N 16.76.
7-Ethoxy-5-methyl-2-phenyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11m). Yield 55%, mp 269–271°C. IR spectrum, ν, cm–1: 691, 743, 1045, 1071, 1115, 1266, 1343, 1470, 1504, 1582, 1612, 2922, 2980, 3059, 3173, 3220, 3502. 1H NMR spectrum, δ, ppm (J, Hz): 8.06 (1H, br. s, NH); 7.90–7.85 (2H, m, H Ar); 7.47–7.30 (3H, m, H Ar); 6.98–6.78 (3H, m, H Ar); 4.59 (1H, br. s, 11-CH); 4.04–3.91 (2H, m, OCH2CH3); 2.41–2.34 (2H, m, 13-CH2); 2.14–2.04 (3H, m, 5-CH3); 1.30 (3H, t, J = 6.3, OCH2CH3). 13C NMR spectrum, δ, ppm: 158.1 (C); 154.4 (C); 147.3 (C); 140.4 (C); 131.4 (C); 129.0 (CH); 128.6 (2CH); 125.7 (2CH); 124.9 (C); 121.2 (CH); 121.1 (CH); 112.8 (CH); 82.7 (C); 63.7 (CH2); 44.3 (CH); 32.1 (CH2); 24.1 (CH3); 14.7 (CH3). Mass spectrum, m/z: 348 [M]+. Found, %: C 68.66; H 5.61; N 15.86. C20H20N4O2. Calculated, %: C 68.95; H 5.79; N 16.08.
9-Bromo-5-methyl-2-phenyl-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11n). Yield 57%, mp 284–285°C. IR spectrum, ν, cm–1: 699, 754, 1047, 1138, 1342, 1480, 1615, 2885, 2925, 3056, 3421. 1H NMR spectrum, δ, ppm: 8.15 (1H, s, NH); 7.91–7.83 (2H, m, H Ar); 7.50 (1H, s, H Ar); 7.42–7.28 (4H, m, H Ar); 6.85–6.78 (1H, m, H Ar); 4.64 (1H, br. s, 11-CH); 2.46–2.33 (2H, m, 13-CH2); 2.03 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 158.7; 154.8; 150.7; 132.7; 132.4; 131.7; 129.4; 128.9 (2C); 127.1; 126.0 (2C); 119.6; 113.0; 83.6; 44.3; 32.1; 24.1. Found, %: C 56.04; H 4.05; N 15.04. C18H15BrN4O. Calculated, %: C 56.41; H 3.95; N 14.62.
5-Methyl-2-(pyridin-2-yl)-11,12-dihydro-5,11-methano-[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11o). Yield 52%, mp 338–337°C. IR spectrum, ν, cm–1: 647, 754, 1089, 1131, 1345, 1481 1586, 2869, 2962, 3040, 3142, 3200. 1H NMR spectrum, δ, ppm: 8.57 (1H, br. s, NH); 8.30–8.19 (1H, m, H Ar); 7.94–7.74 (2H, m, H Ar); 7.40–7.26 (2H, m, H Ar); 7.21–7.11 (1H, m, H Ar); 7.02–6.90 (1H, m, H Ar); 6.87–6.76 (1H, m, H Ar); 4.67–4.56 (1H, m, 11-CH); 2.40 (2H, br. s, 13-CH2); 2.03 (3H, s, 5-CH3). Mass spectrum, m/z: 305 [M]+. Found, %: C 67.13; H 4.51; N 23.09. C17H15N5O. Calculated, %: C 66.87; H 4.95; N 22.94.
7-Methoxy-5-methyl-2-(pyridin-2-yl)-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11p). Yield 54%, mp 333–334°C. IR spectrum, ν, cm–1: 750, 1048, 1131, 1266, 1483, 1605, 2931, 2962, 3061, 3154, 3396. 1H NMR spectrum, δ, ppm: 8.61–8.56 (1H, m, NH); 8.30–8.25 (1H, m, H Ar); 7.91–7.78 (2H, m, H Ar); 7.38–7.32 (1H, m, H Ar); 6.91–6.81 (3H, m, H Ar); 4.63–4.58 (1H, m, 11-CH); 3.69 (3H, s, OCH3); 2.41–2.36 (2H, m, 13-CH2); 2.04 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 155.1 (2C); 154.3; 147.6; 140.7; 130.0; 127.2; 124.9; 124.5; 124.1; 121.7; 121.5; 113.2; 83.4; 64.1; 44.4; 32.1; 24.3. Mass spectrum, m/z: 335 [M]+. Found, %: C 64.17; H 4.81; N 20.83. C18H17N5O2. Calculated, %: C 64.47; H 5.11; N 20.88.
