Abstract
We examined the origins of cultivated stocks of the endangered species Primula sieboldii at the individual plant level by using an assignment test based on eight microsatellite loci and regional features of chloroplast DNA (cpDNA) variation of wild populations. To confirm that we had sufficient information for estimating the origins of the stocks, we performed an assignment test with 920 genets that we collected from 32 wild populations with known origins. The test assigned 99.6% of the genets to the population from which they had been sampled, confirming the suitability of the method. We then performed the assignment test with 29 cultivated stocks. The alleged origins of 19 were confirmed by microsatellite and cpDNA variations. In contrast, the alleged origins of five were rejected by both markers. Five stocks, which do not have a reference population located within 30 km of their reputed origin, were not assigned to any population. Stocks whose alleged origins were rejected are inappropriate as restoration materials, because their introduction might disturb local gene pools. Six stock haplotypes could not be detected in wild populations. This may suggest the loss of genetic diversity in the wild and the value of stocks as a gene bank. The genetic method used in this study will also be helpful to detect cryptic invasion by nonendemic genotypes or to trace the origins of plants collected for commercial purposes, a threat to many endangered species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In restoration programs for endangered plants, ex situ stocks cultivated in botanical or private gardens have occasionally been used because they sometimes preserve invaluable genetic lineages (Demauro 1993; Robichaux et al. 1997; Hogbin and Peakall 1999; Ibanez et al. 1999; Maunder et al. 1999, 2000). However, labeling errors, hybridization between stocks, and confusion about origins have been identified in some ex situ stocks (Friesen et al. 1999; Hogbin and Peakall 1999; Maunder et al. 1999, 2004). A mixing of genetically distinct populations or species disturbs the evolutionary history endemic to an area or species and can cause outbreeding depression due to a loss of local adaptation or the breakup of coadapted gene complexes (Hufford and Mazer 2003). Therefore, If we are to use cultivated stocks for restoration activities, it is vital to confirm their origins.
An assignment test, which is often used to ensure bloodstock integrity in captive propagation programs or to identify hybrid individuals of endangered fishes (Olsen et al. 2000; Congiu et al. 2001), may allow us to infer the origins of endangered plant stocks. The test assigns each individual to a reference population in which its multilocus genotype is most likely to occur (reviewed by Manel et al. 2005). An assignment test would make a significant contribution to tracing the origins of individual stocks. Furthermore, the geographical distribution of maternally inherited organelle genomes might provide insights into tracing origins (Deguilloux et al. 2003). In plant species, the geographical distribution of chloroplast DNA (cpDNA) haplotypes is often restricted (reviewed by Soltis et al. 1997; Newton et al. 1999), allowing us to infer maternal origin. By utilizing both an assignment test based on nuclear DNA markers and cpDNA structure, we can achieve reliable estimation of origins of stocks.
Primula sieboldii E. Morren (Primulaceae) is a perennial clonal herb that is distributed in Japan, the Korean peninsula, northeastern China, and eastern Siberia (Richards 2003). However, it is now listed as vulnerable in the Japanese Red Data Book (Environment Agency of Japan 2000) owing to the rapid decrease of both numbers and sizes of populations due to loss or fragmentation of habitats by human exploitation, abandonment of traditional management of woodlands or grasslands, and commercial collection. Ecological studies and practices for in situ conservation of the species have been extensively implemented (reviewed by Washitani et al. 2005), and restoration activities led by private citizens have been growing in a number of regions. In local residents’ gardens adjacent to declining or extinct populations and in botanical gardens of research institutes there are several clonally cultivated stocks of P. sieboldii that are said to have been collected from wild populations in known areas. These stocks may retain genetic diversity lost from the wild and could be useful resources for the genetic restoration of local wild populations, if their origins can be confirmed.
In previous studies, we investigated nuclear microsatellite and maternally inherited cpDNA variation in representative extant populations of P. sieboldii in Japan to determine conservation units (Honjo et al. 2004, in press; Honjo 2005). Microsatellite analysis revealed a significant genetic isolation-by-distance pattern, and genetic groupings corresponding to the geographical location of populations. cpDNA analysis detected 22 haplotypes belonging to three distinct clades (I, II, and III), and most of the haplotypes were geographically structured. These regional features of genetic variations may allow us to infer whether the stock originated in the region or not.
