Abstract
Trace amines and their primary receptor, Trace Amine-Associated Receptor-1 (TAAR1) are widely studied for their involvement in the pathogenesis of neuropsychiatric disorders despite being found in the gastrointestinal tract at physiological levels. With the emergence of the “brain-gut-microbiome axis,” we take the opportunity to review what is known about trace amines in the brain, the defined sources of trace amines in the gut, and emerging understandings on the levels of trace amines in various gastrointestinal disorders. Similarly, we discuss localization of TAAR1 expression in the gut, novel findings that TAAR1 may be implicated in inflammatory bowel diseases, and the reported comorbidities of neuropsychiatric disorders and gastrointestinal disorders. With the emergence of TAAR1 specific compounds as next-generation therapeutics for schizophrenia (Roche) and Parkinson’s related psychoses (Sunovion), we hypothesize a therapeutic benefit of these compounds in clinical trials in the brain-gut-microbiome axis, as well as a potential for thoughtful manipulation of the brain-gut-microbiome axis to modulate symptoms of neuropsychiatric disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brain-gut-microbiome axis is an emerging area of research highlighting the involvement of gastrointestinal microbes with the comorbidities of several neuropsychiatric disorders. The intestinal tract harbors the most abundant ecosystem of bacteria with concentrations ranging from 103 to 1014 bacteria depending on tissue localization (Hillman et al. 2017). The idea that microbes have a beneficial impact on human health predates our current understanding of the microbiome by 100 years, as E. Metchnikoff associated fermented food products with longevity in a rural population, and suggested that lactobacilli could counteract the effects of illness and aging (Metchnikoff and Mitchell 1908). In 2007, the United States National Institutes of Health established the “Human Microbiome Project” to improve the understanding of the microbial flora in human health. The collective genome of the microbial species living on our body, termed metagenome, outnumbers the human genome by a factor of 200 (Qin et al. 2010; Ray et al. 2019). Thus, it is not surprising that the metagenome and its encoded metabolic activities play a crucial role in all aspects of human health and disease (Marcobal et al. 2012). As such there has been a focus on the role of bacterial metabolic byproducts in human health (Jacobs et al. 2016; Santoru et al. 2017; Smith and Macfarlane 1997; Vandenberg et al. 2003; Kisuse et al. 2018). Meanwhile, advances have been made in animal studies using germ-free mice, suggesting that disturbances in the intestinal microbial flora can alter brain chemistry and behavior (Park et al. 2013). About 60% of anxiety and depression patients are described to have intestinal function disturbance, such as in irritable bowel syndrome (Gupta et al. 1997). Recently, irritable bowel syndrome has also been related to changes in intestinal microbiota, including disruption of the intestinal microflora. While there has been a focus on the role of complex carbohydrates and neuroactive short-chain fatty acids (e.g. butyrate, acetate and propionate) in the brain-gut-microbiome axis, some of these same studies provide evidence that trace amine levels are altered in gastrointestinal disorders and neuropsychiatric disorders. Here, we propose the novel hypothesis, that the putative trace amine receptor, Trace Amine-Associated Receptor-1 (TAAR1) can augment gastrointestinal illness and neuropsychiatric disorders as a result of a dysregulated intestinal microbial flora. This review discusses several elements of the brain-gut-microbiome axis as it relates to trace amines, TAAR1, and the role they may play in both neuropsychiatric and comorbid gastrointestinal disorders.