7-Ethoxy-5-methyl-2-(pyridin-2-yl)-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11q). Yield 53%, mp 333–334°C. IR spectrum, ν, cm–1: 598, 744, 1049, 1198, 1266, 1271, 1346, 1474, 1504, 1582, 1621, 2971, 3095, 3421. 1H NMR spectrum, δ, ppm (J, Hz): 8.65–8.56 (1H, m, NH); 8.50–8.41 (1H, m, H Ar); 7.96–7.86 (1H, m, H Ar); 7.85–7.77 (1H, m, H Ar); 7.41–7.31 (1H, m, H Ar); 6.94–6.77 (3H, m, H Ar); 4.67–4.58 (1H, m, 11-CH); 4.00–3.86 (2H, m, OCH2CH3); 2.45–2.32 (2H, m, 13-CH2); 2.10–2.01 (3H, m, 5-CH3); 1.29 (3H, t, J = 6.8, OCH2CH3). 13C NMR spectrum, δ, ppm: 158.5; 154.9; 150.3; 149.8; 147.6; 140.8; 137.2; 125.2; 124.2; 121.8; 121.6; 121.5; 113.1; 83.2; 64.1; 44.7; 32.4; 24.4; 15.1. Mass spectrum, m/z: 349 [M]+. Found, %: C 65.51; H 5.26; N 20.16. C19H19N5O2. Calculated, %: C 65.32; H 5.48; N 20.04.
5-Methyl-2-(pyridin-3-yl)-11,12-dihydro-5,11-methano-[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11r). Yield 60%, mp 280–282°C. 1H NMR spectrum, δ, ppm: 9.02 (1H, s, H Ar); 8.59–8.50 (1H, m, NH); 8.31 (1H, s, H Ar); 8.20–8.11 (1H, m, H Ar); 7.45–7.37 (1H, m, H Ar); 7.34–7.26 (1H, m, H Ar); 7.21–7.11 (1H, m, H Ar); 6.99–6.90 (1H, m, H Ar); 6.86–6.79 (1H, m, H Ar); 4.62 (1H, br. s, 11-CH); 2.40 (2H, br. s, 13-CH2); 2.04 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 156.5 (C); 155.0 (C); 151.2 (C); 150.3 (CH); 147.2 (CH); 133.3 (CH); 130.3 (CH); 129.9 (CH); 127.5 (C); 124.5 (C); 124.2 (CH); 121.9 (CH); 117.1 (CH); 83.4 (C); 44.6 (CH); 32.5 (CH2); 24.2 (CH3). Mass spectrum, m/z: 305 [M]+. Found, %: C 66.55; H 4.99; N 22.51. C17H15N5O. Calculated, %: C 66.87; H 4.95; N 22.94.
7-Methoxy-5-methyl-2-(pyridin-3-yl)-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11s). Yield 62%, mp 280–281°C. IR spectrum, ν, cm–1: 753, 1072, 1270, 1484, 1583, 2941, 2962, 3017, 3189, 3420. 1H NMR spectrum, δ, ppm: 9.02 (1H, s, H Ar); 8.59–8.51 (1H, m, NH); 8.32 (1H, s, H Ar); 8.21–8.12 (1H, m, H Ar); 7.47–7.37 (1H, m, H Ar); 6.94–6.78 (3H, m, H Ar); 4.60 (1H, br. s, 11-CH); 3.69 (3H, s, OCH3); 2.37 (2H, br. s, 13-CH2); 2.04 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 156.5; 154.9; 150.3; 148.4; 147.1; 140.6; 133.3; 127.5; 125.0; 124.2; 121.6; 121.5; 112.0; 83.2; 55.7; 44.6; 32.4; 24.3. Mass spectrum, m/z: 335 [M]+. Found, %: C 64.21; H 5.14; N 20.51. C18H17N5O2. Calculated, %: C 64.47; H 5.11; N 20.88.