In this study, we examined the origins of 29 stocks of P. sieboldii by analyzing nuclear microsatellite and cpDNA variation. First, we performed an assignment test based on the multilocus genotype of eight microsatellite loci. Second, we examined where cpDNA haplotypes of stocks were distributed in the wild. As far as we know, this study is one of the first that actually demonstrates the traceability of endangered plant stocks at the individual level, which may allow us to devise plans for the effective restoration.
Materials and methods
Study species and materials
Primula sieboldii is a distylous and clonal perennial herb that occurs in moist habitats in northeastern Asia (Richards 2003). As is typical of distylous species, mating between genets having different floral morphs is required for viable seed production. Each genet is composed of physiologically independent ramets originating from short rhizomes.
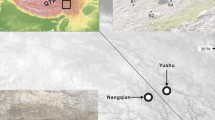
At flowering time (April–May) in 2002–2005, we collected fresh leaves from 29 stocks of P. sieboldii (Fig. 1). Because each stock of P. sieboldii corresponds to a genet and therefore does not have intra-stock genetic variation, we collected leaves from one ramet per stock. Each stock is named after the place from which the stock was reputedly collected, though there was no verification of this. These living stocks have been maintained in local residents’ gardens adjacent to the alleged places of origin, several local botanical gardens, the Agricultural and Forestry Research Center of University of Tsukuba, and Saitama Prefecture Agriculture and Forestry Research Center (Table 1).
The alleged places of origin of the stocks (circled numbers) analyzed in this study. The circles on the map represent the wild populations in which we analyzed both microsatellite and cpDNA variations (Honjo 2005; Honjo et al. in press). Italic numbers next to the plots correspond to the population numbers in M. Honjo et al. (in press), and colors of the plots represent the clades to which the genets within the population belong, as revealed by phylogenetic analysis based on chloroplast DNA sequences: white, clade I; gray, clade II; black, clade III
The alleged originating populations of several stocks had already been lost through human activity. Stocks 4–7, maintained in a local resident’s garden, had been rescued just before the loss of habitat due to farmland consolidation and river improvement in 1970 (T. Yasuno, personal communication, May 19, 2003). Stocks 10–18 reputedly originated in several populations in the Arakawa area near Tokyo (Fig. 1), where there had been many populations until about 1920. Now only two populations (15 and 16 in Fig. 1) remain. Stock 27 had been collected from a now vanished population in Geihoku town in western Honshu and has been cultivated in local resident’s gardens for about 70 years. Although other wild populations exist in the town (such as population 31), the indigenous origin of stock 27 has long been questioned by local people (A. Kodama, personal communication, May 9, 2003). Potentially, as it is found within a site occupied in the historical past by semi-sedentary iron makers, the population may be an ancient translocation.
Table 1 lists wild populations located within 30 km of the alleged place of origin of each stock that we previously analyzed for microsatellite and cpDNA variations (Honjo 2005; Honjo et al. in press). We expected stocks 4–7, 22, and 29 to be rejected from any population in the following assignment test, because these stocks do not have any reference population located within 30 km of the origin.
In addition, we collected new leaf samples from wild populations 9, 14, 15, 16, 24, 26 and 31 (five to 16 individuals per population) to enhance the reference cpDNA data.
Genotyping by nuclear microsatellites
Genomic DNA was extracted from leaves by a modified CTAB method (Murray and Thompson 1980). The samples were genotyped at the following eight microsatellite loci, which were used in a previous analysis of wild populations (Honjo 2005; Honjo et al. in press): PS2 (Isagi et al. 2001), ga0235, ga0381, ga0668, ga1277 (Ueno et al. 2003), ga0653, ga0666 (Ueno et al. 2005), and Pri0146 (Kitamoto et al. 2005b). PCR amplifications were performed in 10 μL reaction mixtures containing 10–15 ng template DNA, 10 mM Tris·HCl (pH 8.3), 50 mM KCl, 100 μM each dNTP, 0.02% Triton X-100, 0.01% gelatin, 1.5 mM MgCl2, 0.25 units Taq polymerase, and 0.2 μM each primer. Thermocycling conditions were as follows: 3 min at 94°C; 30 cycles of 30 s at 94°C, 30 s at a primer-specific annealing temperature (ga0668, 52°C; ga0666 and PS2, 55°C; ga0381, 57°C; others, 60°C) and 30 s at 72°C; and a final extension step at 72°C for 7 min. The PCR products were run on a 3100 Genetic Analyzer with GeneScan software (Applied Biosystems, Foster City, CA, USA).