TAAR1 is a G protein-coupled receptor that was deorphanized in 2001 (Borowsky et al. 2001; Bunzow et al. 2001) and has been widely studied as a major regulator of dopamine in neuropsychiatric disorders and in acute and neuroadaptive responses to drugs of abuse; and extensively reviewed (Berry et al. 2017; Christian and Berry 2018; Grandy et al. 2016; Schwartz et al. 2018). Currently, specific TAAR1 compounds are nearing completion of clinical trials for treatment of schizophrenia and Parkinson’s related psychoses (Roche, Sunovion). The predominant endogenous ligands for TAAR1 are classified as ‘trace amines’ and include p-tyramine, β-phenylethylamine, tryptamine, 3-iodothyronamine, and octopamine as well as ‘classical’ monoamine neurotransmitters including histamine, serotonin, and dopamine (Borowsky et al. 2001; Chiellini et al. 2012; Hoefig et al. 2015; Pugin et al. 2017; Sotnikova et al. 2010; van Kessel et al. 2019). Trace amines activate TAAR1 at nanomolar affinities, whereas classical monoamine neurotransmitters activate the receptor at or near micromolar concentrations (Panas et al. 2012; Xie et al. 2007).
The term trace amine was adopted by a study group at the 1975 meeting of the American College of Neuropsychopharmacology (Usdin and Sandler 1976), and it is now often mentioned that the levels of trace amines are < 10 ng/g (Berry 2004; Gainetdinov et al. 2018). trace amines are classically defined as any monoamine with a physiological level less than 1–100 ng/g of tissue weight (Boulton 1974) though oftentimes higher levels are subsequently identified in new tissue assessments of particular amines. Historical studies of trace amines in the body have correlated imbalances in trace amine levels to neuropsychiatric disorders including schizophrenia, substance abuse, depression, attention-deficit hyperactive disorder, and Parkinson’s, and has been extensively reviewed (e.g. Gainetdinov et al. 2018). A role of trace amines in the gut has not been systematically studied, likely because the identification of trace amines and their hypothesized role in neuropsychiatric disorders was decades before the understanding of the microbiome and metabolome. Perhaps because of the focus of trace amines in psychiatric illness over prior decades, the discovery of TAAR1 in 2001 led to a body of research studying the effects of TAAR1 in modulating monoaminergic signaling in the brain. The proposed and known functions of TAAR1 in neuropsychiatric disorders have been extensively reviewed and as such will only be briefly described here when relevant.
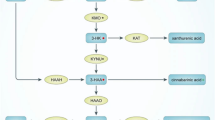
Recent research has increasingly drawn connections between perturbations to the gut microbiota and both gastrointestinal and psychiatric conditions (Felice and O’mahony 2017). The gastrointestinal tract is a heterogeneous layer of tissue comprised of smooth muscle, neuronal cells, immune cells, and epithelial cells. Maintenance of gastrointestinal homeostasis is dynamic and involves the regulation of the epithelial cell monolayer to protect the underlying immune cells and neurons to prevent excessive inflammation (Rao and Wang 2010). There are billions of neurons interconnected via trillions of synapses in the gut and brain, all of which are primarily governed by communication mediated by neuromodulators. One way these modulators are hypothesized to link the gut and brain is by production of aminergic compounds from the gut microbiota—a diverse collection of microbial communities that are thought to influence a wide array of biological processes. Within the realm of neuromodulators originating from the microbiota exist the trace amines (Pugin et al. 2017). Several enteric and food-borne microorganisms are known to produce tyramine and β-phenylethylamine, as listed in Table 1. In fact, around 3 g of un-degraded proteins and peptides enter the human intestine every day from diet, as well as from endogenous sources such as host tissues, pancreatic enzymes and other secretions (Chacko and Cummings 1988). Such large amounts of organic nitrogen-containing compounds are available for catabolism to amino acids, providing essential amino acids to the host (e.g. phenylalanine) and further metabolic degradation by intestinal microorganisms (Fig. 1a) (Rasnik et al. 2017). It has been suggested that in response to an acidification of the environment, microorganisms, such as those listed in Table 1, upregulate several transporters including the tyrosine transporter and a tyrosine decarboxylase (Wolken et al. 2006). Once transported intracellularly, tyrosine is rapidly decarboxylated to tyramine by the bacterial tyrosine decarboxylase (TyrDC), where it is then exported from the microorganism by the tyrosine transporter (TyrP), mechanistically described in Fig. 1b (Wolken et al. 2006). When produced in adequate amounts, gut bacterial-produced trace amines have been shown to have differing effects for the host. For example, β-phenylethylamine is reported as an antimicrobial against pathogenic E. coli (Lynnes et al. 2014), and tyramine has been shown to stimulate fast ileal contractions and neuropeptide Y release (Broadley et al. 2009), as well as increasing synthesis and secretion into circulation of monoamine neurotransmitters (Yano et al. 2015). Modulation of intestinal motility and secretion can have profound effects on luminal pH, mucosal immune response, and delivery of important nutrients to the host cells and enteric microbiota.