7-Ethoxy-5-methyl-2-(pyridin-3-yl)-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11t). Yield 60%, mp 248–249°C. IR spectrum, ν, cm–1: 750, 1050, 1069, 1199, 1268, 1470, 1504, 1582, 2978, 3080, 3369. 1H NMR spectrum, δ, ppm (J, Hz): 9.10 (1H, br. s, H Ar); 8.86–8.82 (1H, m, NH); 8.76–8.71 (1H, m, H Ar); 8.65–8.57 (1H, m, H Ar); 7.98–7.92 (1H, m, H Ar); 6.92–6.80 (3H, m, H Ar); 4.65 (1H, br. s, 11-CH); 3.93 (2H, q, J = 6.9, OCH2CH3); 2.44–2.34 (2H, m, 13-CH2); 2.07 (3H, s, 5-CH3); 1.29 (3H, t, J = 6.9, OCH2CH3). 13C NMR spectrum, δ, ppm: 159.9 (2C); 154.3; 147.6 (2C); 140.6; 140.1; 130.0; 127.2; 125.0; 121.7; 121.6; 113.2; 83.4; 64.1; 44.4; 32.1; 24.3; 15.1. Mass spectrum, m/z: 349 [M]+. Found, %: C 65.00; H 5.14; N 19.96. C19H19N5O2. Calculated, %: C 65.32; H 5.48; N 20.04.
5-Methyl-2-(pyridin-4-yl)-11,12-dihydro-5,11-methano-[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11u). Yield 48%, mp 255–257°C. 1H NMR spectrum, δ, ppm: 8.62–8.54 (2H, m, H Ar); 8.39–8.28 (1H, m, NH); 7.80–7.72 (2H, m, H Ar); 7.05–6.90 (4H, m, H Ar); 4.62 (1H, br. s, 11-CH); 3.34 (2H, br. s); 2.40 (2H, br. s, 13-CH2); 2.03 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 158.7; 155.2; 151.1; 150.6 (2C); 138.8; 124.5; 121.9; 120.4 (2C); 120.2; 120.1; 117.1; 83.5; 44.6; 32.4; 24.2. Found, %: C 66.55; H 4.80; N 23.17. C17H15N5O. Calculated, %: C 66.87; H 4.95; N 22.94.
7-Methoxy-5-methyl-2-(pyridin-4-yl)-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11v). Yield 53%, mp 302–304°C. 1H NMR spectrum, δ, ppm: 8.64–8.55 (2H, m, H Ar); 8.40–8.30 (1H, m, NH); 7.82–7.71 (2H, m, H Ar); 6.93–6.80 (3H, m, H Ar); 4.61 (1H, br. s, 11-CH); 3.70 (3H, br. s, OCH3); 2.38 (2H, br. s, 13-CH2); 2.05 (3H, s, 5-CH3). 13C NMR spectrum, δ, ppm: 156.7 (C); 155.2 (C); 150.6 (2CH); 148.4 (C); 140.6 (C); 138.8 (C); 125.0 (C); 121.7 (CH); 121.5 (CH); 120.2 (2CH); 112.0 (CH); 83.3 (C); 55.7 (CH3); 44.5 (CH); 32.3 (CH2); 24.3 (CH3). Found, %: C 64.30; H 4.90; N 20.59. C18H17N5O2. Calculated, %: C 64.47; H 5.11; N 20.88.
7-Ethoxy-5-methyl-2-(pyridin-4-yl)-11,12-dihydro-5,11-methano[1,2,4]triazolo[1,5- c ][1,3,5]benzoxadiazocine (11w). Yield 58%, mp 273–274°C. 1H NMR spectrum, δ, ppm (J, Hz): 8.64–8.55 (2H, m, H Ar); 8.40–8.32 (1H, m, NH); 7.81–7.74 (2H, m, H Ar); 6.92–6.79 (3H, m, H Ar); 4.60 (1H, br. s, 11-CH); 3.93 (2H, q, J = 6.9, OCH2CH3); 2.38 (2H, br. s, 13-CH2); 2.05 (3H, s, 5-CH3); 1.28 (3H, t, J = 6.6, OCH2CH3). 13C NMR spectrum, δ, ppm: 158.7 (C); 155.1 (C); 150.6 (2CH); 147.6 (C); 140.7 (C); 138.8 (C); 125.1 (C); 121.7 (CH); 121.5 (CH); 120.2 (2CH); 113.2 (CH); 83.3 (C); 64.1 (CH2); 44.5 (CH); 32.3 (CH2); 24.3 (CH3); 15.1 (CH3). Found, %: C 64.88; H 5.41; N 20.23. C19H19N5O2. Calculated, %: C 65.32; H 5.48; N 20.04.