Assignment test based on eight microsatellite loci
To infer the origin of the stocks, we performed an assignment test using Rannala and Mountain (1997) criterion with the ‘leave one out’ procedure on the basis of microsatellite variation with the program GeneClass2 (Piry et al. 2004). This test calculates the probability that an individual’s multilocus genotype occurs in each reference population. After we found the population with highest probability, we evaluated rejection and acceptance of membership of the genotype to the population by the Monte Carlo resampling method of Paetkau et al. (2004). The probability of given individual multilocus genotype was compared to the distribution of probabilities of multilocus genotypes (10,000 replicates) generated based on the allele frequencies of the population, and if the value was below α < 0.01, the individual was rejected from the population. Such a combination of an assignment test and exclusion-method is used for other studies (Manel et al. 2005; Frantz et al. 2006; Hare et al. 2006).
First, to confirm that there was sufficient information for estimating the origin of the stocks, we tested the multilocus genotypes of 920 genets collected from 32 wild populations in a previous study (Honjo 2005; Honjo et al. in press) and whose origins we therefore know. In these populations, we sampled leaves from 10 to 49 genets per population corresponding to population size. Because P. sieboldii has marked visible interclonal variations in floral morphology (i.e., size, color, shape of petal, and heterostyly) we collected leaves by visually distinguishing genets in the field. In this assignment test, we amalgamated populations 1–6 in the Hidaka area and populations 18–21 in the Karuizawa area into one population each, because they were located within 10 km of one another and microsatellite and cpDNA variations indicated that they were genetically closely related (Honjo 2005). The resultant information was used to infer the origins of the cultivated stocks of P. sieboldii.
We performed an additional assignment test in which we amalgamated populations 15 and 16 in the Arakawa area (Fig. 1) and populations 27–30 in western Honshu (Fig. 1) into one population each, to depict average profiles of genetic variation in the areas. The fragmentation and decline of populations in those areas are especially extensive (M. Honjo et al., unpublished), likely biasing the genetic composition of the populations through genetic drift and reduced interpopulation gene flow. We considered that we might have been able to outline the intrinsic genetic composition in the areas this way. We did not integrate population 31 into the assemblage of western Honshu populations, because it belongs to a different maternal lineage (clade I) from populations 27 to 30 (clade II; Fig. 1).
Sequencing of cpDNA
The following five noncoding regions of cpDNA, which were used in a previous analysis of wild populations (Honjo et al. 2004), were sequenced in all stocks and newly analyzed wild individuals: (1) trnT (UGU) and trnL (UAA) 5′ exon; (2) trnL (UAA) intron; (3) trnL (UAA) 3′ exon and trnF (GAA); (4) trnH (GUG) and psbA; and (5) trnD (GUC) and trnT (GGU). PCR and sequencing using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems) were performed as described by Honjo et al. (2004). Sequencing data were aligned manually with CLUSTAL W (Thompson et al. 1994). Insertions/deletions (indels) were generally placed so as to increase the number of matching nucleotides in a sequence position. We determined cpDNA haplotypes from nucleotide substitutions and indels.
Phylogenetic relationships among cpDNA haplotypes
We inferred phylogenetic relationships among newly detected cpDNA haplotypes and those reported in wild populations by the maximum parsimony method using both substitution data and indel characters. As an outgroup, we used the closely related species P. kisoana Miq. Indels of more than 1 bp were treated as a single character resulting from one mutational event in the data matrix. However, a tandem repeat of mononucleotides in the intergenic spacer between trnT and trnL was omitted from the phylogenetic analysis because its homology was ambiguous (Honjo et al. 2004). The most parsimonious trees were obtained by PAUP 4.0b10 (Swofford 2002) using the branch-and-bound search options with bootstrap values based on 10,000 replicates. All character states, including indels, were specified as unordered and equally weighted.
