Abstract
A regioselective three-component reaction of α,β-unsaturated aldehydes, cyclic 1,3-dicarbonyls and 6-aminouracils in the presence of FeCl3·6H2O as catalyst under microwave irradiation has been demonstrated. Three-component reaction of α,β-unsaturated aldehydes like cinnamaldehyde/crotonaldehyde, cyclic 1,3-diketones such as 2-hydroxy-1,4-naphthaquinone/dimedone and 6-aminouracils provides regioselective pyrimidine-fused tetrahydropyridines tethered with cyclic 1,3-diketones. On the other hand, replacing cyclic 1,3-diketones by 4-hydroxycoumarin and keeping all other conditions the same provided a two-component pyrimidine-fused pyridines. The salient features of this methodology are operational simplicity, short reaction time, good-to-moderate yields of the products, easy purification method and regioselective products having medicinally important heterocyclic rings such as pyrimidine, tetrahydropyridine or pyridine.
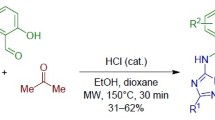
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) are one of the popular synthetic strategies in organic chemistry for the synthesis of large libraries of diverse heterocycles using minimum pot and steps [1,2,3,4,5]. Considering their easy execution, environment-friendly nature, better atom economy and green characteristics, MCRs have gained significant interest in organic synthesis. Microwave (MW) heating reduces reaction time drastically and many a time provides cleaner products compared to conventional heating [6,7,8,9]. Thus, the combination of MW in MCRs has many advantages in organic chemistry, especially for the synthesis of diverse biologically active heterocycles [10,11,12].
Fused heterocyclic compounds, especially pyrimidine-fused pyridine derivatives, are important molecules found in several natural and synthetic compounds with wide pharmacological applications. They exhibit anti-microbial [13,14,15], anti-inflammatory [16], anti-bacterial, [17] anti-tumour [18] and antifungal [19] activities. Pyrimidine-fused pyridine derivatives also find applications as versatile biological redox co-enzyme [20]. In addition, they have been identified as potential acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzyme inhibitors [21] as well as anticancer agents [22]. Some of the bioactive pyrimidine-fused pyridine derivatives along with their medicinal properties are shown in Fig. 1.
Considering the wide applications of these heterocyclic motifs, synthesis of pyrimidine-fused pyridine derivatives has found significant attention in recent years using multicomponent reactions. Recently, Zhang et al. [23] reported synthesis of a series of pyrido[2,3-d]pyrimidine derivatives using carbonaceous material as a catalyst from the reaction of 2,6-diaminopyrimidin-4(3H)-one, nitroolefin and aldehydes in water. Similarly, the multicomponent reaction of 2,4-thiazolidinedione, aromatic aldehydes and N,N-dimethyl-6-aminouracil was reported for the synthesis of pyrido[2,3-d]pyrimidine derivatives in the presence of heterogeneous ionic liquid catalyst [24]. Likewise, pyrido[2,3-d]pyrimidines were synthesized electrochemically using NaBr as supporting electrolyte and ethanol as solvent via multicomponent reaction of aromatic aldehydes, dimedone/malononitrile and 6-aminouracil [25]. Recently, we have reported the synthesis of pyrimidine-fused quinolines via the domino reaction of 6-aminouracils and 2-bromobenzaldehydes or 2-bromobenzyl bromide in the presence of Cu(II) catalyst [26]. We have also reported the synthesis of 1,4-dihydropyridines fused with naphthaquinone and pyrimidines from the three-component reaction of 2-hydroxy-1,4-naphthaquinone, aldehydes and 6-aminouracils in acetic acid/water (1:1; v/v) under microwave heating conditions [27]. FeCl3·6H2O is an inexpensive, environment-friendly, versatile catalyst used in various organic reactions [28,29,30,31]. In continuation of our research work [32,33,34,35,36,37] for the synthesis of diverse heterocycles, herein we report an efficient methodology for the synthesis of fused heterocycles from the reaction of α,β-unsaturated aldehydes, cyclic 1,3-dicarbonyls and 6-aminouracils in ethanol medium in the presence of FeCl3·6H2O as catalyst under microwave irradiation (Scheme 1).
Results and discussion
Initially, we have chosen the three-component reaction of 2-hydroxy-1,4-naphthaquinone (1a), cinnamaldehyde (2a) and 1,3-dimethyl-6-aminouracil (3a) as the model reaction.
In the absence of any catalyst, when we did the reaction under MW heating for 30 min in ethanol medium, we observed a three-component product with 50% yield (Table 1, entry 1).
MCRs can generate multiple products; thus, first, we tried to characterize the obtained product. In 1H NMR spectrum, two singlets of N-CH3 were observed at 3.13 and 3.29 ppm, respectively. In the aliphatic region, another four peaks were found with one hydrogen for each peak. Similarly, in 13C NMR, we observed two peaks for the N-CH3 at 27.8 and 29.7 ppm along with other three aliphatic peaks in 31.8, 35.8 and 43.6 ppm, respectively. Initially, we were not sure about the structure of our three-component product. From this three-component reaction, two regioisomers 4a or 4a′ (Scheme 2) are possible.
The structure of our product was unambiguously confirmed as 4a with the help of 1H, 13C and DEPT as well as 2D NMR experiments such as 1H–1H COSY, 1H–13C HSQC and 1H–13C HMBC NMR. Based on these experiments, the assignment of proton and carbons of 4a was done and is shown in Fig. 2a. Similarly, the structure of 4a with atom numbering, and HMBC correlation table are shown in Fig. 2b. In 1H–13C HMBC NMR spectra, the proton B is correlated with carbon nos. 6, 7, 10, 22, 26, 21, 4 and 9. On the other hand, the proton C is correlated with carbon nos. 6, 12, 13 and 11. This clearly proves that the phenyl ring is attached to carbon no. 5, and the 2-hydroxynaphthaquinone is attached to carbon no. 7. Therefore, the structure of our obtained product is 4a not 4a′. This interesting regioselectivity prompted us to fully study this reaction methodology by optimization of yield and checking substrate scope of the reaction.