a 2 Metabolic byproducts of dietary amino acid metabolism by mammalian and microbial enzymatic systems. Phenylalanine and tyrosine are decarboxylated by the microbial TyrDC & TyrP system and mammalian aromatic amino acid decarboxylases (AADC) to phenylethylamine and tyramine respectively. b Microbial transport and decarboxylation of tyrosine. Tyrosine is taken in by the tyrosine transporter (tyrP) into microbes where the enzymatic decarboxylation of tyrosine to tyramine occurs by the tyrosine decarboxylase (TyrDC) enzyme. Tyramine is then transported to the extracellular space of the microbe by tyrP (Bargossi et al. 2017; Bargossi et al. 2015; Pessione et al. 2009; Wolken et al. 2006). A similar mechanism occurs for decarboxylation of phenylalanine to β-phenylethylamine by tyrosine decarboxylase
TAAR1 localization has been identified in both the stomach and the intestine in mouse and human (Chiellini et al. 2012; Adriaenssens et al. 2015; Ito et al. 2009; Kidd et al. 2008; Ohta et al. 2017; Raab et al. 2015; Revel et al. 2013), but the exact function in the polarized gastrointestinal epithelium remains largely unexplored. Here, it is worth noting that despite no definitive function being identified in the polarized epithelium of the stomach and intestine, TAAR1 functionality has been described in the beta-cells of the pancreas, co-localized with PYY and GLP-1 in duodenal cells, and selective TAAR1 agonists resulted in elevated plasma levels of PYY and GLP-1 (Raab et al. 2015), though these studies are not relevant to our discussion the brain-gut-microbiome axis. Functional TAAR1 was found in almost all peripheral immune cells (Babusyte et al. 2013; Panas et al. 2012; Sriram et al. 2016; Wasik et al. 2012), with evidence that TAAR1 can modulate not only intracellular signaling (Panas et al. 2012), but also immune cell functions such as chemotaxis (Babusyte et al. 2013), phagocytosis [unpublished abstract, Miller Lab (Gwilt et al. 2018)] and altered expression of cytokines (Bugda Gwilt et al. 2019a; Babusyte et al. 2013). Immune cells are known to infiltrate the gastrointestinal tract with epithelial damage and inflammation, and given the reported chemotactic capacity of TAAR1 positive cells towards trace amines, TAAR1 positive immune cells should be noted in the gastrointestinal microenvironment given the propensity of patients with neuropsychiatric disorders to also present with peripheral inflammation.
Our lab has identified TAAR1 expression in the gastrointestinal tract of C57BL/6 mice, summarized in Fig. 2 (Bugda Gwilt et al. 2019b; Ito et al. 2009), in human intestinal epithelial cell lines (unpublished data), as well as enteric glia (Fig. 2c). Interestingly, TAAR1 localization is primarily intracellularly in colonic epithelium (Fig. 2a) whereas in the small intestine it is predominantly found on epithelial membranes (Fig. 2b). The dynamic expression and localization of TAAR1 has been previously described (Bugda Gwilt et al. 2019a; Sriram et al. 2016; Stavrou et al. 2018) and the production of trace amines by several species in the microbiome (e.g. Bargossi et al. 2015; Pessione et al. 2009; van Kessel et al. 2019) may account for the predominant membranous localization of TAAR1 compared to colonic tissue. In the brain-gut-microbiome axis, gut microbes are able to signal by the vagal nerve, mediating behavioral effects in animals (Forsythe et al. 2014). While vagal nerve signaling is demonstrated to have roles in these effects, studies are lacking that investigate the role of non-neuronal-like tissues in the brain-gut-microbiome axis. Given evidence for the localization of TAAR1 in the gastrointestinal epithelium (Fig. 2a), enteric glia (Fig. 2c) and all peripheral immune cells, it is important to understand the role that these tissues have in mediating gut health and modulation of inflammation by the varied sources of trace amines in the gut. Perhaps, TAAR1 activation in these tissues can be damaging, exposing the sensitive underlying tissue to pathogenic microbes, providing a mechanism of exposure of the vagal nerve pathways to microorganism, or food byproducts.