Results
Assignment test based on microsatellite variation
All eight loci were amplified in every stock. Stocks 4–7 and stocks 13 and 20 showed the same genotype, respectively, suggesting that these stocks belong to the same genet.
To confirm that there was sufficient information for estimating the origins of the stocks, we first performed an assignment test with 920 genets, which we collected from 32 wild populations. As a result (Table 2), 916 genets (99.6% of total) showed the highest probability of occurrence of their genotypes in the population from which they had been sampled, three in a population located within 30 km of their origins (each one genet in populations 3, 20, 28 were assigned to populations 7, 23, 27, respectively), and one in the most geographically adjacent population, which is located 90 km away from the origin (one genet in population 10 was assigned to population 9). In the evaluation of membership by the Monte Carlo resampling method of Paetkau et al. (2004), 919 genets showed the values larger than 0.01 and were accepted for membership, though one genet in population 6 was not. This high probability of correct assignment affords us an opportunity to infer the origins of the stocks.
Fifteen stocks (2, 8, 9, 10–19, 21, 28) among the 29 showed the highest probability of occurrence of their genotypes in a population located within 30 km of the alleged places of origin with α > 0.01, but eight (1, 3, 20, 23–27) in populations in other areas. Among the six stocks with no reference population within 30 km of the reputed places of origin, five (4–7, 29) were not assigned to any populations with α > 0.01, but stock 22 was assigned to population 27, which grows in the same region (i.e., western Honshu) as the alleged place of origin of stock 22.
In the additional assignment test based on the average profiles of genetic composition in the Arakawa area and western Honshu, stocks 24 and 26 showed the highest probability in the assemblage of populations 27–30 in western Honshu with α > 0.01, where those stocks reputedly originated. Stock 23, which also reputedly originated in western Honshu, showed the highest probability in the assemblage, but α < 0.01. Other stocks showed the highest probability in the same reference populations as the former test showed.
Chloroplast DNA haplotypes detected in the stocks
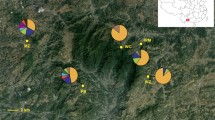
We sequenced the five noncoding spacers from the several additional wild samples and 29 stocks of P. sieboldii. In the wild samples, we detected nine distinct cpDNA haplotypes. Four haplotypes were newly detected (haplotypes X, Y, ε and λ in populations 9, 31, 14 and 15, respectively; Fig. 2), and others had already been reported in previous studies (Honjo et al. 2004; Kitamoto et al. 2005a). Among the 29 stocks analyzed, 14 showed haplotypes distributed in the alleged places of origin, but five did not (Table 1). The other 10 stocks showed haplotypes that were not detected in wild populations (viz., G, W, α, π, θ, σ). Among these, haplotype G was already reported in Honjo et al. (2004), and others were newly reported in this study. The nucleotide sequence data of new haplotypes will appear in the DDBJ database under accession numbers AB161511, AB277083 to AB277095.
One of the two most parsimonious trees among the cpDNA haplotypes of Primula sieboldii based on the sequences of the five noncoding regions but omitting a tandem repeat of mononucleotides between trnT and trnL. The strict consensus tree of the two most parsimonious trees shows the same topology. Numbers above branches are percentage bootstrap values based on 10,000 replicates. Solid and open bars represent nucleotide substitutions and indels, respectively. Haplotypes that were not detected in wild populations are underlined
We constructed most parsimonious trees incorporating both substitutions and indels by using the branch-and-bound search option. We obtained two most parsimonious trees that required 93 steps (consistency index = 0.9892; retention index = 0.9865). The topology of the strict consensus tree of these two trees was identical to one of the two most parsimonious trees (Fig. 2). Haplotypes G and π belonged to clade I, haplotypes θ and σ to clade II, and haplotype α to clade III. Haplotype W showed a unique variation at all five regions and formed a distinct clade.