References
(a) Biginelli, P. Gazz. Chim. Ital. 1891, 21, 487. (b) Tron, G. C.; Minassi, A.; Appendino, G. Eur. J. Org. Chem. 2011, 5541.
(a) Kappe, C. O. Tetrahedron 1993, 49, 6937. (b) Suresh; Sandhu, J. S. ARKIVOC 2012, (i), 66. (c) Heravi, M. M.; Asadi, S.; Lashkariani, B. M. Mol. Diversity 2013, 17, 389. (d) Barbero, M.; Cadamuro, S.; Dughera, S. Green Chem. 2017, 19, 1529.
(a) Panda, S. S.; Khanna, P.; Khanna, L. Curr. Org. Chem. 2012, 16, 507. (b) Alvim, H. G. O.; Lima, T. B.; de Oliveira, A. L.; de Oliveira, H. C. B.; Silva, F. M.; Gozzo, F. C.; Souza, R. Y.; da Silva, W. A.; Neto, B. A. D. J. Org. Chem. 2014, 79, 3383.
(a) Kappe, C. O. Eur. J. Med. Chem. 2000, 35, 1043. (b) Slobbe, P.; Ruijter, E.; Orru, R. V. A. MedChemComm 2012, 3, 1189. (c) de Fátima, Â.; Braga, T. C.; Neto, L. d. S.; Terra, B. S.; Oliveira, B. G. F.; da Silva, D. L.; Modolo, L. V. J. Adv. Res. 2015, 6, 363. (d) Sepehri, S.; Sanchez, H. P.; Fassihi, A. J. Pharm. Pharm. Sci. 2015, 18, 1.
(a) Dondoni, A.; Massi, A. Acc. Chem. Res. 2006, 39, 451. (b) Isambert, N.; Lavilla, R. Chem.–Eur. J. 2008, 14, 8444. (c) Wan, J.-P.; Liu, Y. Synthesis 2010, 3943.
(a) Svetlik, J.; Hanus, V.; Bella, J. J. Chem. Res., Synop. 1991, 4. (b) Abbas, E. M. H.; Abdallah, S. M.; Abdoh, M. H.; Tawfik, H. A.; El-Hamouly, W. S. Turk. J. Chem. 2008, 32, 297. (c) Světlík, J.; Veizerová, L.; Kettmann, V. Tetrahedron Lett. 2008, 49, 3520. (d) El-Hamouly, W. S.; Tawfik, H. A.; Abbas, E. M. H. Green Chem. Lett. Rev. 2009, 2, 213. (e) Cheng, Q.; Wang, Q.; Xu, X.; Ruan, M.; Yao, H.; Yang, X. J. Heterocycl. Chem. 2010, 47, 624.
(a) Chebanov, V. A.; Gura, K. A.; Desenko, S. M. Top. Heterocycl. Chem. 2010, 23, 41. (b) Chebanov, V. A.; Desenko, S. M. Diversity-Oriented Synth. 2012, 1, 43.
(a) Svetlik, J.; Veizerová, L.; Mayer, T. U.; Catarinella, M. Bioorg. Med. Chem. Lett. 2010, 20, 4073. (b) Světlík, J.; Prónayová, N.; Švorc, L.; Frecer, V. Tetrahedron 2014, 70, 8354. (c) Tkachenko, V. V.; Muravyova, E. A.; Desenko, S. M.; Shishkin, O. V.; Shishkina, S. V.; Sysoiev, D. O.; Müller, T. J. J.; Chebanov, V. A. Beilstein J. Org. Chem. 2014, 10, 3019. (d) Murlykina, M. V.; Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoiev, D. O.; Kornet, M. N.; Schols, D.; Goeman, J. L.; Van der Eycken, J.; Van der Eycken, E. V.; Chebanov, V. A. Eur. J. Org. Chem. 2015, 4481. (e) Svĕtlík, J.; Prónayová, N.; Frecer, V.; Cież, D. Tetrahedron 2016, 72, 7620.