Next, the same model reaction was tried in the presence of 10 mol% FeCl3·6H2O as catalyst without changing the solvent and microwave heating. Interestingly, in this case within 30 min, we ended with very good yield (85%) of 4a (Table 1, entry 2). We also tried our model reaction in the presence of 10 mol% FeCl3·6H2O in ethanol medium under reflux conditions by conventional heating. In this case, even after 8-h reflux, we observed only 74% yield (Table 1, entry 3). After having these results, the three-component reaction was screened under microwave irradiation in the presence of various other Lewis acid catalysts such as CeCl3·7H2O, I2 and Bi(NO3)3·5H2O (Table 1, entries 4–6). Among all these, the best result was observed in the presence of FeCl3·6H2O (Table 1, entry 2). We also screened the same reaction in various other solvents such as H2O, CH3CN, DMF, DMSO, DCM and toluene under microwave irradiation for 30 min (Table 1, entries 7–12). Among all the screened solvents, EtOH was found to be the best solvent for this reaction in terms of yield obtained.
After optimizing the reaction conditions, the generality and scope of this three-component reaction was studied by varying α,β-unsaturated aldehydes, cyclic 1,3-dicarbonyls and 6-aminouracils. The results are summarized in Fig. 3. Various α,β-unsaturated aldehydes such as 4-methoxycinnamaldehyde, 4-bromocinnamaldehyde, 4-fluorocinnamaldehyde, 4-nitrocinnamaldehyde and crotonaldehyde were tested with 2-hydroxy-1,4-naphthaquinone and 6-amino uracil derivatives, and the corresponding products 4a–4j were observed in good-to-moderate yields (Fig. 3). Both 6-aminouracil and 1,3-dimethyl-6-aminouracil are suitable for this reaction. Cyclic 1,3-diketone such as dimedone was also found suitable for this MCR and provided the corresponding products 4k–4m in good yields. Interestingly, when ortho-substituted cinnamaldehyde such as 2-nitro cinnamaldehyde was reacted with 6-amino uracil and 2-hydroxy-1,4-naphthaquinone under the similar reaction conditions, we ended up with a mixture of compounds (Scheme 3). Along with our expected product 4n, we have also isolated other two products 4n′ and 4n″.
Three-component reaction of cyclic 1,3-diketones, α,β-unsaturated aldehydes and 6-aminouracils. Reaction conditions: cyclic 1,3-dicarbonyls (1.0 mmol), α,β-unsaturated aldehydes (1.0 mmol) and 6-aminouracils (1.0 mmol) in 2.0 ml EtOH in the presence of 10 mol% FeCl3·6H2O in MW irradiation under sealed condition for 30 min
Encouraged by these results, next, we wanted to explore 4-hydroxycoumarin as cyclic 1,3-dicarbonyl derivative. When we performed the reaction of 4-hydroxycoumarin, cinnamaldehyde and 1,3-dimethyl-6-aminouracil under the optimized reaction conditions, surprisingly, we observed only a two-component product 5a rather than our expected three-component product 4o. 4-Hydroxycoumarin may be involved in the reaction, but got detached from the expected product as it is a very good leaving group and leads to the formation of two-component product 5a (Scheme 4).
Based on this observation, our next endeavour was to check the feasibility of this reaction with other α,β-unsaturated aldehydes such as 4-methoxycinnamaldehyde and crotonaldehyde with 6-aminouracils and the results are summarized in Fig. 4. All the reactions went smoothly and provided corresponding two-component products in moderate-to-good yields (Fig. 4).
Synthesis of two-component products 5 from the reaction of 4-hydroxycoumarin, α,β-unsaturated aldehydes and 6-aminouracils. Reaction conditions: 4-hydroxycoumarin (1.0 mmol), α,β-unsaturated aldehydes (1.0 mmol) and 6-aminouracils (1.0 mmol) in 2.0 ml EtOH in the presence of 10 mol% FeCl3·6H2O in MW irradiation under sealed condition for 30 min
The plausible reaction pathway for the formation of pyrimidine-fused tetrahydropyridines 4 from the three-component reaction of cyclic 1,3-dicarbonyls, α,β-unsaturated aldehydes and 6-aminouracils in the presence of FeCl3·6H2O as Lewis acid catalyst under microwave irradiation is illustrated in Scheme 5. It is believed that in the initial step, the cyclic 1,3-dicarbonyl 1 undergoes Knoevenagel-type reaction with the α,β-unsaturated aldehyde 2 to generate the intermediate A. Next, 6-aminouracil 3 attacks A by 1,6-addition to give the intermediate B which on tautomerization followed by 1,4-addition provides our desired three-component product 4.
Conclusions
In conclusion, we have developed an efficient microwave-assisted methodology for the synthesis of pyrimidine-fused tetrahydropyridines and pyridines by the three-component reaction of cyclic 1,3-dicarbonyls, α,β-unsaturated aldehydes and 6-aminouracils in ethanol in the presence of FeCl3·6H2O as catalyst. The key advantages of the present methodology include short reaction time, moderate-to-good yields of the products, operational simplicity, inexpensive starting materials and easy purification process. Considering the presence of bioactive pyrimidine-fused pyridine moiety in the products, it is expected that these molecules will show potent biological activity and will be useful in medicinal chemistry.
Experimental section
General
All the starting materials used in these reactions are commercially available. The capillary tube method was used for the determination of melting point of synthesized products. Shimadzu FTIR spectrophotometer was used to record IR. 1H and 13C NMR spectra were recorded in CDCl3 and DMSO-d6 on Bruker Avance II 400 MHz spectrophotometer. The chemical shifts of NMR are reported on the δ scale (ppm) downfield from tetramethyl silane (δ = 0.0 ppm). Data are reported as follows: chemical shift and multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = double doublet and brs = broad singlet). HRMS analysis data were recorded in Bruker Impact HD mass spectrometer. Initiator 2.5 Microwave Synthesizers from Biotage, Uppsala, Sweden, were used for performing the reactions.