Discussion
Garden stocks retain genetic diversity possibly lost in the wild
In the 29 stocks analyzed, we detected two new microsatellite alleles and six cpDNA haplotypes that have not been found from wild populations. Haplotype W of stock 22, whose western Honshu origin was supported in the assignment test (Table 1), formed a unique clade (Fig. 2, clade IV). This suggests that a genetically distinct, unknown population once existed in western Honshu. New haplotype π was found from stocks 4–7, which were grown in the same garden as each other. It was suggested that these stocks belong to the same genet because they showed the same genotypes at all eight microsatellite loci. Though it is difficult for one genet alone to produce viable seeds due to self-incompatibility, these are the only stocks retaining endemic variation in the area. Although it is extremely difficult to sample all genetic variation remaining in extant wild populations, this high number of newly detected haplotypes may suggest the loss of genetic diversity in the wild and the value of stocks as a gene bank.
Geographical origin of the stocks
An assignment test is a useful tool to trace the origins of individuals (Manel et al. 2005). In this study, we first performed the test with genets from wild populations with known origins, and 99.6% of the genets were assigned to the population from which they had been sampled (Table 2). Although four genets were misassigned, however, they were assigned to geographically adjacent populations probably because of genetic proximity among those populations (Honjo 2005; Honjo et al. in press). This considerably high probability of correct assignment and parallel use of cpDNA information would afford us an opportunity to infer the origins of the stocks.
Among the stocks, 11 (2, 8, 9, 10, 13–15, 17–19, 21) were assigned to populations located in the alleged area of origin in the assignment test and showed cpDNA haplotypes distributed there (Table 1). We thus consider these stocks to have originated in the alleged areas, and so they could be used in restoration projects if needed.
In contrast, five stocks (1, 3, 20, 25, 27) were rejected as having originated in the alleged areas by both microsatellite and cpDNA variation. Among them, stocks cultivated in Saitama Prefecture Agriculture and Forestry Research Center (1, 3) might have been mixed up during cultivation, because many stocks have been grown there. Stocks cultivated in private gardens in each region (20, 25, 27) might have been transferred from other regions by commerce or whim. Stocks 20 and 25 were judged to have originated in Arakawa from both markers. Because populations in the Arakawa area, the nearest populations to Tokyo, are known to have been the major source of horticultural P. sieboldii from 350 years ago, commercial transfer from the area is very likely. Indeed, stock 20 showed the same genotype and haplotype as stock 13, which confirmed its Arakawa origin. Stock 27, whose origin has long been questioned, also appears to have experienced a translocation. In the assignment test, stock 27 showed no probability of occurrence of the genotype in the local population (population 31). Further, it showed haplotype I, which is distributed in the Arakawa area and is not found in population 31 (Honjo 2005; Honjo et al. in press). These stocks, whose alleged origins were rejected by both markers, should not be used for restoration activity so as not to change local gene pools.
Stocks 11, 12, 16, and 28 were assigned to their alleged populations of origin in the assignment test, but showed haplotypes (α, G, and θ) not detected in the wild. Haplotypes α and G are closely related to haplotypes C and E (Fig. 2), respectively. Haplotypes C and E are distributed in the alleged populations of origin (populations 15 and 16) of stocks 11, 12, and 16. Therefore, it should not be a surprise that haplotypes α and G are distributed in populations 15 and 16. More samples are needed to examine whether these haplotypes exist in extant wild populations.
Stocks 23, 24, and 26 showed haplotype B, which is common in western Honshu, where these stocks reputedly originated. However, in the assignment test, these stocks were not assigned to any populations in western Honshu. One possible cause of the discrepancy between microsatellite and cpDNA markers is a degeneration of genetic composition of populations as population size is reduced. Previous work revealed that allelic variation at microsatellite loci significantly decreases as population size reduces (Honjo 2005; Honjo et al. in press). Because the population decline in western Honshu is extensive (M. Honjo et al., unpublished), the genetic composition of each population may have been changed greatly. Integrating remnant populations in the area may afford us an opportunity to depict the intrinsic allelic composition of the region. So we performed an assignment test with an assemblage of populations 27–30 in western Honshu. As a result, every stock showed the highest probability of occurrence in the assemblage (Table 1), although stock 23 was excluded from membership (α = 0.006). This result supports the western Honshu origin of stocks 24 and 26. It seems effective to integrate remnant populations in the assignment test when populations in the reference area have been highly fragmented. Stocks 25 and 26, which grow in the same garden and are said to have been collected from nearby declining habitat in western Honshu, were demonstrated to differ in their origins. The alleged western Honshu origin of stock 25 was clearly rejected by both markers. This result strongly suggests the importance of confirming the origin of stocks before they are used in restoration projects.
