Abstract
Sevoflurane is a widely used obstetric general anesthetic, but the neurotoxic effects of late-pregnancy exposure to one minimum alveolar concentration ([MAC], 2.5%) of sevoflurane on offspring remain unclear. We investigated whether exposure to 2.5% sevoflurane during late pregnancy would affect offspring hippocampal neuronal development and neurocognitive function. On gestational day 18 (G18), rats were randomly treated with 2.5% sevoflurane in 50% oxygen for 1 (Sev × 1), 3 (Sev × 3), or 6 h (Sev × 6). The neuronal apoptosis rate and mature brain-derived neurotrophic factor (mBDNF) and postsynaptic density protein 95 (PSD-95) expression levels were measured in offspring hippocampi on postnatal day 1 (P1) and P35. Dendritic spine formation and cognitive function were examined on P35. The neuronal apoptosis rate was enhanced, and mBDNF and PSD-95 levels were reduced in the Sev × 3 and Sev × 6 groups on P1. mBDNF and PSD-95 levels were also decreased in the Sev × 6 group on P35. The error rate was elevated in the maze test, whereas dendritic spine density and long-term potentiation (LTP) were reduced in the Sev × 6 group on P35. To determine whether exposure to an enriched environment (EE) would ameliorate sevoflurane’s neurotoxic effects, offspring from another Sev × 6 group were exposed to either a standard environment (SE) or an EE. Lower error rates and greater dendritic spine densities and LTP were found in the Sev × 6 + EE vs. Sev × 6 + SE group. Collectively, we showed that exposing rats to 1 MAC sevoflurane for 3 h during late pregnancy increased neuronal apoptosis in neonates but did not impair neuronal development or cognitive function in juvenile rats, whereas a 6-h exposure impaired neuronal development and cognitive function in juvenile rats, effects that were attenuated by an EE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous animal studies have demonstrated that exposing neonates to anesthetic agents triggers neurodegeneration and subsequent learning and memory impairments, suggesting that similar effects may occur during fetal exposure (Ji et al. 2017; Gui et al. 2017; Zhou et al. 2016). Exposure to volatile anesthetics during pregnancy induces neuronal apoptosis and cognitive dysfunction in a dose- and time-dependent manner in offspring. To date, human studies in this area are limited, and most animal studies have focused on the mid-pregnancy period (Fang et al. 2017; Zheng et al. 2013; Song et al. 2017). However, late pregnancy is the period of peak of brain development, and the brain and central nervous system are particularly sensitive to drugs at this stage.
In humans, brain growth and development are complex. Neuronal apoptosis or programmed cell death occurs at a high rate from the 24th week of gestation to 4 weeks after birth, the proliferation of neuronal synapses begins at around the 20th week of gestation and continues rapidly, and synapse formation accelerates during late pregnancy, with the synapse number reaching a peak at about 1–2 years of age (Andropoulos 2018). Neurons are extremely sensitive during the synaptic plasticity phase; extensive dendritic arborization, cortical lamination, and myelination also occur during this phase of synaptogenesis (De Tina and Palanisamy 2017). Changes in internal or external environments may accelerate neuronal apoptosis and reduce synapse formation in late pregnancy. In 2016, the United States Food and Drug Administration issued a warning regarding impairments in the developing brain when mothers have repeated or prolonged (> 3 h) exposure to certain general anesthetic agents during late pregnancy (Olutoye et al. 2018), but the underlying mechanisms were not explained in detail. Although emergent non-obstetric surgeries may occur at any time during gestation, various emergency operations or fetal surgeries that necessitate general anesthetics may be required in late pregnancy. Most general anesthetics are lipophilic and thus cross the placenta easily. As such, the duration of fetal exposure to general anesthesia may exceed 3 h. While volatile anesthetics are commonly used in obstetric anesthesia, only a few studies on fetal exposure to volatile anesthetics during late pregnancy exist, and the results are contradictory (Suehara et al. 2016; Shan et al. 2018). Hence, the effects of anesthetic exposure in late pregnancy on offspring remain controversial.
The minimum alveolar concentration (MAC) of sevoflurane in rats is approximately 2.5%; this is also the concentration typically used in human obstetric surgery. In both animals and humans, the hippocampus is crucial for cognitive functions such as learning and memory, and several animal studies have indicated that exposure to an enriched environment (EE) ameliorates sevoflurane-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity (Ji et al. 2015; Nithianantharajah and Hannan 2006). In the present study, we aimed to investigate whether late-pregnancy exposure to 1 MAC sevoflurane would impair the neuronal development and neurocognitive function of offspring. Further, we used different exposure times to determine the range of safe exposure. We also investigated whether an EE would ameliorate any damage caused by such exposure. Collectively, these metrics may guide the clinical use and investigation of anesthetics.
Materials and Methods
Animals and Anesthesia
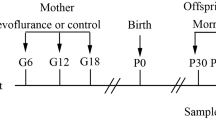
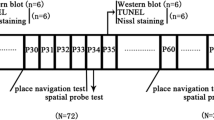
The protocol was approved by the Tianjin Medical University Committee on the Use of Animals in Research and Teaching. Three-month-old pregnant Sprague Dawley rats were provided by the Chinese Academy of Military Sciences. They were housed individually in a room maintained at 22 °C under a 12-h light cycle and had access to food and water ad libitum. Pregnant rats (n = 32) at gestational day 18 (G18) were included in the experiments and assigned randomly to the anesthesia group or control group (n = 8 per group). Anesthesia group rats received 2.5% sevoflurane in 50% oxygen for 1 (Sev × 1), 3 (Sev × 3), or 6 h (Sev × 6) in an anesthetizing chamber (Datex-Ohmeda Inc., Tewksbury, MA, USA) (Fig. 1a). Control group rats were only exposed to 50% oxygen. The rats breathed spontaneously and the concentrations of sevoflurane, oxygen, and carbon dioxide were monitored by a gas analyzer (Drager, Lübeck, Germany); arterial blood gas analyses were conducted continuously. The temperature of the anesthetizing chamber was controlled to maintain each animal’s rectal temperature at 37 ± 0.5 °C. Upon anesthesia termination, rats were left in a chamber containing 50% oxygen until 20 min after the righting reflex returned. All pregnant rats were raised to delivery, which generated a sufficient number of rat offspring for experiments. Rat offspring were randomly divided into two arms in every group. In one arm, the bilateral hippocampal tissues of neonatal rats were harvested on P1 for apoptosis assays and biochemistry studies. Offspring in the other arm were raised to P31, whereupon they underwent behavioral testing with the eight-arm radial maze for 5 days. On P35, bilateral hippocampal tissues were harvested for flow cytometry, biochemistry, Golgi staining, and electrophysiology studies. All rats were culled under pentobarbital anesthesia.
