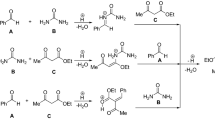
A brief review of the publications over the past 5 years devoted to the synthesis of 3,4-dihydropyrimidin-2(1H)-one or -thione derivatives using Biginelli reaction is presented. The impact of reaction conditions (solvents, catalysts, energy input) on the yield is discussed. New combinations of reagents enabling the synthesis of a wide range of 3,4-dihydropyrimidin-2(1H)-one derivatives with various functional groups are considered.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction

The three-component reaction between aromatic aldehydes, urea, and acetoacetic esters was discovered by the Italian chemist P. Biginelli in 1893. The products of this reaction are 3,4-dihydropyrimidin-2(1H)-ones (DHPMs). For a long time the Biginelli reaction was not commonly used, but in the last 20–30 years this class of heterocyclic compounds received considerable attention as a type of privileged heterocyclic scaffolds with a significant pharmacological potential.1 It has been shown that some dihydropyrimidines exhibit high antiviral, anti-inflammatory, anticancer, and antihypertensive activity.2 Therefore, the number of publications and patents devoted to the synthesis of DHPMs derivatives by means of the Biginelli reaction is growing every year. This can be explained by the simplicity of the synthetic procedure, the possibility to vary the starting reagents, catalysts, and solvents, as well as the possibility of introducing substituents that are easily converted into various functional groups. The rapid development of combinatorial chemistry also led to an increased interest in Biginelli reaction.3 Several reviews are devoted to the reaction.1 , 2a , 4

Reaction conditions

The classic method of carrying out the Biginelli reaction assumes one-pot condensation of ethyl acetoacetate, benzaldehyde, and urea under strongly acidic conditions. The reaction proceeds with low yields and requires relatively long time (15–20 h).
A significant number of works has been devoted to the optimization of reaction conditions in order to increase the yields of target DHPMs. The influence of solvents and catalysts on the yields of the target products obtained in Biginelli reaction has been recently studied.5 One approach is optimization of the solvents (acetic acid, acetonitrile, THF, DMFA, etc.) and the selection of appropriate catalyst systems (organic and inorganic acids,6 Lewis acids,7 ionic liquids8). In order to accelerate the reaction, experiments have been performed with microwave irradiation, infrared irradiation, as well as ultrasonication, thereby reducing the reaction time to few minutes and increasing yields up to 98%.9



Nathaliia Simurova was born in 1962 in Kyiv, Ukraine. She received her PhD in chemistry in 1995 under supervision of Prof. A. Sinitsa (Institute of Organic Chemistry of the NAS of Ukraine). Currently she is Associate Professor at the Organic Chemistry Department, National University of Food Technologies of Ukraine. She is the author of over 50 scientific publications, textbook on organic synthesis of medicines. Her scientific interests – chemistry of heterocyclic compounds, bioactive compounds, as well as the chemistry of trivalent phosphorus compounds.

Olena Maiboroda was born in 1969 in Chernigіv, Ukraine. She graduated with honors from the Chernigiv State Pedagogical Institute in 1991 and obtained her PhD in chemistry under supervision of Prof. O. Tolmachev at the Institute of Organic Chemistry of the NAS of Ukraine in 2002. At present, she is Associate Professor at the Organic Chemistry Department, National University of Food Technologies of Ukraine. Her scientific interests include organic synthesis, chemistry and applications of heterocyclic compounds, and medicinal chemistry.
New combinations of reagents

Changing the three building blocks has allowed to synthesize a large number of new multifunctional pyrimidines. Sugar aldehydes have been employed in the Biginelli reaction by Ali.10 Using samarium chloride as catalyst, dihydropyrimidine sugar derivatives were prepared in good yields.
The range of CH-acidic carbonyl compounds used in this condensation has been significantly expanded over the past years. Shubha et al. introduced cyanoacetic ester and guanidine nitrate, resulting in the isolation of aminopyrimidines in high yields.11
Different components, such as guanidine, barbituric acid derivatives, and other heterocyclic systems containing the desired motifs have been introduced into the reaction during recent studies. El-Saghier with coworkers carried out the interaction of barbituric acid, aromatic aldehyde, and urea (thiourea) resulting in the formation of pyrimidopyrimidinones.12 These reactions proceeded with high efficiency in water in the presence of cerium ammonium nitrate (CAN) as catalyst.
Ryabukhin et al. demonstrated3b the possibility of using aminoheterocycles in the cyclization, while employing chlorotrimethylsilane as an efficient promoter and water scavenger. A series of 89 triazolopyrimidines and tetrazolopyrimidines were obtained in 25–90% yields as a result.


Green chemistry

A new, powerful trend in the field of organic chemistry in recent years is the application of "green chemistry", which often envisions the synthesis of complex molecules without solvents or by using water as a solvent. A simple and efficient method for the synthesis of DHPMs was reported by Priyadharsini and Chitra.13 They suggested Sn(IV) oxide as a catalyst that was highly active, inexpensive, environmentally friendly, convenient in handling, and nontoxic.
A selective synthesis of DHPM derivatives was recently illustrated by Hajipour and coworkers8 by a reaction of aromatic aldehydes with cyclopentanone and urea (thiourea) in the presence of N,N,N-triethyl-4-sulfobutan-1-aminium hydrogen sulfate as a Brønsted acidic ionic liquid and effective catalyst.
Previously described CAN-catalyzed Biginelli reaction by El-Saghier et al. can be viewed as green method.12
The application of polyphosphoric acid on silica gel allowed to carry out the reaction under mild conditions, gave high yields, and the catalyst could be easily recovered.14 A significant number of works have been devoted to the application of environmentally friendly natural clays and silica gel as catalysts.15