All six stocks that do not have a reference population within 30 km of the alleged places of origin (4–7, 22, 29) showed new haplotypes. Stocks 4–7 and 29 were not assigned to any populations with α > 0.01. These results support the conclusion that these stocks originated from genetically distinct local populations in each area.
Conclusions
Stocks of P. sieboldii preserved in private or botanical gardens retain genetic diversity that might have been lost in the wild. If these stocks are to be used for restoration, it is preferable to first confirm the origin of each plant. By using both the assignment test and the geographical distribution of maternally inherited cpDNA haplotypes, we were able to infer the origins of individual stocks. This system of tracing will also help to detect cryptic invasion by nonendemic genotypes (Saltonstall 2002; Hufford and Mazer 2003) or to trace the origins of plants collected for commercial purposes, one of the crucial causes of extinction of the species.
References
Congiu L, Dupanloup I, Patarnello T, Fontana F, Rossi R, Arlati G, Zane L (2001) Identification of interspecific hybrids by amplified fragment length polymorphism: the case of sturgeon. Mol Ecol 10:2355–2359
Deguilloux MF, Pemonge MH, Bertel L, Kremer A, Petit J (2003) Checking the geographical origin of oak wood: molecular and statistical tools. Mol Ecol 12:1629–1636
Demauro MM (1993) Relationship of breeding system to rarity in the lakeside daisy (Hymenoxys acaulis var. glabra). Conserv Biol 7:542–550
Environment Agency of Japan (2000) Threatened wildlife of Japan, red data book, vascular plants. Environment Agency of Japan, Tokyo (in Japanese)
Frantz AC, Pourtois T, Heuertz M, Schley L, Flamand MC, Krier A, Bertouille S, Chaumont F, Burke T (2006) Genetic structure and assignment tests demonstrate illegal translocation of red deer (Cervus elaphus) into a continuous population. Mol Ecol 15:3191–3203
Friesen N, Pollner S, Bachmann K, Blattner FR (1999) RAPDs and noncoding chloroplast DNA reveal a single origin of the cultivated Allium fistulosum from A. altaicum (Alliaceae). Am J Bot 86:554–562
Hare MP, Allen SK Jr, Bloomer P, Camara MD, Carnegie RB, Murfree J, Luckenbach M, Meritt D, Morrison C, Paynter K, Reece KS, Rose CG (2006) A genetic test for recruitment enhancement in Chesapeake Bay oysters, Crassostrea virginica, after population supplementation with a disease tolerant strain. Conserv Genet 7:717–734
Hogbin PM, Peakall R (1999) Evaluation of the contribution of genetic research to the management of the endangered plant Zieria prostrata. Conserv Biol 13:514–522
Honjo M (2005) Molecular ecological genetics for the conservation of genetic diversity in Primula sieboldii. Doctoral dissertation, University of Tsukuba (in Japanese)
Honjo M, Kitamoto N, Ueno S, Tsumura Y, Washitani I, Ohsawa R (in press) Managing units of the endangered herb Primula sieboldii based on microsatellite variation among and within populations throughout Japan. Conserv Genet doi:10.1007/s10592-007-9292-4
Honjo M, Ueno S, Tsumura Y, Washitani I, Ohsawa R (2004) Phylogeographic study based on intraspecific sequence variation of chloroplast DNA for the conservation of genetic diversity in the Japanese endangered species Primula sieboldii. Biol Conserv 120:215–224
Hufford KM, Mazer SJ (2003) Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends Ecol Evol 18:147–155
Ibanez O, Calero C, Mayol M, Rossello JA (1999) Isozyme uniformity in a wild extinct insular plant, Lysimachia minoricensis J.J. Rodr. (Primulaceae). Mol Ecol 8:813–817
Isagi Y, Honjo M, Washitani I (2001) Development of microsatellite markers for Primula sieboldii using degenerate oligonucleotide-primed PCR-amplified DNA. Mol Ecol Notes 1:22–24
Kitamoto N, Honjo M, Ueno S, Takenaka A, Tsumura Y, Washitani I, Ohsawa R (2005a) Spatial genetic structure among and within populations of Primula sieboldii growing beside separate streams. Mol Ecol 14:149–157