General procedure for the synthesis of pyrimidine-fused tetrahydropyridines 4
To a mixture of cyclic 1,3-dicarbonyl (1.0 mmol) and α,β-unsaturated aldehyde (1.0 mmol) in a 5.0-ml reaction vial, 2.0 ml ethanol and FeCl3·6H2O (10 mol%) were added and the mixture was stirred for 10 min at room temperature. Then, 6-aminouracil (1.0 mmol) was added and the reaction mixture was irradiated in MW (74–75 W) under sealed and stirring condition for 30 min keeping the temperature at 100 °C. After completion of the reaction, the reaction mixture was cooled in an ice bath for 20 min, and solid precipitate was observed. The crude solid was separated by simple filtration and recrystallized in ethanol or ethyl acetate to obtain the pure product 4.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-1,3-dimethyl-5-phenylpyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (4a)
Yield: 85%; Red solid; mp: 302–303 °C; IR (ATR, cm−1): 3366, 3159, 1683, 1621, 1520, 1355, 1219, 999, 727; 1H NMR (400 MHz, DMSO): δ ppm; 1.81–1.84 (m, 1H), 2.44–2.49 (m, 1H), 3.13 (s, 3H), 3.29 (s, 3H), 4.18–4.19 (m, 1H), 4.60–4.64 (m, 1H), 6.89 (s, 1H), 7.21 (t, J = 8.0 Hz, 3H), 7.31 (t, J = 8.0 Hz, 2H), 7.79–7.82 (m, 1H), 7.85–7.88 (m, 1H), 7.96 (d, J = 8.0 Hz, 1H), 8.02 (d, J = 8.0 Hz, 1H), 11.36 (brs, 1H); 13C NMR (100 MHz, DMSO-d6): δ 27.8, 29.7, 31.8, 35.8, 43.6, 82.9, 121.2, 126.2, 126.3, 126.4, 128.1, 128.6, 130.2, 132.6, 133.7, 135.4, 146.1, 150.3, 151.6, 157.0, 160.8, 181.7, 183.6 ppm; HRMS (ESI-TOF) calcd for C25H22N3O5 [M + H]+ 444.1554, found 444.1560.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-5-phenylpyrido[2,3-d]pyrimidine-2,4 (1H,3H)-di one (4b)
Yield: 70%; Yellow solid; mp: 238–240 °C; IR (ATR, cm−1): 3379, 3230, 3199, 1695, 1546, 1536, 1432, 1328, 1254, 1093, 965, 876, 787; 1H NMR (400 MHz, DMSO-d6): δ 2.04–2.07 (m,1H), 2.34–2.41 (m, 1H), 3.81–3.84 (m, 1H), 4.81–4.83 (m, 1H), 6.25 (s, 1H), 6.88 (t, J = 8.0 Hz, 1H), 7.00–7.03 (m, 2H), 7.07–7.08 (m, 2H), 7.73–7.76 (m, 1H), 7.80–7.83 (m, 1H), 7.91 (t, J = 8.0 Hz, 2H), 10.09 (s, 1H), 10.12 (s, 1H), 11.22 (brs, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 36.2, 36.3, 47.0, 83.5, 120.8, 125.5, 125.9, 126.2, 127.0, 127.9, 130.0, 130.1, 132.6, 133.5, 135.1, 145.9, 150.9, 152.1, 163.0, 181.4, 183.9 ppm; HRMS (ESI-TOF) calcd for C23H18N3O5 [M + H]+ 416.1241, found 416.1236.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-5-(4-methoxyphenyl)-1,3-dimethyl pyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (4c)
Yield: 88%; Orange solid; mp: 209–210 °C; IR (ATR, cm−1): 3367, 3019, 1674, 1620, 1573, 1512, 1473, 1354, 1273, 1211, 1126, 999, 873, 736; 1H NMR (400 MHz, DMSO-d6): δ 1.76–1.80 (m, 1H), 2.40–2.45 (m, 1H), 3.12 (s, 3H), 3.28 (s, 3H), 3.74 (s, 3H), 4.11–4.12 (m, 1H), 4.60–4.64 (m, 1H), 6.84 (brs, 1H), 6.86 (d, J = 8.0 Hz, 2H), 7.09 (d, J = 8.0 Hz, 2H), 7.80 (t, J = 8.0 Hz, 1H), 7.86 (t, J = 8.0 Hz, 1H), 7.96 (d, J = 8.0 Hz, 1H), 8.02 (d, J = 8.0 Hz, 1H), 11.35 (brs, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 27.3, 29.2, 31.4, 34.4, 43.1, 55.0, 82.8, 113.4, 120.8, 125.7, 125.9, 128.5, 129.7, 132.1, 133.2, 134.9, 137.5, 149.7, 151.1, 156.3, 157.5, 160.3, 181.1, 183.2 ppm; HRMS (ESI-TOF) calcd for C26H24N3O6 [M + H]+ 474.1660, found 474.1663.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-5-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (4d)
Yield: 75%; Brown solid; mp: 219–220 °C; IR (ATR, cm−1): 3367, 3275, 3212, 2945, 1693, 1593, 1539, 1473, 1338, 1273, 1215, 1056, 964, 852, 729; 1H NMR (400 MHz, DMSO-d6): δ 1.72–1.75 (m, 1H), 2.36–2.44 (m, 1H), 3.74 (s, 3H), 3.98–3.99 (m, 1H), 4.51–4.55 (m, 1H), 6.20 (s, 1H), 6.87 (d, J = 8.0 Hz, 2H), 7.06–7.09 (m, 2H), 7.79 (t, J = 8.0 Hz, 1H), 7.85 (t, J = 8.0 Hz, 1H), 7.94 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 9.99 (s, 1H), 10.22 (s, 1H), 11.46 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 32.0, 33.4, 42.3, 55.0, 82.0, 113.5, 120.3, 125.7, 125.9, 128.4, 129.7, 132.0, 133.2, 134.9, 137.5, 150.1, 150.5, 156.7, 157.4, 162.6, 181.0, 183.1 ppm; HRMS (ESI-TOF) calcd for C24H20N3O6 [M + H]+ 446.1347, found 446.1351.