Sedash, Y. V.; Gorobets, N. Yu.; Chebanov, V. A.; Konovalova, I. S.; Shishkin, O. V.; Desenko, S. M. RSC Adv. 2012, 2, 6719.
(a) Gorobets, N. Yu.; Sedash, Y. V.; Ostras, K. S.; Zaremba, O. V.; Shishkina, S. V.; Baumer, V. N.; Shishkin, O. V.; Kovalenko, S. M.; Desenko, S. M.; Van der Eycken, E. V. Tetrahedron Lett. 2010, 51, 2095. (b) Kondratiuk, M.; Gorobets, N. Yu.; Sedash, Y. V.; Gümüş, M. K.; Desenko, S. M. Molbank 2016, 2016, M898.
Komykhov, S. A.; Bondarenko, A. A.; Musatov, V. I.; Diachkov, M. V.; Gorobets, N. Yu.; Desenko, S. M. Chem. Heterocycl. Compd. 2017, 53, 378. [Khim. Geterotsikl. Soedin. 2017, 53, 378.]
Světlík, J.; Kettmann, V. Tetrahedron Lett. 2011, 52, 1062.
Frolova, L. V.; Malik, I.; Uglinskii, P. Y.; Rogelj, S.; Kornienko, A.; Magedov, I. V. Tetrahedron Lett. 2011, 52, 6643.
Claridge, T. D. W.; Davies, S. G.; Polywka, M. E. C.; Roberts, P. M.; Russell, A. J.; Savory, E. D.; Smith, A. D. Org. Lett. 2008, 10, 5433.
Chen, Q.; Jiang, L.-L.; Chen, C.-N.; Yang, G.-F. J. Heterocycl. Chem. 2009, 46, 139.
(a) Kappe, C. O. J. Org. Chem. 1997, 62, 7201. (b) Alvim, H. G. O.; da Silva Júnior, E. N.; Neto, B. A. D. RSC Adv. 2014, 4, 54282.
(a) Muravyova, E. A.; Desenko, S. M.; Rudenko, R. V.; Shishkina, S. V.; Shishkin, O. V.; Sen'ko, Y. V.; Vashchenko, E. V.; Chebanov, V. A. Tetrahedron 2011, 67, 9389. (b) Sakhno, Y. I.; Desenko, S. M.; Shishkina, S. V.; Shishkin, O. V.; Sysoyev, D. O.; Groth, U.; Kappe, C. O.; Chebanov, V. A. Tetrahedron 2008, 64, 11041.
Gümüş, M. K.; Gorobets, N. Yu.; Sedash, Y. V.; Shishkina, S. V.; Desenko, S. M. Tetrahedron Lett. 2017, 58, 3446.
Cieplak, A. S. In Structure Correlation; Bürgi, H. B.; Dunitz, J. D., Eds.; Wiley-VCH: Weinheim, 2008, p. 205.
Gilmore, K.; Alabugin, I. V. Chem. Rev. 2011, 111, 6513.
Světlík, J.; Tureček, F.; Hanuš, V. J. Chem. Soc., Perkin Trans. 1 1987, 563.
The work was supported by Artvin Coruh University research project (BAP-2012.F19.02.24) and the Council of Higher Education of Turkey, Mevlana Exchange Program (MEV-2016-027).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing 1H and 13C NMR spectral data of the obtained compounds 11a–w is available at the journal website at http://springerlink.bibliotecabuap.elogim.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(11), 1261–1267
Electronic supplementary material
ESM 1
(PDF 3891 kb)
Rights and permissions
About this article
Cite this article
Gümüş, M.K., Gorobets, N.Y., Sedash, Y.V. et al. A modified Biginelli reaction toward oxygen-bridged tetrahydropyrimidines fused with substituted 1,2,4-triazole ring. Chem Heterocycl Comp 53, 1261–1267 (2017). https://doi.org/10.1007/s10593-018-2204-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2204-3