Study design and testing for the first (a) and second (b) experimental arms. G gestational day, SD Sprague Dawley, P postnatal day, Sev sevoflurane, mBDNF mature brain-derived neurotrophic factor, PSD-95 postsynaptic density protein, LTP long-term potentiation, SE standard environment, EE enriched environment
Flow Cytometry Assay for Apoptosis
Since sevoflurane-induced neuronal apoptosis can cause long-term cognitive impairment (Tian et al. 2018), we assessed the apoptosis rate using flow cytometry. Rat hippocampi weighing ~ 1 g were collected on a 150-μm copper net, cut with tweezers, and gently rubbed and filtered. A 300-μm nylon mesh was used to filter the cell suspension to remove large clumps. The cell suspension was washed and collected by centrifugation. The supernatant was removed and the cells were resuspended in 500 μL annexin-V binding buffer (Beijing Biosea Biotechnology, Beijing, China), to which 5 μL annexin-V-fluorescein isothiocyanate was added, followed by 10 μL propidium iodide (PI). The cells were incubated at a density of 5 × 105 cells/mL in the dark for 15 min before detection. The labeling intensity was recorded by a flow cytometer (Beckman Coulter, Brea, CA, USA), and the percentage of positive cells was assessed.
Western Blot Analysis
Brain-derived neurotrophic factor (BDNF) is the most widely distributed neurotrophin in the brain. It is essential for brain development and critical for neuronal regeneration and synaptic plasticity. BDNF protein is synthesized as pre-proBDNF and cleaved into a proBDNF protein, proBDNF is either cleaved intracellularly or extracellularly to mature BDNF (mBDNF), and mBDNF binds to the tropomyosin-related kinase B (TrkB) receptor, leading to neuronal survival, differentiation, and positive regulation of learning and memory (Sen et al. 2017; Park and Poo 2013). Therefore, using western blot analyses, we measured the protein expression of mBDNF to establish the effects of sevoflurane on brain development. We also measured the expression of postsynaptic density protein 95 (PSD-95), since light and electron microscopy data indicate that PSD-95 is contained in a significant percentage of synapses (McLeod et al. 2017), the density and strength of which are important for maintaining neuronal network connections, in turn supporting learning and memory. Indeed, deficits in synapse number can lead to cognitive dysfunction. For the western blot analyses, total protein was extracted by lysing the bilateral whole hippocampus tissues. Anti-mBDNF (1:1000, molecular weight 14 kDa; Abcam, Cambridge, UK) and anti-PSD-95 (1:1000, molecular weight 80 kDa; Cell Signaling Technology, Danvers, MA, USA) antibodies were used to evaluate the expression levels of mBDNF and PSD-95, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was employed as a loading control using rabbit anti-GAPDH antibody (1:3000; CWBIO, Beijing, China). The results normalized for each membrane are expressed as percentages of saline-treated controls. Protein bands were quantified using ImageJ software (NIH Image, Bethesda, MD, USA).
Golgi Staining
Dendritic spines are neuronal specializations that are the major sites of synaptic transmission. Most excitatory synapses in the mammalian central nervous system are located on dendritic spines (Chidambaram et al. 2019). Dendritic spines are responsible for receiving input from excitatory synapses and are thought to be closely related to synaptic function (Zhou et al. 2004). To establish sevoflurane’s effects on synapses, we performed Golgi staining with dendritic spine quantification. After the behavioral tests, several P35 rats were randomly selected from each group. After anesthesia, rats were perfused transcardially with saline followed by 4% paraformaldehyde. The hippocampus was collected bilaterally and immersed in Golgi solution. Golgi staining was performed on 150 µm-thick frozen hippocampal sections using an FD Rapid Golgi Stain Kit (FD Neurotechnologies, Inc., Columbia, MD, USA) according to the manufacturer’s protocol. After slides were dehydrated with a gradient of ethanol and cleared in xylene, the specimens were covered with a coverslip, which was then sealed with Permount. The slides were viewed in detail using a light microscope with a 100 × oil-immersion objective lens. Pyramidal neurons that were well-impregnated and clearly distinguishable from others in each hippocampus were analyzed (× 400 magnification). Spine density for each neuron (spine number per 10 µm) was determined using ImageJ software. Spines were quantified on three segments of secondary dendrites. All analyses were completed by an investigator blinded to the experimental conditions.
Eight-Arm Radial Maze
The eight-arm radial maze is a reliable method for assessing learning and memory. This test was used herein to evaluate the learning and memory of offspring in each group, as previously described (Servick 2014; Ilieva et al. 2019; Xu et al. 2019). The maze consisted of a central platform and eight arms (length: 425 mm; width: 145 mm; height: 225 mm). During the training stage, offspring were trained from P26 to 30. Chocolates were randomly placed at the ends of four arms. After a fasting period of 16–18 h, the rats were placed on the platform and allowed to choose arms with/without food freely. When the rats had completed consumption of all food or when the time reached a 10-min cap, the rats were removed. Training was performed once a day for 5 days. During the testing stage on P31 to P35, chocolates were placed at the ends of four arms as was done during the training stage. The test ended when the rats had completed consuming all chocolates or when the time reached a 10-min cap. The rats were tested twice a day for 5 days with an interval of > 1 h between sessions. In total, 10 tests were conducted. Each rat’s maze performance was recorded by the EthoVision XT video tracking software (Noldus Information Technology, Wageningen, Netherlands). When the rat entered an arm without food, it was defined as a reference memory error (RME), which reflected long-term memory. When the rat entered an arm that had already been entered, it was defined as a working memory error (WME), which reflected short-term memory. When the rat turned back halfway, it was defined as a half error. The total number of arm entries and total amount of time spent entering arms over the 5 days were recorded. The percentages of the following were calculated for each group: > 3 RMEs, ≥ 1 WME, and > 8 total arm entries. Data from rats that did not consume all chocolates within 10 min were excluded from further analysis.