5-(4-Bromophenyl)-5,6,7,8-tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-1,3-dimethylpyrido [2,3-d]pyrimidine-2,4(1H,3H)-dione (4e)
Yield: 80%; Orange solid; mp: 264–265 °C; IR (ATR, cm−1): 3369, 3257, 1674, 1616, 1521, 1473, 1355, 1273, 1161, 1037, 999, 837, 734; 1H NMR (400 MHz, DMSO-d6): δ 1.79–1.82 (m, 1H), 2.45–2.49 (m, 1H), 3.13 (s, 3H), 3.28 (s, 3H), 4.15–4.16 (m, 1H), 4.56–4.60 (m, 1H), 6.92 (s, 1H), 7.16–7.19 (m, 2H), 7.49 (d, J = 8.0 Hz, 2H), 7.81 (t, J = 8.0 Hz, 1H), 7.87 (t, J = 8.0 Hz, 1H), 7.96 (d, J = 8.0 Hz, 1H), 8.02 (d, J = 8.0 Hz, 1H), 11.39 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 27.8, 29.7, 31.6, 35.4, 43.5, 82.6, 119.4, 121.0, 126.2, 126.4, 130.2, 130.4, 131.4, 132.6, 133.8, 135.4, 145.7, 150.4, 151.6, 156.9, 160.9, 181.6, 183.6 ppm; HRMS (ESI-TOF) calcd for C25H21 BrN3O5 [M + H]+ 522.0659, found 522.0661.
5-(4-Fluorophenyl)-5,6,7,8-tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-1,3-dimethylpyrido [2,3-d]pyrimidine-2,4(1H,3H)-dione (4f)
Yield: 84%; Yellow solid; mp: 248–250 °C; IR (ATR, cm−1): 3381, 3182, 1680, 1618, 1527, 1475, 1355, 1274, 1170, 1037, 999, 835, 731; 1H NMR (400 MHz, DMSO-d6): δ 1.79–1.82 (m, 1H), 2.44–2.48 (m, 1H), 3.13 (s, 3H), 3.28 (s, 3H), 4.18 (m, 1H), 4.57–4.61 (m, 1H), 6.90 (s, 1H), 7.10–7.15 (m, 2H), 7.23 (t, J = 8.0 Hz, 2H), 7.81 (t, J = 8.0 Hz, 1H), 7.87 (t, J = 8.0 Hz, 1H), 7.96–7.97 (m, 1H), 8.02 (d, J = 8.0 Hz, 1H), 11.38 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 27.8, 29.7, 31.9, 35.1, 43.5, 83.0, 115.1, 115.3, 121.2, 126.2, 126.4, 129.7, 129.8, 130.2, 132.6, 135.4, 142.2, 150.3, 151.6, 156.8, 159.9, 160.9, 162.3, 181.6, 183.7 ppm; HRMS (ESI-TOF) calcd for C25H21 FN3O5 [M + H]+ 462.1460, found 462.1469.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-1,3-dimethyl-5-(4-nitrophenyl)pyrido [2,3-d]pyrimidine-2,4(1H,3H)-dione (4g)
Yield: 78%; Orange solid; mp: 259–261 °C; IR (ATR, cm−1): 3233, 1673, 1592, 1504, 1437, 1378, 1302, 1237, 1056, 946, 849, 762; 1H NMR (400 MHz, DMSO-d6): δ 1.84–1.87 (m, 1H), 2.57–2.59 (m, 1H), 3.09 (s, 3H), 3.31 (s, 3H), 4.52–4.53 (m, 1H), 4.76–4.79 (m, 1H), 7.06 (s, 1H), 7.43 (d, J = 8.0 Hz, 1H), 7.50–7.53 (m, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.80–7.83 (m, 1H), 7.86–7.89 (m, 1H), 7.96 (t, J = 8.0 Hz, 2H), 8.03 (d, J = 8.0 Hz, 1H), 11.45 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 27.7, 29.8, 30.4, 32.3, 43.5, 82.6, 120.5, 124.9, 126.2, 126.4, 128.1, 130.7, 133.3, 133.7, 135.4, 140.3, 149.4, 151.0, 151.5, 157.3, 160.8, 181.6, 183.6 ppm; HRMS (ESI-TOF) calcd for C25H21N4O7 [M + H]+ 489.1405, found 489.1416.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-1,3-dimethyl-5-(2-nitrophenyl)pyrido [2,3-d]pyrimidine-2,4(1H,3H)-dione (4h)
Yield: 74%; Orange solid; mp: 218–220 °C; IR (ATR, cm−1): 3356; 3284, 2941, 1686, 1601, 1524, 1336, 1268, 1206, 1041, 727; 1H NMR (400 MHz, DMSO-d6): δ 1.87–1.91 (m, 1H), 2.58–2.65 (m, 1H), 3.10 (s, 3H), 3.31 (s, 3H), 4.56–4.57 (m, 1H), 4.79–4.83 (m, 1H), 7.01 (s, 1H), 7.42–7.45 (m, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.63–7.66 (m, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.83 (t, J = 8.0 Hz, 1H), 7.91 (d, J = 8.0 Hz, 1H), 8.00 (t, J = 8.0 Hz, 2H), 11.35 (brs, 1H) ppm; 13C NMR (100 MHz, CDCl3 + DMSO-d6): δ 27.1, 29.1, 29.9, 31.8, 43.0 82.3, 120.1, 124.3, 125.5, 125.9, 127.3, 129.6, 130.1, 132.0, 132.5, 132.9, 134.6, 139.9, 148.9, 150.4, 150.9, 156.5, 160.3, 181.0, 183.0 ppm; HRMS (ESI-TOF) calcd for C25H21N4O7 [M + H]+ 489.1405, found 489.1408.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-1,3,5-trimethyl pyrido[2,3-d]pyrimidine-2,4(1H, 3H)-dione (4i)
Yield: 76%; Red solid; mp: 247–249 °C; IR (ATR, cm−1): 3421, 2958, 1674, 1577, 1554, 1504, 1373, 1273, 1165, 941, 767; 1H NMR (400 MHz, DMSO-d6): δ 1.11 (d, J = 8.0 Hz, 3H), 1.55–1.58 (m, 1H), 2.15–2.20 (m, 1H), 2.92–2.98 (m, 1H), 3.14 (s, 3H), 3.21 (s, 3H), 4.93–4.97 (m, 1H), 6.77 (s, 1H), 7.83 (t, J = 8.0 Hz, 1H), 7.89 (t, J = 8.0 Hz, 1H), 8.02–8.07 (m, 2H), 11.41 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 21.2, 24.8, 27.7, 29.5, 30.6, 43.5, 86.3, 121.6, 126.2, 126.5, 130.2, 132.6, 133.8, 135.4, 149.1, 151.4, 156.9, 160.9, 181.7, 183.8 ppm; HRMS (ESI-TOF) calcd for C20H20N3O5 [M + H]+ 382.1397, found 382.1400.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-5-methylpyrido[2,3-d]pyrimidine-2,4 (1H,3H)-dione (4j)
Yield: 66%; Orange solid; mp: 224–226 °C; IR (ATR, cm−1): 3298, 2954, 2924, 1712, 1674, 1643, 1589, 1458, 1396, 1296, 1138, 1083, 983, 856, 775; 1H NMR (400 MHz, DMSO-d6): δ 1.08–1.09 (m, 3H), 1.50–1.53 (m, 1H), 2.11–2.16 (m, 1H), 2.79–2.83 (m, 1H), 4.84–4.88 (m, 1H), 6.09 (s, 1H), 7.82 (t, J = 8.0 Hz, 1H), 7.89 (t, J = 8.0 Hz, 1H), 8.01–8.04 (m, 2H), 9.83 (s, 1H), 10.14 (s, 1H), 11.56 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 20.9, 23.4, 30.6, 42.3, 85.2, 120.6, 125.7, 125.9, 129.8, 132.0, 133.3, 134.8, 149.1, 150.3, 156.6, 162.8, 181.0, 183.3 ppm; HRMS (ESI-TOF) calcd for C18H16N3O5 [M + H]+ 354.1084, found 354.1077.