Kitamoto N, Ueno S, Tsumura Y, Washitani I, Ohsawa R (2005b) Development of microsatellite markers in Primula sieboldii E. Morren, a threatened herb. Jpn J Conserv Ecol 10:47–51 (in Japanese with English summary)
Manel S, Gaggiotti OE, Waples RS (2005) Assignment methods: matching biological questions with appropriate techniques. Trends Ecol Evol 20:136–142
Maunder M, Culham A, Bordeu A, Allainguillaume J, Wilkinson M (1999) Genetic diversity and pedigree for Sophora toromiro (Leguminosae): a tree extinct in the wild. Mol Ecol 8:725–738
Maunder M, Culham A, Alden B, Zizka G, Orliac C, Lobin W, Bordeu A, Ramirez JM, Glissmann-Gough S (2000) Conservation of the toromiro trees: case study in the management of a plant extinct in the wild. Conserv Biol 14:1341–1350
Maunder M, Hughes C, Hawkins JA, Culham A (2004) Hybridization in ex situ plant collections: conservation concerns, liabilities, and opportunities. In: Guerrant EO Jr, Havens K, Maunder M (eds) Ex situ plant conservation. Island Press, Washington DC
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Newton AC, Allnutt TR, Gillies ACM, Lowe AJ, Ennos RA (1999) Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends Ecol Evol 14:140–145
Olsen JB, Bentzen P, Banks MA, Shaklee JB, Young S (2000) Microsatellites reveal population identity of individual pink salmon to allow supportive breeding of a population at risk of extinction. Trans Am Fish Soc 129:232–242
Paetkau D, Slade R, Burden M, Estoup A (2004) Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation based exploration of accuracy and power. Mol Ecol 13:55–65
Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A (2004) GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Rannala B, Mountain JL (1997) Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA 94:9197–9221
Richards AC (2003) PRIMULA, 2nd edn. Timber Press, Portland
Robichaux RH, Friar EH, Mount DW (1997) Molecular genetic consequences of a population bottleneck associated with reintroduction of the Mauna Kea silversword (Argyroxiphium sandwicense ssp. sandwicense [Asteraceae]). Conserv Biol 11:1140–1146
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99:2445–2449
Soltis DE, Gitzendanner MA, Strenge DD, Soltis PS (1997) Chloroplast DNA intraspecific phylogeography of plants from the Pacific Northwest of North America. Plant Syst Evol 206:353–373
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (* and other methods). Version 4. Sinauer Associates, Sunderland
Thompson JD, Higgings DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequencing weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Ueno S, Tsumura Y, Washitani I (2003) Development of microsatellite markers in Primula sieboldii E. Morren, a threatened Japanese perennial herb. Conserv Genet 4:809–811
Ueno S, Kitamoto N, Ohsawa R, Tsumura Y, Washitani I (2005) Nine additional microsatellite markers for Primula sieboldii E. Morren. Conserv Genet 6:1063–1064
Washitani I, Ishihama F, Matsumura C, Nagai M, Nishihiro J, Nishihiro MA (2005) Conservation ecology of Primula sieboldii: Synthesis of information toward the prediction of the genetic/demographic fate of a population. Plant Species Biol 20:3–15
Acknowledgments
We express our sincere thanks to M. Aoto, C. Kikuchi, T. Kinebuchi, A. Kodama, T. Konishi, H. Koyano, K. Kuwata, T. Matsumoto, Y. Matsumoto, A. Notsu, K. Shirakawa, and T. Yasuno for collection of plant materials, and to anonymous reviewers for their valuable comments on this paper. This work was partly supported by the Fundamental Research Fund for Future Environment from the Japanese Ministry of the Environment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honjo, M., Ueno, S., Tsumura, Y. et al. Tracing the origins of stocks of the endangered species Primula sieboldii using nuclear microsatellites and chloroplast DNA. Conserv Genet 9, 1139–1147 (2008). https://doi.org/10.1007/s10592-007-9427-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-007-9427-7