Electrophysiology
Synaptic plasticity is critical for memory formation and storage and is closely related to the structural modification of synapses and synaptic transmission. Long-term potentiation (LTP) reflects the strength of postsynaptic neurotransmission, which is an alteration in synaptic strength that is viewed as a potential functional mechanism forming the neuronal basis of learning and memory (Titley et al. 2017). By performing electrophysiological recordings, we were able to determine sevoflurane’s effects on LTP. To do this, offspring brains were removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF), which was carbonated with 95% O2 and 5% CO2. Acute coronal hippocampal slices were prepared using a vibratome. The slices were placed in ACSF for 1 h at 32 °C, and then held in ACSF at 22–24 °C before recording the extracellular field potential.
Extracellular Field Potential Recordings
Field evoked postsynaptic potentials (fEPSPs) were obtained from the CA1 region. Brain slices were perfused with ACSF (1.5–2.0 mL/min) in a recording chamber, which was bubbled with 95% O2/5% CO2 continually. ACSF-filled theta-glass stimulating pipettes and recording pipettes were placed in Schaffer collaterals and the hippocampal CA1 region, respectively. The distance from the stimulating to recording electrodes was 400–500 μm. fEPSPs were elicited once every 30 s and recorded at a sampling rate of 10 kHz (Molecular Devices, San Jose, CA, USA). To induce LTP, high-frequency stimulation (HBS) was applied. Input/output curves were created, and HBS (100 Hz) was adjusted to evoke 50–60% of the maximum fEPSP. The fEPSP slope was recorded for 20 min before and 50–60 min after the LTP induction protocol was applied, and data were analyzed with Clampfit 9 (Molecular Devices).
Enriched Environment Experiment
An EE provides the opportunity for social interaction, exercise, and somatosensory and cognitive stimulation, which enhances synaptic plasticity in the brain (Mahati et al. 2016). For this experiment, six rats (G18) received 2.5% sevoflurane in 50% oxygen for 6 h. Offspring were randomly divided into the Sev × 6 + EE and Sev × 6 + SE (standard environment) groups. Dendritic spine formation and cognitive function were examined using methods similar to those described above (Fig. 1b).
Environment Exposure
Offspring were subjected to an EE or SE daily for 15 days from P16 to P30 for 6 h (from 09:00 to 15:00). The offspring were kept with their mothers from P16 to P21 and weaned from P22 to P35. The EE was a large cage (120 cm × 80 cm × 80 cm) containing different toys, wheels, plastic tunnels, and ladders. The types and locations of tunnels and ladders were changed every 2 days. EE cages provided scope for visual, motor, somatosensory, and cognitive stimulation. The SE was the exact cage with the equipment removed. Food and water were available ad libitum in all cages.
Statistical Analysis
All data were analyzed using GraphPad Prism software (version 7.00; GraphPad Software, La Jolla, CA, USA; www.graphpad.com). Data are expressed as the mean ± standard deviation. Two-way analyses of variance followed by Tukey post-hoc tests were used to analyze the differences in arterial blood gas, cell apoptosis, mBDNF and PSD-95 expression, total time spent entering arms, dendritic spine density, and extracellular electrophysiological parameters of the hippocampus among the control, Sev × 1, Sev × 3, and Sev × 6 groups. Two-way analyses of variance were also used to assess differences in the total time spent entering arms, dendritic spine density, and extracellular electrophysiological parameters between the Sev × 6 + EE and Sev × 6 + SE groups. The percentages of > 3 RMEs, ≥ 1 WME, and > 8 total arm entries in the maze test were analyzed with the chi-squared test. Differences were deemed statistically significant at P < 0.05.
Results
Arterial Blood Gas Analysis
As shown in Table 1, there were no significant differences in pH value, arterial oxygen tension, and arterial carbon dioxide tension between the four groups. The possibility of impairment induced by arterial blood gas alterations was therefore considered minimal.
Maternal Sevoflurane Exposure-Induced Hippocampal Neuronal Apoptosis in Offspring
We investigated whether impairments in cognitive function were associated with hippocampal apoptosis by quantifying the number of annexin-V-positive/PI-negative and annexin-V-positive/PI-positive cells (Fig. 2a). On P1, there was no significant difference in neuronal apoptosis between the control and Sev × 1 groups. However, the apoptosis rate was significantly greater in the Sev × 3 group than in the control and Sev × 1 groups (F[2, 21] = 12.64, P < 0.001; Fig. 2b), and significantly greater in the Sev × 6 group than in the other three groups (F[3, 29] = 27.09, P < 0.001; Fig. 2b). On P35, the apoptosis rate was not significantly different among the groups.
Effects of late-pregnancy sevoflurane exposure on offspring brain development on P1. Flow cytometry and western blots were used to evaluate neuronal apoptosis (a) and protein expression (c), respectively. Exposure to 2.5% sevoflurane for 3 h and 6 h induced neuronal apoptosis (b), and reduced PSD-95 (d) and mBDNF (e) protein levels in the hippocampus. Data are expressed as the mean ± standard deviation (n = 8–10). ***P < 0.001
Maternal Sevoflurane Exposure-Induced Changes in Hippocampal PSD-95 and mBDNF Levels in Offspring
Hippocampal PSD-95 and mBDNF protein levels were assessed using western blots on P1 (Fig. 2c) and P35 (Fig. 3a) and quantified. On P1, PSD-95 and mBDNF levels between the Sev × 1 and control groups were not significantly different. However, the levels of PSD-95 (F[2, 22] = 86.91, P < 0.001; Fig. 2d) and mBDNF (F[2, 22] = 225.5, P < 0.001; Fig. 2e) were significantly lower in the Sev × 3 group than in the Sev × 1 and control groups. Moreover, the levels of PSD-95 (F[3, 30] = 144.8, P < 0.001; Fig. 2d) and mBDNF (F[3, 30] = 262.3, P < 0.001; Fig. 2e) were significantly lower in the Sev × 6 group than in the other three groups. On P35, the levels of PSD-95 (F[3, 18] = 40.36, P < 0.001; Fig. 3b) and mBDNF (F[3, 18] = 34.39, P < 0.001; Fig. 3c) were significantly reduced in the Sev × 6 group, but no differences among the other three groups were identified.