5,6,7,8-Tetrahydro-7-(2-hydroxy-4,4-dimethyl-6-oxocyclo hex-1-enyl)-1,3-dimethyl-5-phenylpyrido[2,3d]pyrimidine-2,4(1H, 3H)-dione (4k)
Yield: 80%; Colourless solid; mp: 242–244 °C; IR (ATR, cm−1): 3264, 2955, 2870, 1692,1689, 1589, 1533, 1469, 1350, 1254, 1172, 1003, 887, 756; 1H NMR (400 MHz, DMSO-d6): δ 0.98 (s, 6H), 1.56–1.60 (m, 1H), 2.19 (s, 4H), 2.33–2.38 (m, 1H), 3.10 (s, 3H), 3.30 (s, 3H), 4.06–4.07 (m, 1H), 4.37–4.41 (m, 1H), 6.53 (s, 1H), 7.13–7.17 (m, 3H), 7.26 (t, J = 8.0 Hz, 2H), 10.72 (brs, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 27.2, 27.9, 29.1, 31.3, 31.9, 35.5, 42.4, 82.7, 111.9, 125.6, 127.5, 127.9, 146.0, 150.2, 151.2, 160.4 ppm; HRMS (ESI-TOF) calcd for C23H28N3O4 [M + H]+ 410.2074, found 410.2094.
5,6,7,8-Tetrahydro-7-(2-hydroxy-4,4-dimethyl-6-oxocyclo hex-1-enyl)-5-(4-methoxyphenyl)-1,3-dimethylpyrido [2,3-d]pyrimidine-2,4(1H,3H)-dione (4l)
Yield: 84%; Colourless solid; mp: 312–315 °C; IR (ATR, cm−1): 3250, 2965, 2858, 1635, 1596, 1520, 1438, 1382, 1254, 1013, 956, 867, 735; 1H NMR (400 MHz, DMSO-d6): δ 0.98 (s, 6H), 1.53–1.56 (m, 1H), 2.19 (s, 4H), 2.27–2.34 (m, 1H), 3.10 (s, 3H), 3.29 (s, 3H), 3.72 (s, 3H), 3.99–4.00 (m, 1H), 4.36–4.40 (m, 1H), 6.51 (s, 1H), 6.82 (d, J = 8.0 Hz, 2H), 7.03 (d, J = 8.0 Hz, 2H), 10.75 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 27.2, 27.9, 29.1, 31.3, 32.1, 34.6, 42.4, 54.9, 83.0, 112.0, 113.3, 128.4, 137.9, 150.1, 151.2, 157.3, 160.3 ppm; HRMS (ESI-TOF) calcd for C24H30N3O5 [M + H]+ 440.2180, found 440.2172.
5-(4-Fluorophenyl)-5,6,7,8-tetrahydro-7-(2-hydroxy-4,4-dimethyl-6-oxocyclo hex-1-enyl)-1,3-dimethyl pyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (4m)
Yield: 78%; Yellow solid; mp: 248–250 °C; IR (ATR, cm−1): 3280, 2952, 2931, 1687, 1586, 1537, 1471, 1380. 1310, 1209, 1006, 859,775; 1H NMR (400 MHz, DMSO-d6): δ 0.99 (s, 6H), 1.55–1.58 (m, 1H), 2.20 (brs, 4H), 2.30–2.37 (m, 1H), 3.11 (s, 3H), 3.29 (s, 3H), 4.06 (s, 1H), 4.33–4.37 (m, 1H), 6.56 (s, 1H), 7.05–7.10 (m, 2H), 7.15–7.18 (m, 2H), 10.74 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 27.7, 28.4, 29.6, 31.8, 32.5, 35.3, 42.9, 83.1, 112.4, 114.9, 115.1, 129.7, 129.8, 142.6, 150.7, 151.7, 159.8, 160.8, 162.2 ppm; HRMS (ESI-TOF) calcd for C23H27 FN3O4 [M + H]+ 428.1980, found 428.1984.