Recent progress in asymmetric Biginelli reaction

Compounds containing the DHPM ring are asymmetrical, but the impact of the absolute configuration of such molecules on their biological activity has been insufficiently studied. It is known that the enantiomers of 4-arylsubstituted DHPMs may have pharmacological activity of different strength or even provide the opposite biological effects. In recent years there has been a considerable progress in the study of enantiopure DHPMs.16,17, – 18
Enantiomeric purity of the products can be achieved through the application of chiral organocatalysts on titanium dioxide or


Recent progress in asymmetric Biginelli reaction (continued) 
References
(a) Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083. (b) Merugu, R.; Garimella, S.; Balla, D.; Sambaru, K. Int. J. PharmTech Res. 2015, 8(6), 88.
(a) Slobbe, P.; Ruijter, E.; Orru, R. V. A. Med. Chem. Commun. 2012, 3, 1189. (b) Selvam, T. P.; James, C. R.; Dniandev, P. V.; Valzita, S. K. Res. Pharm. 2012, 2, 1.
(a) Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. ACS Sustainable Chem. Eng. 2014, 2, 1228. (b) Ryabukhin, S. V.; Plaskon, A. S.; Boron, S. Y.; Volochnyuk, D. M.; Tolmachev, A. A. Mol. Diversity 2011, 15, 189.
(a) Sandhu, S.; Sandhu, J. S. ARKIVOC 2012, (i), 66. (b) Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J. N. Tetrahedron Lett. 2016, 57, 5135.
Alvim, H. G. O; Lima, T. B.; de Oliveira, A. L.; de Oliveira, H. C. B.; Silva, F. M.; Gozzo, F. C.; Souza, R. Y.; da Silva, W. A.; Neto, B. A. D. J. Org. Chem. 2014, 79, 3383.
(a) Mazhukina, O. A.; Platonova, A. G.; Fedotova, O. V.; Reshetov, P. V. Chem. Heterocycl. Compd. 2012, 48, 1278. [Khim. Geterotsikl. Soedin. 2012, 1368.] (b) Sharma, R.; Jadav, S. S.; Yasmin, S.; Bhatia, S.; Khalilullah, H.; Ahsan, M. J. Med. Chem. Res. 2015, 24, 636.
(a) Kurmach, M. N.; Ryabitskiy, A. B.; Britsun, V. N. Chem. Heterocycl. Compd. 2014, 49, 1770. [Khim. Geterotsikl. Soedin. 2013, 1910.] (b) Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. ACS Sustainable Chem. Eng. 2014, 2, 1228.
(a) Hajipour, A. R.; Ghayeb, Y.; Sheikhan, N.; Ruoho, A. E. Synth. Commun. 2011, 41, 2226. (b) Alvim, H. G. O.; de Lima, T. B.; de Oliveira, H. C. B.; Gozzo, F. C.; de Macedo, J. L.; Abdelnur, P. V.; Silva, W. A.; Neto, B. A. D. ACS Catal. 2013, 3, 1420.
(a) Flores-Conde, M. I.; Reyes, L.; Herrera, R.; Rios, H; Vazquez, M. A.; Miranda, R.; Tamariz, J.; Delgado, F. Int. J. Mol. Sci. 2012, 13, 2590. (b) Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566. [Khim. Geterotsikl. Soedin. 2012, 607.] (c) Kaur, N. Synth. Commun. 2015, 45, 1145.
Ali, I. A. I. Rev. Chim. (Bucharest) 2013, 64, 1413.
Bhatewara, A.; Jetti, S. R.; Kadre, T.; Paliwal, P.; Jain, S. Arch. Appl. Sci. Res. 2012, 4, 1274.
Mohamed, M. A. A.; Mahmoud N. F. H.; El-Saghier A. M. M. Chem. J. 2012, 2(2), 64.
Priyadharsini, M.; Chitra, S. IJNCSE 2015, 2(6), 18.
Zeinali-Dastmalbaf, M.; Davoodnia, A.; Heravi, M. M.; Tavakoli-Hoseini, N.; Khojastehnezhad, A.; Zamani, H. A. Bull. Korean Chem. Soc. 2011, 32(2), 656.
(a) Darehkordi, A.; Hosseini, S. S.; Tahmooresi M. Iran. J. Mater. Sci. Eng. 2012, 9(3), 49. (b) Agarwal, S.; Aware, U.; Patil, A.; Rohera, V.; Ghate, M.; Jain, M.; Patel, P. Bull. Korean Chem. Soc. 2012, 33, 377.
Fedorova, O. V.; Titova, Y. A.; Vigorov, A. Y.; Toporova, M. S.; Alisienok, O. A.; Murashkevich, A. N.; Krasnov, V. P.; Rusinov, G. L.; Charushin, V. N. Catal. Lett. 2016, 146, 493.
(a) Saha, S.; Moorthy, J. N. J. Org. Chem. 2011, 76, 396. (b) Phillips, A. M. F. Eur. J. Org. Chem. 2014, 33, 7291.
(a) Rao, H.; Quan, Z.; Bai, L.; Ye, H. Chin. J. Org. Chem. 2016, 36, 283. (b) Wan, J.-P.; Lin, Y.; Hu, K.; Liu, Y. Beilstein J. Org. Chem. 2014, 10, 287.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2017, 53(4), 413–415
Rights and permissions
About this article
Cite this article
Simurova, N., Maiboroda, O. Biginelli reaction – an effective method for the synthesis of dihydropyrimidine derivatives (microreview). Chem Heterocycl Comp 53, 413–415 (2017). https://doi.org/10.1007/s10593-017-2067-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-017-2067-z