Effects of late-pregnancy sevoflurane exposure on offspring brain development on P35. Western blots were used to evaluate protein expression (a–c). Golgi staining was used to detect dendritic spine density (d, e). The eight-arm radial maze (f, g) and electrophysiological recordings (h, i) were used to evaluate cognition. Data are expressed as the mean ± standard deviation. Percentages were analyzed using the chi-squared test. *P < 0.05, **P < 0.01, ***P < 0.001
Maternal Sevoflurane Exposure Reduced Hippocampal CA1 Dendritic Spine Density in Offspring
Fully impregnated CA1 pyramidal cells were detected by Golgi staining (Fig. 3d), and the spines of secondary dendrites were visualized using a light microscope. Only the dendritic spine density was determined, since other spine types were not always clearly distinguishable. The hippocampal CA1 spine density in the Sev × 6 group was significantly lower than that in the other three groups (F[3, 18] = 8.509, P < 0.001; Fig. 3e), but no differences in spine density were found between the control, Sev × 1, and Sev × 3 groups.
Maternal Sevoflurane Exposure-Induced Learning and Memory Impairments in Offspring
The total time spent entering the arms of the eight-arm radial maze over the 5 test days was longer in the Sev × 6 group than in the other groups (F[3, 70] = 4.185, P = 0.006; Fig. 3f), but no other differences were identified among the control, Sev × 1, and Sev × 3 groups. The percentages of > 3 RMEs (χ2 = 9.825, P = 0.020; Fig. 3g), ≥ 1 WME (χ2 = 8.624, P = 0.034; Fig. 3g), and > 8 total arm entries (χ2 = 13.07, P = 0.005; Fig. 3g) were higher in the Sev × 6 group than they were in the other groups. No differences in these percentages were found between the control, Sev × 1, and Sev × 3 groups.
Maternal Sevoflurane Exposure Reduced Hippocampal CA1 LTP in Offspring
We evaluated whether fetal exposure to 2.5% sevoflurane would induce LTP changes in the hippocampal CA1 region (Fig. 3h). No differences in the magnitude of LTP (the mean fEPSP slope during the last 20 min of recording) induced by HBS were observed among the control, Sev × 1, and Sev × 3 groups. The magnitude of LTP induced by HBS was significantly lower in the Sev × 6 group than in the other three groups (F[3, 18] = 15.57, P < 0.001; Fig. 3i).
Sevoflurane Exposure-Induced Dendritic Spine Abnormalities and Impairments in LTP and Cognition were Ameliorated by an EE
In the EE experiment, Golgi staining (Fig. 4a), the eight-arm radial maze, and electrophysiological recordings (Fig. 4e) were used to evaluate dendritic spine density and cognition. The hippocampal CA1 spine density was greater in the Sev × 6 + EE vs. Sev × 6 + SE group (F[1, 9] = 11.67, P = 0.008; Fig. 4b). The total time spent entering arms (F[1, 23] = 35.29, P < 0.001; Fig. 4c), and the percentages of > 3RMEs (χ2 = 8.003, P = 0.005; Fig. 4d), ≥ 1 WME (χ2 = 4.147; P = 0.042; Fig. 4d), and > 8 total arm entries (χ2 = 4.066, P = 0.044; Fig. 4d) were lower in the Sev × 6 + EE vs. Sev × 6 + SE group. The magnitude of LTP induced by HBS was significantly higher in the Sev × 6 + EE vs. Sev × 6 + SE group (F[1, 10] = 69.82, P = 0.0007; Fig. 4f).
Sevoflurane exposure-induced dendritic spine abnormalities and impairments in LTP and cognition were ameliorated by an EE on P35. The Sev × 6 + EE group exhibited a greater dendritic spine density (a, b), shorter total time spent entering maze arms (c), and greater LTP in the hippocampus (e, f) than did the Sev × 6 + SE group. Data are expressed as the mean ± standard deviation. Percentages were analyzed using the chi-squared test. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
In this study, exposing maternal rats to 1 MAC sevoflurane for 3 h during late pregnancy induced toxic effects on offspring hippocampal neuronal development on P1. Learning and memory deficits were observed in P35 rats after 6 h of maternal exposure, and these cognitive function impairments were ameliorated by an EE.
Findings from the few published animal studies on the effects of volatile anesthetic exposure in late pregnancy are contradictory. One study indicated that exposing G21 rats to 1.3% isoflurane for 6 h is not neurotoxic to the fetal brain and does not impair learning and memory in juvenile or adult offspring (Li et al. 2007). In contrast, other researchers have reported that exposing rats to 1.5% isoflurane for 2 h or 3% isoflurane for 1 h during late pregnancy affects the hippocampal structure and impairs the cognitive function of offspring (Wang et al. 2009; Luo et al. 2016). In a study by Seuhara et al. (2016), anesthetizing G17 pregnant mice with 1.5% sevoflurane for 6 h did not affect the neuronal development and learning and memory abilities of offspring. Although the methods used in that study were similar to ours, the results differed. The disparate results may be related to the fact that we used 2.5% sevoflurane, which approximates the MAC and is the most commonly used concentration, albeit higher, than that employed in the previous study. Another report demonstrated that maternal exposure to 3% sevoflurane for 4 h on G20 causes neuronal damage to the hippocampus of rat pups (Shan et al. 2018), but offspring learning and memory were not examined. The findings of a mid-pregnancy study suggested that repeated, but not single, maternal exposure(s) to 3% sevoflurane for 2 h affects offspring BDNF expression, dendritic density, and memory (Wu et al. 2018). However, herein, we found that late-pregnancy exposure to 2.5% sevoflurane for 3 h changed BDNF expression in the neonatal hippocampus, while exposure for 6 h impaired dendritic density, memory, and cognitive function in juvenile rats. The different gestation and exposure time between the studies may explain the discrepant results. Furthermore, we observed that an EE improved the sevoflurane-induced cognitive function impairments of offspring. The gestation period of rat is about 19 to 22 days, late pregnancy is from G17 or G18 to G22, but parts of the pregnant rats began to deliver on G19, so we exposed rats to sevoflurane on G18.