5,6,7,8-Tetrahydro-7-(1,4-dihydro-2-hydroxy-1,4-dioxo naphthalen-3-yl)-5-(2-nitrophenyl)pyrido[2,3-d]pyrimidine-2,4 (1H,3H)-dione (4n)
Yield: 22%; Orange solid; mp: 221–223 °C; IR (ATR, cm−1): 3387, 3296, 3145, 1683, 1560, 1468, 1398, 1273, 1098, 947, 843, 749; 1H NMR (400 MHz, DMSO-d6): δ 1.81–1.85 (m, 1H), 2.56–2.58 (m, 1H), 4.47–4.48 (m, 1H), 4.67–4.70 (m, 1H), 6.38 (s, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.82 (t, J = 8.0 Hz, 1H), 7.92–7.95 (m, 2H), 8.01 (d, J = 8.0 Hz, 1H), 10.10 (s, 1H), 10.26 (s, 1H), 11.57 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 31.0, 31.2, 42.8, 81.7, 120.0, 125.0, 126.2, 126.4, 128.1, 130.3, 130.6, 132.6, 133.4, 133.7, 135.3, 140.1, 149.3, 150.9, 151.4, 157.5, 163.0, 181.5, 183.6 ppm; HRMS (ESI-TOF) calcd for C23H17N4O7 [M + H]+ 461.1092, found 461.1096.
(E)-5-(2-nitrostyryl)benzo[g]pyrimido[4,5-b]quinoline-2,4,6,11(1H,3H,5H,12H)-tetraone (4n′)
Yield: 45%; Red Solid; mp: 312–315 °C; IR (ATR, cm−1): 3382, 3259, 3133, 2802, 1702, 1579, 1378, 1268, 1015, 857, 721; 1H NMR (400 MHz, DMSO-d6): δ 4.78 (d, J = 8.0 Hz, 1H), 6.37–6.42 (m, 1H), 6.65 (d, J = 16 Hz, 1H), 7.45 (t, J = 8.0 Hz, 1H), 7.60 (t, J = 8.0 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.84 (t, J = 8.0 Hz, 1H), 7.87–7.92 (m, 2H), 8.03–––8.07 (m, 2H), 9.32 (s, 1H), 10.16 (s, 1H), 11.03 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 31.7, 86.4, 117.3, 124.7, 125.3, 126.4, 126.5, 128.6, 128.9, 130.6, 131.8, 132.4, 133.7, 133.9, 135.5, 136.1, 138.9, 144.6, 148.0, 149.9, 163.1, 179.4, 182.2 ppm; HRMS (ESI-TOF) calcd for C23H15N4O6 [M + H]+ 443.0986, found 443.0992.
(E)-13-(2-nitrostyryl)-5H-dibenzo[b,i]xanthene-5,7,12,14 (13 H)-tetraone (4n”)
Yield: 22%; Yellow Solid; mp: 198–202 °C; IR (ATR, cm−1): 2982, 1702, 1579, 1378, 1268, 1015, 857, 721; 1H NMR (400 MHz, DMSO-d6): δ 5.43 (d, J = 8.0 Hz, 1H), 6.72 (d, J = 16 Hz, 1H), 7.06 (dd, J = 16 Hz, J = 8.0 Hz, 1H), 7.46–7.49 (m, 1H), 7.70 (t, J = 8.0 Hz, 1H), 7.78 (t, J = 8.0 Hz, 2H), 7.86 (t, J = 8.0 Hz, 3H), 7.91 (d, J = 8.0 Hz, 1H), 7.98 (d, J = 8.0 Hz, 4H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 37.7, 121.9, 123.5, 124.7, 126.1, 126.5, 128.5, 128.8, 130.2, 132.5, 132.7, 133.6, 133.8, 135.2, 135.8, 147.8, 156.0, 181.6, 183.7 ppm; Anal. Calcd. For C29H15NO7 (489.43): C, 71.17; H, 3.09; N, 2.86%; Found: C, 71.22; H, 3.12; N, 2.98%. HRMS (ESI-TOF) calcd for C29H16NO7 [M + H]+ 490.0927, found 490.0930.
General procedure for the synthesis of pyrimidine-fused pyridines 5
To a mixture of 4-hydroxycoumarin (1.0 mmol) and α,β-unsaturated aldehyde (1.0 mmol) in a 5.0-ml reaction vial, 2.0 ml ethanol and FeCl3·6H2O (10 mol%) were added and the mixture was stirred for 10 min at room temperature. Then, 6-aminouracil (1.0 mmol) was added to the reaction mixture and it was irradiated in MW (74–75 W) under sealed and stirring condition for 30 min keeping temperature at 100 °C. After completion of the reaction, the reaction mixture was cooled in an ice bath for 20 min, and solid precipitate was observed. The crude solid was washed with ethanol to get the pure product 5.
1,3-Dimethyl-5-phenylpyrido[2,3-d]pyrimidine-2,4 (1H,3H)-dione (5a)
Yield: 80%; Pale Yellow Solid; mp: 224–226 °C; IR (ATR, cm−1): 2954, 1711, 1651, 1547, 1458, 1332, 1279, 1174, 999, 841; 1H NMR (400 MHz, DMSO-d6): δ 3.19 (s, 3H), 3.63 (s, 3H), 7.09–7.10 (m, 1H), 7.31–7.33 (m, 2H), 7.41–7.42 (m, 2H), 8.68–8.69 (m, 2H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 28.6, 30.3, 108.1, 122.2, 128.0, 128.3, 128.6, 139.7, 151.3, 152.1, 152.7, 153.6, 160.4 ppm; HRMS (ESI-TOF) calcd for C15H14N3O2 [M + H]+ 268.1081, found 268.1091.
5-Phenylpyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (5b)
Yield: 75%; Colourless Solid; mp: 330–333 °C; IR (ATR, cm−1): 3283, 3170, 2810, 1737, 1707, 1574, 1399, 1251, 843, 754 693; 1H NMR (400 MHz, DMSO-d6): δ 6.99 (d, J = 8.0 Hz, 1H), 7.33–7.35 (m, 2H), 7.38–7.41 (m, 3H), 8.54–8.55 (m, 1H), 11.21 (s, 1H), 11.67 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 107.5, 122.1, 127.9, 128.4, 128.8, 139.2, 150.6, 153.2, 153.4, 154.0, 162.0 ppm; HRMS (ESI-TOF) calcd for C13H10N3O2 [M + H]+ 240.0768, found 240.0766.