Summary of TAAR1 expression in mouse gastrointestinal tissue Formalin fixed paraffin embedded mouse tissue (a, b) was stained with specific TAAR1 antibody, “D274” designed and published by the Miller lab (Bugda Gwilt et al. 2019a). a TAAR1 localization is seen in apical epithelial cells and is both intracellularly and membrane localized in colon tissue. Colon tissue morphology is as expected, and crypts shapes are depicted by dashed lines for clarity. b TAAR1 localization is seen primarily on membranes of the villus (finger-like projections) and in the base of the crypts. c Ex vivo isolated enteric glial cultures were stained with TAAR1 (green) neurofilament medium (NEFM Red), Actin (pink) and DAPI (blue), demonstrating profound intracellular localization of TAAR1 in ex vivo enteric glial cultures
In addition to bacterial origins, another prominent source of trace amines in the human body is through consumption of fermented food like cheese, pickles, and wine, where the lactic acid bacteria are responsible for production of trace amines in these food products (Marcobal et al. 2012). While the entire Lactobacillus species are considered producers of tyramine (Pessione et al. 2009), only specific Lactobacillus species are found in food products, and some have been found to survive transit through the gastrointestinal tract (Pugin et al. 2017). Bacterial jejunal contents were found to coincide with production of tyramine in the presence of tyrosine decarboxylase ex vivo (Fernandez De Palencia et al. 2011; Van Kessel et al. 2019). When fermented food products are ingested, levels of trace amines in the gut can be raised to undesirable levels (Pugin et al. 2017). As previously discussed, acidification of an environment enriches tyramine production through the TyrP and TyrDC enzymatic systems. Accordingly, tyramine production by Enterococcus species in food is enhanced by lowered pH in the small intestine that can not only simulate rapid passage through the gastrointestinal tract (Fernandez de Palencia et al. 2011), but is common in patients with inflammatory bowel disease (Press et al. 1998).
Dietary trace amines were first described as having a physiological relevance with the advent of a new class of antidepressants: the monoamine oxidase inhibitors. This phenomenon –known as “The Cheese Effect”—has been attributed to accumulation of very high levels of tyramine and β-phenylethylamine in patients being treated with monoamine oxidase inhibitors (Anderson et al. 1993; Price and Smith 1971; Shalaby 1996; Stratton et al. 1991). Patients suffer from severe vasoconstriction as a result of accumulation of very high levels of consumed and bacterial-produced tyramine and phenylethylamine (Anderson et al. 1993; Price and Smith 1971; Shalaby 1996; Stratton et al. 1991). Though the mechanism of the so-called “cheese effect” is not mediated by TAAR1, a similar decrease in monoamine oxidase activity may be present in patients with gastrointestinal illness. A hallmark of several gastrointestinal diseases leads to an ablation of the polarized epithelium, commonly seen in inflammatory bowel diseases, or an altered microbial composition. In either case, it is reasonable to predict that monoamine oxidase enzymatic activity can be affected by an overabundance of aminergic compounds, or an ablation of the cells harboring the enzyme.