Effects of 2.5% Sevoflurane Exposure During Late Pregnancy on the Brain Development of P1 Offspring
Inhalation anesthetics can induce neuronal apoptosis, or programmed cell death, in the developing brain and lead to long-term cognitive impairments (Noguchi et al. 2017; Chung et al. 2015; Lv et al. 2017), but the underlying mechanisms are poorly understood. The current study revealed that exposing fetal rats to 1 MAC sevoflurane for 3 h and 6 h in late pregnancy induced greater amounts of neuronal apoptosis (Fig. 2b). Hence, the number of functional neuronal cells was reduced, potentially leading to abnormal brain function. Therefore, a relationship may exist between early neuronal apoptosis and the cognitive defects observed on P35.
BDNF is associated with the neurodegeneration or neuronal apoptosis caused by anesthetic agent exposure in the developing rodent brain (Ibla et al. 2009; Nieto et al. 2013). The gene encoding BDNF is expressed during development and adulthood. Decreased mBDNF expression at protein level may lead to synaptic dysfunction, neuroplasticity and LTP failures, and impaired memory formation (Park and Poo 2013; Yeh et al. 2012). Additionally, PSD-95, an excitatory postsynaptic marker associated with several receptor systems, plays an essential role in neuroplasticity (Baucum 2017). PSD-95 reductions are associated with decreases in synapse number or synaptic loss. Here, mBDNF and PSD-95 protein expression levels were reduced in the Sev × 3 and Sev × 6 groups on P1 (Fig. 2d, e). Thus, late-pregnancy exposure to 1 MAC sevoflurane for 3 h and 6 h may induce neuronal apoptosis, impair neuronal development, and affect synaptogenesis in the neonatal rat hippocampi.
Effects of 2.5% Sevoflurane Exposure During Late Pregnancy on the Brain Development of P35 Offspring
Neuronal apoptosis induced by low concentrations and short exposure times of sevoflurane may not contribute to long-term cognitive dysfunction (Lu et al. 2016). Indeed, late-pregnancy exposure to 2.5% sevoflurane for 3 h did not yield differences in neuronal apoptosis and mBDNF and PSD-95 protein levels (Fig. 3b, c) on P35, which was in contrast to the P1 findings. In addition, no differences in dendritic spine structure, LTP magnitude, and maze test results were found between the Sev × 3 group and Sev × 1 and control groups on P35. We conjectured that late-pregnancy exposure to 2.5% sevoflurane for 3 h led to short-term neuronal damage in the offspring, but that these adverse effects recovered during development and ultimately did not impair learning and memory in juvenile rats.
When the exposure time was 6 h, mBDNF and PSD-95 protein levels were significantly reduced on P35 (Fig. 3b, c), dendritic spine density was reduced (Fig. 3e), LTP was inhibited (Fig. 3i), and the results of the maze test were abnormal. mBDNF increases the number of excitatory synapses, regulates axonal morphology, directly promotes synapse formation, participates in LTP, and is associated with learning and memory (Sen et al. 2017; Li et al. 2019). Decreased mBDNF protein expression levels adversely affect LTP generation and cognitive function. The anesthetic sevoflurane reduces the PSD-95 level, leading to cognitive impairments in young mice (Zhang et al. 2015; Ju et al. 2016). Reductions in PSD-95 expression also adversely affect LTP and cognitive function. Abnormal dendritic spine structure and function is one of the most prominent features associated with neurodevelopmental disorders; defects in spine density and morphology may lead to LTP alterations, ultimately affecting cognitive function. Studies using animal models have demonstrated a close correlation between dendritic spine abnormalities and learning and memory deficits (Amrock et al. 2015). Sevoflurane exposure may impair hippocampal synaptic plasticity and learning and memory abilities (Shen et al. 2018; Hirase and Shinohara 2014). In the present study, we observed that maze error rates were increased, and both long- and short-term memory were damaged in the maze test (Fig. 3f, g). Neurotoxicity depends on three main factors: exposure concentration, duration, and brain development stage. Therefore, we speculated that when rats were exposed to 1 MAC sevoflurane for a specific temporal window, this damaged the neuronal development and cognitive function of the offspring during late pregnancy. If the exposure time was 6 h, permanent damage on offspring neuronal development and cognitive function likely occurred.
Beneficial Effects of an EE on Offspring Neuronal Development and Cognitive Function
Studies have indicated that an EE has positive effects on the nervous system at anatomical, molecular, and functional levels. An EE has been shown to promote hippocampal neurogenesis (Nithianantharajah and Hannan 2006; Hirase and Shinohara 2014). In a study that exposed P7 rats to 1 MAC sevoflurane anesthesia for 4 h, long-term impairments in short-term spatial memory were induced, which were mitigated by a delayed EE (Shih et al. 2012). Here, compared with an SE, an EE ameliorated the neuronal development and cognitive function impairments of rat offspring that were induced by maternal late-pregnancy exposure to sevoflurane for 6 h, and enhanced dendritic spine density (Fig. 4b), LTP (Fig. 4f), and spatial learning and memory (Fig. 4c, d). These findings are consistent with the previous study that repeated sevoflurane exposure in P6 mice induced reductions in synaptic markers, dendritic spine abnormalities, and impairments in LTP and cognition on P35; these were ameliorated by an EE, which also enhanced neural plasticity, learning, and memory (Olutoye et al. 2018).