5-(4-Methoxyphenyl)pyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (5c)
Yield: 78%; Colourless Solid; mp: 358–360 °C; IR (ATR, cm−1): 3383, 3165, 2963, 1693, 1595, 1392, 1291, 1016, 811; 1H NMR (400 MHz, DMSO-d6): δ 3.81 (s, 3H), 6.95 (d, J = 8.0 Hz, 2H), 6.98 (d, J = 8.0 Hz, 1H), 7.30–7.33 (m, 2H), 8.50–8.51 (m, 1H), 11.19 (s, 1H), 11.61 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 55.6, 107.4, 113.4, 122.1, 130.6, 131.1, 150.6, 153.0, 153.3, 154.1, 159.8, 162.1 ppm; HRMS (ESI-TOF) calcd for C14H12N3O3 [M + H]+ 270.0873, found 270.0877.
1,3,5-Trimethyl pyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (5d)
Yield: 78%; Colourless Solid; mp: 330–333 °C; IR (ATR, cm−1): 2948, 1696, 1645, 1422, 1335, 1273, 1028, 855; 1H NMR (400 MHz, DMSO-d6): δ 2.70 (s, 3H), 3.25 (s, 3H), 3.52 (s, 3H), 7.12 (d, J = 8.0 Hz, 1H), 8.47–8.48 (m, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 22.2, 28.4, 30.1, 109.2, 122.5, 151.1, 151.8, 152.5, 152.7, 161.8 ppm; HRMS (ESI-TOF) calcd for C10H12N3O2 [M + H]+ 206.0924, found 206.0938.
5-Methylpyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (5e)
Yield: 75%; Colourless Solid; mp: 289–291 °C; IR (ATR, cm−1): 3389, 3159, 2989, 1666, 1580, 1398, 1267, 1025, 841; 1H NMR (400 MHz, DMSO-d6): δ 2.68 (s, 3H), 7.03–7.04 (m, 1H), 8.36–8.37 (m, 1H), 11.27 (s, 1H), 11.50 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d6): δ 21.6, 108.7, 122.2, 150.5, 152.3, 153.3, 153.8, 163.7 ppm; HRMS (ESI-TOF) calcd for C8H8N3O2 [M + H]+ 178.0611, found 178.0604.
References
Boukis AC, Reiter K, Frolich M, Hofheinz D, Meier MAR (2018) Multicomponent reactions provide key molecules for secret communication. Nat Commun 9:1439–1448. https://doi.org/10.1038/s41467-018-03784-x
Touré BB, Hall DG (2009) Natural product synthesis using multicomponent reaction strategies. Chem Rev 109:4439–4486. https://doi.org/10.1021/cr800296p
Ganem B (2009) Strategies for innovation in multicomponent reaction design. Acc Chem Res 42:463–472. https://doi.org/10.1021/ar800214s
Singh MS, Chowdhury S (2012) Recent developments in solvent-free multicomponent reactions: a perfect synergy for eco-compatible organic synthesis. RSC Adv 2:4547–4592. https://doi.org/10.1039/C2RA01056A
Levi L, Müller TJJ (2016) Multicomponent syntheses of functional chromophores. Chem Soc Rev 45:2825–2846. https://doi.org/10.1039/C5CS00805K
Lidström P, Tierney J, Wathey B, Westman J (2001) Microwave assisted organic synthesis—a review. Tetrahedron 57:9225–9283. https://doi.org/10.1016/S0040-4020(01)00906-1
Kokel A, Schäfer C, Török B (2017) Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem 19:3729–3751. https://doi.org/10.1039/C7GC01393K
Roberts BA, Strauss CR (2005) Toward rapid, “Green”, predictable microwave-assisted synthesis. Acc Chem Res 38:653–661. https://doi.org/10.1021/ar040278m
Polshettiwar V, Varma RS (2008) Microwave-assisted organic synthesis and transformations using benign reaction media. Acc Chem Res 41:629–639. https://doi.org/10.1021/ar700238s
Jiang B, Shi F, Tu S-J (2010) Microwave-assisted multicomponent reactions in the heterocyclic chemistry. Curr Org Chem 14:357–378. https://doi.org/10.2174/138527210790231892
Dalvi PB, Lin S-F, Paike V, Sun C-M (2015) Microwave-assisted multicomponent synthesis of dihydroquinoxalinones on soluble polymer support. ACS Comb Sci 17:421–425. https://doi.org/10.1021/acscombsci.5b00053
Hügel HM (2009) Microwave multicomponent synthesis. Molecules 14:4936–4972. https://doi.org/10.3390/molecules14124936
Donkor IO, Klein CL, Liang L, Zhu N, Bradley E, Clark AM (1995) Synthesis and antimicrobial activity of 6,7-annulated pyrido[2,3-d]pyrimidine. J Pharm Sci 84:661–664. https://doi.org/10.1002/jps.2600840526
Ziarani GM, Nasab NH, Rahimifard M, Soorki AA (2015) One-pot synthesis of pyrido[2,3-d]pyrimidine derivatives using sulfonic acid functionalized SBA-15 and the study on their antimicrobial activities. J Saudi Chem Soc 19:676–681. https://doi.org/10.1016/j.jscs.2014.06.007
Rabie ST, Abdel-Monem RA, Mohamed NR, Hashem AI, Nada AA (2014) Utility of 6-amino-2-thiouracils as a core of biologically potent polynitrogen–sulfur fused heterocycles. J Heterocycl Chem 51:E189–E196. https://doi.org/10.1002/jhet.1936
Youssouf MS, Kaise P, Singh GD, Singh S, Bani S, Gupta VK, Satti NK Suri, Suri KA, Johri RK (2008) Anti-histaminic, anti-inflammatory and broncho relaxant activities of 2,7-dimethyl-3-nitro-4H pyrido[1,2-a]pyrimidine-4-one. Int Immunopharmacol 8:1049–1055. https://doi.org/10.1016/j.intimp.2008.03.015
Narayana BL, Rao ARR, Rao PS (2009) Synthesis of new 2-substituted pyrido[2,3-d]pyrimidin-4(1H)-ones and their antibacterial activity. Eur J Med Chem 44:1369–1376. https://doi.org/10.1016/j.ejmech.2008.05.025
Cordeu L, Cubedo E, Bandrés E, Rebollo A, Sáenz X, Chozas H, Domínguez MV, Echeverría M, Mendivil B, Sanmartin C, Palop JA, Font M, Foncillas JG (2007) Biological profile of new apoptotic agents based on 2,4-pyrido[2,3-d]pyrimidine derivatives. Bioorg Med Chem 15:1659–1669. https://doi.org/10.1016/j.bmc.2006.12.010