Interestingly, fecal metabolomic studies have identified a higher relative abundance of the TyrDC gene and its harboring bacteria Enterococcus in Parkinson’s disease patients who require higher frequency of the levodopa daily dosage regime compared to other Parkinson’s disease patients (van Kessel et al. 2019). TyrDC has the capacity to decarboxylate levodopa into dopamine, which coincides with the conversion of tyrosine into tyramine (van Kessel et al. 2019). Tyramine has been recently been suggested as an early stage biomarker for Parkinson’s due to increased urine tyramine compared to healthy controls (D’andrea et al. 2019). Thus, higher availability of tyrosine or TyrDC in the intestinal tract of those patients may result in accumulation of tyramine, causing detrimental side effects. For example: many patients experience dyskinesias, which have been previously correlated with modulation of the β-arrestin 2 signaling pathway, a pathway that has been previously linked to TAAR1 signaling (Espinoza et al. 2015; Harmeier et al. 2015; Urs et al. 2015).
Tyramine has additional known functions in human intestinal epithelial cell lines (Del Rio et al. 2017), though there are currently no published functional links to a receptor-mediated mechanism by TAAR1 in these epithelial cell models. Briefly—tyramine transiting the gut, presumably from consumption of tyramine rich food—can promote the adherence of microbes to the intestinal epithelial cells (Fernandez De Palencia et al. 2011; Luqman et al. 2018) and can modulate inflammatory cytokine signaling in intestinal epithelial cells (Fernandez de Palencia et al. 2011). Tyramine can also increase the synthesis of serotonin by enteroendocrine cells in the gut, elevating its release into circulation (Yano et al. 2015). Additionally, work from our lab has demonstrated that tyramine activation of bone marrow derived macrophages from C57BL/6 mice augments secretion of inflammatory cytokine gene expression, an effect that is attenuated by the specific TAAR1 antagonist EPPTB (Bugda Gwilt et al. 2019a). Based on reports that dietary trace amines can activate human TAAR1 in primary epithelial cells (Ohta et al. 2017), these specific effects of tyramine in in vitro human epithelial cell models may be attributed to a specific receptor-mediated mechanism by TAAR1 activation. Our ongoing work is currently seeking to delineate this effect.
Both mouse studies and human patients present with elevated tyramine levels compared to healthy controls in gastrointestinal diseases with comorbid neuropsychiatric disorders including: celiac disease (De Angelis et al. 2016; Di Cagno et al. 2011), colorectal cancer (Goedert et al. 2014; Sinha et al. 2016) and inflammatory bowel disease (Santoru et al. 2017; Nagao-Kitamoto et al. 2016). Metabolomic studies have also identified a role of β-phenylethylamine in the fecal metabolome, and altered phenylalanine metabolism in inflammatory bowel disease (Kolho et al. 2017; Paley 2019; Santoru et al. 2017; Yuan et al. 2018). In a human cohort, the fat composition of the diet can mediate the levels of β-phenylethylamine in the fecal metabolome (Kisuse et al. 2018). No receptor-mediated mechanism has been confirmed for either tyramine or β-phenylethylamine to act on the polarized epithelia of the gut, though a recent review by Christian et al. suggests that TAAR1 may mediate some effects in inflammatory bowel diseases (Christian and Berry 2018).
Trace amines from dietary or microbial synthetic pathways have many potential fates in the gut, some of which may be context specific. In the brain, trace amines are rapidly degraded by tissue monoamine oxidases of neurons and supportive cells, although production and circulation in the brain may provide a more limited source of amines than the plentiful sources in the gut. On a cellular level, trace amines can be absorbed by simple diffusion (Berry et al. 2013; Blakeley and Nicol 1978; Tchercansky et al. 1994), facilitated diffusion (Blakeley and Nicol 1978) or by specific monoamine transporters (Xie and Miller 2008). In vitro studies investigating the small intestine epithelial cell line report β-phenylethylamine absorption to be pH dependent, and showing minimal degradation of luminal β-phenylethylamine by intestinal bacteria (Fischer et al. 2010). Similarly, Tchercansky et al. (1994) showed tyramine is absorbed by rat small intestine epithelium by simple diffusion (Tchercansky et al. 1994), and tyramine plasma levels are reported to reach levels of 0.2 μM after ingestion of 200 mg of tyramine in healthy individuals (Vandenberg et al. 2003). Reports of absorption of trace amines in vitro and in ex vivo systems suggests that trace amines in the gut may escape the degradative effects of monoamine oxidase enzymes, even in healthy epithelium.