Currently, the mechanisms of action of EE on the brain are unclear. Gamma-aminobutyric acid (GABA) and N-methyl-d-aspartate receptor systems play important roles in neurodevelopment. Sevoflurane activates GABA and N-methyl-d-aspartate receptors, which may have detrimental effects on the brain (Fang et al. 2012). Studies indicate that EE exposure increases GABA and glutamate levels and neurotrophic factor expression, as well as improves synaptic dendritic arborization in CA1 and ameliorates impaired LTP (He et al. 2010; Bhagya et al. 2017). An EE also reportedly attenuates sevoflurane-induced neurotoxicity and learning and memory impairments via the peroxisome proliferator-activated receptor-γ signaling pathway (Zhao et al. 2015), but the exact mechanisms by which an EE reduces sevoflurane’s effects remain to be determined. In the present study, the EE was employed preweaning and postweaning, and babies in all groups remained with their mothers from P16 to P21 and were weaned from P22 to P35. Several studies have investigated the effects of preweaning EE on the developing brain; most of these studies indicated that preweaning EE is beneficial to brain development, learning, and memory, which are consistent with our observation. In six day-old C57BL/6 male mice, exposure to 3% sevoflurane, 2 h daily, for 3 days from P6 to P8 induced cognitive impairments compared to the mice exposed to sevoflurane and EE every day for 2 h from P8 to P90 (Ji et al. 2017) or from P8 to P42 (Ji et al. 2015), and mice that were allowed to stay with their mothers from P8 to P21. The two studies found that sevoflurane exposure-induced cognitive impairments in mice were reversed or ameliorated by EE. Pregnant mice (G14) that were treated with 2.5% sevoflurane for 2 h had offspring with impaired learning and memory at P 31. Pregnant mice treated with sevoflurane were exposed to an EE every day, and two hours before delivery. After delivering their offspring at G21, the mother and babies were exposed to an EE again every day for two hours, from P4 to P30, and the babies were weaned from P22. They found that maternal, preweaning, and postweaning EE attenuated sevoflurane-induced learning and memory impairment (Zheng et al. 2013). Another study also indicated that preweaning EE can enhance hippocampus-dependent learning and memory function in developing rats as well as postweaning EE does (Lu et al. 2017). The experimental rats that were exposed to an EE for 20 min each day, from P10 to 24, that were separated from their mothers were later picked up and returned to their cages. And the authors did not discuss the effects of separation on mothers and their offspring. In our study, we found that the spine density and the magnitude of LTP were increased, and the maze performances were better in the Sev × 6 + EE group compared to the Sev × 6 + SE group. Therefore, we believe that exposing the dam and pup to an EE has beneficial effects on offspring cognition. However, a report presented contrary conclusions on the effects of preweaning EE on the developing brain. When EE was provided from postnatal day 1 until weaning, dams reduced nursing time and showed more non-maternal appetitive behavior in the EE group. This study suggested that preweaning EE can interfere with maternal behavior in rats and can possibly lead to increased anxiety in the offspring. Although cognition capability was not assessed, the findings suggest that enrichment procedures can have potentially unintended effects, interfering with the development of emotional behavior in rats (Li et al. 2016). Hence, the effects of preweaning exposure to an EE on the developing brain need to be further studied.
Our study has several limitations. First, although a previous study demonstrated sex differences in the vulnerability to neurotoxicity caused by anesthetics (Zhao et al. 2015), we conducted the behavioral tests on all offspring, irrespective of sex. Second, we did not separate the hippocampus into subregions in our analysis of BDNF and PSD-95; given that the dorsal and ventral regions of the hippocampus regulate cognitive and emotional outputs separately, future studies should evaluate these regions independently. Third, other groups have reported that fetal exposure to sevoflurane during late pregnancy affects offspring brain development, although their methods and conclusions were different. Fourth, although exact evaluations of neurodevelopmental outcomes are essential, we only examined neuronal apoptosis and synaptic number using flow cytometry and limited biochemical parameters.
Conclusions
Herein, we demonstrated that late-pregnancy exposure to 2.5% sevoflurane for 3 h caused neuronal developmental abnormalities in the neonatal hippocampus. This damage recovered during development. However, exposure to 2.5% sevoflurane for 6 h enhanced neonatal hippocampal neuronal apoptosis and reduced synaptogenesis and dendritic spine formation, leading to cognitive function impairments in juvenile rats. We observed that these neurodevelopmental impairments could be improved by learning and exercise. Nevertheless, as human and rodent brain development differs, further work is required to confirm the translational relevance of our findings.