Quiroga J, Cisneros C, Insuasty B, Abonia R, Cruz S, Nogueras M, Torre JM, Sortino M, Zacchino S (2006) Microwave-assisted three-component synthesis and in vitro antifungal evaluation of 6-cyano-5,8-dihydropyrido[2,3-d]pyrimidin-4(3H) ones. J Heterocycl Chem 43:299–306. https://doi.org/10.1002/jhet.5570430208
Walsh C (1986) Naturally occurring 5-deazaflavin coenzymes: biological redox roles. Acc Chem Res 19:216–221. https://doi.org/10.1021/ar00127a004
Basiri A, Murugaiyah V, Osman H, Kumar RS, Kia Y, Ali MA (2013) Microwave assisted synthesis, cholinesterase enzymes inhibitory activities and molecular docking studies of new pyridopyrimidine derivatives. Bioorg Med Chem 21:3022–3031. https://doi.org/10.1016/j.bmc.2013.03.058
Mohamed AM, El-Sayed WA, Alsharari MA, Al-Qalawi HRM, Germoush MO (2013) Anticancer activities of some newly synthesized pyrazole and pyrimidine derivatives. Arch Pharm Res 36:1055–1065. https://doi.org/10.1007/s12272-013-0163-x
Zhang F, Li C, Liang X (2018) Solid acid-catalyzed domino cyclization reaction: regio- and diastereoselective synthesis of pyrido[2,3-d]pyrimidine derivatives bearing three contiguous stereocenters. Green Chem 20:2057–2063. https://doi.org/10.1039/C7GC03812G
Ghorbani-Vaghei R, Sarmast N (2018) Hexamethylenetetramine grafted layered double hydroxides as a novel and green heterogeneous ionic liquid catalyst for the synthesis of pyrido[2,3-d]pyrimidine derivatives. Res Chem Intermed 44:4483–4501. https://doi.org/10.1007/s11164-018-3399-8
Upadhyay A, Sharma LK, Singh VK, Singh RKP (2016) An efficient one pot three component synthesis of fused pyridines via electrochemical approach. Tetrahedron Lett 57:5599–5604. https://doi.org/10.1016/j.tetlet.2016.10.111
Panday AK, Mishra R, Jana A, Parvin T, Choudhury LH (2018) Synthesis of pyrimidine fused quinolines by ligand-free copper-catalyzed domino reactions. J Org Chem 83:3624–3632. https://doi.org/10.1021/acs.joc.7b03272
Bharti R, Kumari P, Parvin T, Choudhury LH (2017) Molecular diversity from the three-component reaction of 2-hydroxy-1,4-naphthaquinone, aldehydes and 6-aminouracils: a reaction condition dependent MCR. RSC Adv 7:3928–3933. https://doi.org/10.1039/c6ra18828a
Diaz DD, Miranda PO, Padron JI, Martin VS (2006) Recent uses of iron (III) chloride in organic synthesis. Curr Org Chem 10:457–476. https://doi.org/10.2174/138527206776055330
Cornil J, Guérinot A, Reymond S, Cossy J (2013) FeCl3·6H2O, a catalyst for the diastereoselective synthesis of cis-isoxazolidines from N-protected δ-hydroxylamino allylic acetates. J Org Chem 78:10273–10287. https://doi.org/10.1021/jo401627p
Rana S, Brown M, Mukhopadhyay C (2013) FeCl3 catalysed multicomponent divergent synthesis of a library of indeno-fused heterocycles. RSC Adv 3:3291–3303. https://doi.org/10.1039/C2RA23332K
Zhao F, Jia X, Li P, Zhao J, Huang J, Li H, Li L (2017) FeCl3·6H2O/TMSBr-catalyzed rapid synthesis of dihydropyrimidinones and dihydro pyrimidinethiones under microwave irradiation. Molecules 22:1503–1519. https://doi.org/10.3390/molecules22091503
Bharti R, Kumari P, Parvin T, Choudhury LH (2018) Recent advances of aminopyrimidines in multicomponent reactions. Curr Org Chem 22:417–445. https://doi.org/10.2174/1385272822666171212152406
Kumari P, Bharti R, Parvin T (2019) Synthesis of aminouracil tethered trisubstituted methanes in water by iodine catalyzed multicomponent reactions. Mol Divers 23:205–213. https://doi.org/10.1007/s11030-018-9862-z
Jana A, Panday AK, Mishra R, Parvin T, Choudhury LH (2017) Synthesis of thio and selenoethers of cyclic β-hydroxy carbonyls and amino uracils: a metal-free regioselective I2/DMSO mediated reaction. Chem Select 2:9420–9424. https://doi.org/10.1002/slct.201702066
Bharti R, Parvin T (2016) Multicomponent synthesis of diverse pyrano-fused benzophenazines using bifunctional thiourea-based organocatalyst in aqueous medium. Mol Divers 20:867–876. https://doi.org/10.1007/s11030-016-9681-z
Bharti R, Parvin T (2015) Diversity oriented synthesis of tri-substituted methane containing aminouracil and hydroxynaphthoquinone/hydroxy coumarin moiety using organocatalysed multi component reactions in aqueous medium. RSC Adv 5:66833–66839. https://doi.org/10.1039/c5ra13093j
Parvin T, Choudhury LH (2011) Recent advances in the chemistry of imine-based multicomponent reactions (MCRs). Tetrahedron 67:8213–8228. https://doi.org/10.1016/j.tet.2011.07.020
Acknowledgements
We are grateful to NIT, Patna. The authors are also grateful to SAIF-IIT Patna, and SAIF-Panjab University, for providing analytical facilities for characterization of compounds.
Funding
Funding was provided by Science and Engineering Research Board, DST, Govt. of India (EMR/2016/000960).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, P., Yadav, R., Bharti, R. et al. Regioselective synthesis of pyrimidine-fused tetrahydropyridines and pyridines by microwave-assisted one-pot reaction. Mol Divers 24, 107–117 (2020). https://doi.org/10.1007/s11030-019-09929-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09929-4