References
Amrock LG, Starner ML, Murphy KL, Baxter MG (2015) Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology 122(1):87–95. https://doi.org/10.1097/ALN.0000000000000477
Andropoulos DB (2018) Effect of anesthesia on the developing brain: infant and fetus. Fetal Diagn Therapy 43(1):1–11. https://doi.org/10.1159/000475928
Baucum AJ 2nd (2017) Proteomic analysis of postsynaptic protein complexes underlying neuronal plasticity. ACS Chem Neurosci 8(4):689–701. https://doi.org/10.1021/acschemneuro.7b00008
Bhagya VR, Srikumar BN, Veena J, Shankaranarayana Rao BS (2017) Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J Neurosci Res 95(8):1602–1610. https://doi.org/10.1002/jnr.23992
Chidambaram SB, Rathipriya AG, Bolla SR, Bhat A, Ray B, Mahalakshmi AM, Manivasagam T, Thenmozhi AJ, Essa MM, Guillemin GJ, Chandra R, Sakharkar MK (2019) Dendritic spines: revisiting the physiological role. Progr Neuro-psychopharmacol Biol Psychiatry 92:161–193. https://doi.org/10.1016/j.pnpbp.2019.01.005
Chung W, Park S, Hong J, Park S, Lee S, Heo J, Kim D, Ko Y (2015) Sevoflurane exposure during the neonatal period induces long-term memory impairment but not autism-like behaviors. Paediatr Anaesth 25(10):1033–1045. https://doi.org/10.1111/pan.12694
De Tina A, Palanisamy A (2017) General anesthesia during the third trimester: any link to neurocognitive outcomes? Anesthesiol Clin 35(1):69–80. https://doi.org/10.1016/j.anclin.2016.09.007
Fang F, Xue Z, Cang J (2012) Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function. Neurosci Bull 28(5):499–508. https://doi.org/10.1007/s12264-012-1260-4
Fang F, Song R, Ling X, Peng M, Xue Z, Cang J (2017) Multiple sevoflurane anesthesia in pregnant mice inhibits neurogenesis of fetal hippocampus via repressing transcription factor Pax6. Life Sci 175:16–22. https://doi.org/10.1016/j.lfs.2017.03.003
Gui L, Lei X, Zuo Z (2017) Decrease of glial cell-derived neurotrophic factor contributes to anesthesia- and surgery-induced learning and memory dysfunction in neonatal rats. J Mol Med 95(4):369–379. https://doi.org/10.1007/s00109-017-1521-9
He S, Ma J, Liu N, Yu X (2010) Early enriched environment promotes neonatal GABAergic neurotransmission and accelerates synapse maturation. J Neurosci 30(23):7910–7916. https://doi.org/10.1523/JNEUROSCI.6375-09.2010
Hirase H, Shinohara Y (2014) Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 280:282–298. https://doi.org/10.1016/j.neuroscience.2014.09.031
Ibla JC, Hayashi H, Bajic D, Soriano SG (2009) Prolonged exposure to ketamine increases brain derived neurotrophic factor levels in developing rat brains. Curr Drug Saf 4(1):11–16
Ilieva K, Tchekalarova J, Atanasova D, Kortenska L, Atanasova M (2019) Antidepressant agomelatine attenuates behavioral deficits and concomitant pathology observed in streptozotocin-induced model of Alzheimer's disease in male rats. Hormon Behav 107:11–19. https://doi.org/10.1016/j.yhbeh.2018.11.007
Ji MH, Wang XM, Sun XR, Zhang H, Ju LS, Qiu LL, Yang JJ, Jia M, Wu J, Yang J (2015) Environmental enrichment ameliorates neonatal sevoflurane exposure-induced cognitive and synaptic plasticity impairments. J Mol Neurosci 57(3):358–365. https://doi.org/10.1007/s12031-015-0627-1
Ji MH, Wang ZY, Sun XR, Tang H, Zhang H, Jia M, Qiu LL, Zhang GF, Peng YG, Yang JJ (2017) Repeated neonatal sevoflurane exposure-induced developmental delays of parvalbumin interneurons and cognitive impairments are reversed by environmental enrichment. Mol Neurobiol 54(5):3759–3770. https://doi.org/10.1007/s12035-016-9943-xx
Ju LS, Jia M, Sun J, Sun XR, Zhang H, Ji MH, Yang JJ, Wang ZY (2016) Hypermethylation of hippocampal synaptic plasticity-related genes is involved in neonatal sevoflurane exposure-induced cognitive impairments in rats. Neurotox Res 29(2):243–255. https://doi.org/10.1007/s12640-015-9585-1
Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H (2007) Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology 53(8):942–950. https://doi.org/10.1016/j.neuropharm.2007.09.005
Li KA, Lund ET, Voigt JP (2016) The impact of early postnatal environmental enrichment on maternal care and offspring behaviour following weaning. Behav Process 122:51–58. https://doi.org/10.1016/j.beproc.2015.11.008
Li J, Chen J, Ma N, Yan D, Wang Y, Zhao X, Zhang Y, Zhang C (2019) Effects of corticosterone on the expression of mature brain-derived neurotrophic factor (mBDNF) and proBDNF in the hippocampal dentate gyrus. Behav Brain Res 365:150–156. https://doi.org/10.1016/j.bbr.2019.03.010
Lu Y, Huang Y, Jiang J, Hu R, Yang Y, Jiang H, Yan J (2016) Neuronal apoptosis may not contribute to the long-term cognitive dysfunction induced by a brief exposure to 2% sevoflurane in developing rats. Biomed Pharmacother 78:322–328. https://doi.org/10.1016/j.biopha.2016.01.034
Lu CQ, Zhong L, Yan CH, Tian Y, Shen XM (2017) Effects of preweaning environmental enrichment on hippocampus-dependent learning and memory in developing rats. Neurosci Lett 640(117–1):22. https://doi.org/10.1016/j.neulet.2016.12.053
Luo F, Hu Y, Zhao W, Zuo Z, Yu Q, Liu Z, Lin J, Feng Y, Li B, Wu L, Xu L (2016) Maternal exposure of rats to isoflurane during late pregnancy impairs spatial learning and memory in the offspring by up-regulating the expression of histone deacetylase 2. PLoS ONE 11(8):e0160826. https://doi.org/10.1371/journal.pone.0160826
Lv X, Yan J, Jiang J, Zhou X, Lu Y, Jiang H (2017) MicroRNA-27a-3p suppression of peroxisome proliferator-activated receptor-gamma contributes to cognitive impairments resulting from sevoflurane treatment. J Neurochem 143(3):306–319. https://doi.org/10.1111/jnc.14208
Mahati K, Bhagya V, Christofer T, Sneha A, Shankaranarayana Rao BS (2016) Enriched environment ameliorates depression-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity. Neurobiol Learn Mem 134(Pt B):379–391. https://doi.org/10.1016/j.nlm.2016.08.017
McLeod F, Marzo A, Podpolny M, Galli S, Salinas P (2017) Evaluation of synapse density in hippocampal rodent brain slices. J Vis Exp. https://doi.org/10.3791/56153
Nieto R, Kukuljan M, Silva H (2013) BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front Psychiatry 4:45. https://doi.org/10.3389/fpsyt.2013.00045
Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7(9):697–709. https://doi.org/10.1038/nrn1970
Noguchi KK, Johnson SA, Dissen GA, Martin LD, Manzella FM, Schenning KJ, Olney JW, Brambrink AM (2017) Isoflurane exposure for three hours triggers apoptotic cell death in neonatal macaque brain. Br J Anaesth 119(3):524–531. https://doi.org/10.1093/bja/aex123
Olutoye OA, Baker BW, Belfort MA, Olutoye OO (2018) Food and Drug Administration warning on anesthesia and brain development: implications for obstetric and fetal surgery. Am J Obstet Gynecol 218(1):98–102. https://doi.org/10.1016/j.ajog.2017.08.107
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23. https://doi.org/10.1038/nrn3379
Sen A, Nelson TJ, Alkon DL (2017) ApoE isoforms differentially regulates cleavage and secretion of BDNF. Mol Brain 10(1):19. https://doi.org/10.1186/s13041-017-0301-3
Servick K (2014) Biomedical research. Researchers struggle to gauge risks of childhood anesthesia. Science 346(6214):1161–1162. https://doi.org/10.1126/science.346.6214.1161
Shan Y, Sun S, Yang F, Shang N, Liu H (2018) Dexmedetomidine protects the developing rat brain against the neurotoxicity wrought by sevoflurane: role of autophagy and Drp1-Bax signaling. Drug Des Dev Therapy 12:3617–3624. https://doi.org/10.2147/DDDT.S180343
Shen FY, Song YC, Guo F, Xu ZD, Li Q, Zhang B, Ma YQ, Zhang YQ, Lin R, Li Y, Liu ZQ (2018) Cognitive impairment and endoplasmic reticulum stress induced by repeated short-term sevoflurane exposure in early life of rats. Front Psychiatry 9:332. https://doi.org/10.3389/fpsyt.2018.00332
Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G (2012) Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology 116(3):586–602. https://doi.org/10.1097/ALN.0b013e318247564d
Song R, Ling X, Peng M, Xue Z, Cang J, Fang F (2017) Maternal sevoflurane exposure causes abnormal development of fetal prefrontal cortex and induces cognitive dysfunction in offspring. Stem Cells Int 2017:6158468. https://doi.org/10.1155/2017/6158468
Suehara T, Morishita J, Ueki M, Ueno M, Maekawa N, Mizobuchi S (2016) Effects of sevoflurane exposure during late pregnancy on brain development of offspring mice. Paediatr Anaesth 26(1):52–59. https://doi.org/10.1111/pan.12785
Tian Y, Chen KY, Liu LD, Dong YX, Zhao P, Guo SB (2018) Sevoflurane exacerbates cognitive impairment induced by abeta 1–40 in rats through initiating neurotoxicity, neuroinflammation, and neuronal apoptosis in rat hippocampus. Mediat Inflamm 2018:3802324. https://doi.org/10.1155/2018/3802324
Titley HK, Brunel N, Hansel C (2017) Toward a neurocentric view of learning. Neuron 95(1):19–32. https://doi.org/10.1016/j.neuron.2017.05.021
Wang S, Peretich K, Zhao Y, Liang G, Meng Q, Wei H (2009) Anesthesia-induced neurodegeneration in fetal rat brains. Pediatr Res 66(4):435–440. https://doi.org/10.1203/PDR.0b013e3181b3381b
Wu Z, Li X, Zhang Y, Tong D, Wang L, Zhao P (2018) Effects of sevoflurane exposure during mid-pregnancy on learning and memory in offspring rats: beneficial effects of maternal exercise. Front Cell Neurosci 12:122. https://doi.org/10.3389/fncel.2018.00122
Xu H, Baracskay P, O'Neill J, Csicsvari J (2019) Assembly responses of hippocampal CA1 place cells predict learned behavior in goal-directed spatial tasks on the radial eight-arm maze. Neuron 101(1):119–132. https://doi.org/10.1016/j.neuron.2018.11.015
Yeh CM, Huang CC, Hsu KS (2012) Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. J Physiol 590(4):991–1010. https://doi.org/10.1113/jphysiol.2011.222042
Zhang J, Dong Y, Zhou C, Zhang Y, Xie Z (2015) Anesthetic sevoflurane reduces levels of hippocalcin and postsynaptic density protein 95. Mol Neurobiol 51(3):853–863. https://doi.org/10.1007/s12035-014-8746-1
Zhao Y, Chen K, Shen X (2015) Environmental enrichment attenuated sevoflurane-induced neurotoxicity through the PPAR-gamma signaling pathway. BioMed Res Int 2015:107149. https://doi.org/10.1155/2015/107149
Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, Xie Z (2013) Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology 118(3):516–526. https://doi.org/10.1097/ALN.0b013e3182834d5d
Zhou Q, Homma KJ, Poo MM (2004) Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44(5):749–757. https://doi.org/10.1016/j.neuron.2004.11.011
Zhou X, da Li W, Yuan BL, Niu LJ, Yang XY, Zhou ZB, Chen XH, Feng X (2016) Lithium treatment prevents apoptosis in neonatal rat hippocampus resulting from sevoflurane exposure. Neurochem Res 41(8):1993–2005. https://doi.org/10.1007/s11064-016-1909-x
Acknowledgements
We thank Yuhua Cui for her assistance in designing of the figures.
Funding
This work was supported by grants from the National Natural Science Foundation of China [Grant No. 81571054] and Tianjin Major Support Program of Science and Technology [Grant No. 18YFZCSY00530].
Author information
Authors and Affiliations
Contributions
All authors have made substantive intellectual contributions to the manuscript. ZY and HW designed the study. JW and JW were responsible for conducting the study and analyzing the data. ZY and PZ wrote the manuscript. JC revised the manuscript. All authors have seen the original study data, reviewed the data analysis, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that there is no conflict of interest.
Ethical Approval
All procedures involving animals in this study were performed in accordance with the Regulations for Research at the Tianjin Medical University and with the approval of the local Animal Care Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Z., Wang, J., Wang, H. et al. Effects of Sevoflurane Exposure During Late Pregnancy on Brain Development and Beneficial Effects of Enriched Environment on Offspring Cognition. Cell Mol Neurobiol 40, 1339–1352 (2020). https://doi.org/10.1007/s10571-020-00821-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00821-6