Trace amine signaling has historically been studied in a wide spectrum of neuropsychiatric disorders, including attention-deficit hyperactive disorder, major depressive disorder, and schizophrenia. TAAR1 is strongly implicated in schizophrenia diagnoses and progression. Several studies have found that patients with schizophrenia have increased levels of tyramine or β-phenylethylamine in the urine (Potkin et al. 1979) and plasma (O’reilly et al. 1991; Shirkande et al. 1995), as well as an increase in comorbid inflammatory bowel disease or irritable bowel syndrome diagnoses (Gupta et al. 1997; Hemmings 2004). Perturbations to the microbiome are reported in both inflammatory bowel disease (e.g. Santoru et al. 2017) and schizophrenia (Severance et al. 2015, 2017), a phenomenon that is reversed with the successful administration of antipsychotics.
Attention-deficit hyperactive disorder (ADHD) is commonly associated with a dysregulation of the trace amine β-phenylethylamine (Baker et al. 1991). Extensive studies on the comorbidities of ADHD and gastrointestinal diagnoses are lacking, though current studies are suggestive that disruption to the gut-brain axis may plays a role in ADHD. Children diagnosed with ADHD exhibit changes to their microbiome compared to healthy controls, and administration of certain strains of bacteria within the first 6 months of life has been shown to have protective effects against ADHD (Felice and O’Mahony 2017). Additionally, preliminary studies indicate an increased level of pro-inflammatory cytokines and decreased levels of both tyramine and β-phenylethylamine in patients with ADHD (Baker et al. 1991; Sandgren and Brummer 2018), indicating a potential connection between the psychological condition and trace amine levels in the body.
Major depressive symptoms are also correlated with decreased urinary levels of β-phenylethylamine (Wolf and Mosnaim 1983), and therapeutics seeking to increase β-phenylethylamine levels naturally with exercise (Szabo et al. 2001) or replacement therapy with β-phenylethylamine (Sabelli and Javaid 1995) both appear to provide relief of major depressive disorder symptoms. Conversely, elevated urine β-phenylethylamine levels are correlated with manic disorders including bipolar affective disorder (Karoum et al. 1982; O’Reilly et al. 1991). Interestingly, there is a correlation of either a diagnosis of irritable bowel syndrome or inflammatory bowel disease within 1 year of diagnoses of depression (Kurina et al. 2001).
Discussion
The recognition of TAAR1 as a mediator for trace amines to act as chemical modulators of the brain-gut-microbiome axis opens up a new avenue for investigation on psychiatric and gastrointestinal disorder comorbidity as well as new treatment avenues for these common disorders. The prevailing hypotheses in neuropsychiatric and gastrointestinal disorders suggest an interplay of genetic and environmental factors in the onset and propagation of disease. Trace amines and their primary receptor, TAAR1, have been widely studied for their involvement in the pathogenesis of neuropsychiatric disorders, which have high comorbidity with gastrointestinal disorders. With the emergence of greater understanding of the brain-gut-microbiome axis, it is now clear that both brain and gut share common communication molecules which can originate endogenously in the host or resident microbiome, or exogenously from ingested food. Here, we take the opportunity to review what is known about trace amines in the brain, the defined sources of trace amines in the gut, and our emerging understanding on the levels of trace amines in various gastrointestinal disorders. We summarize evidence that trace amines are ingested as well as produced by the microbiome, and that their receptor, TAAR1, is present in the gastrointestinal tract. Accordingly, novel TAAR1-targetted therapeutic compounds being advanced in clinical trials as new treatments for neuropsychiatric disorders could potentially have a therapeutic benefit through manipulation of the brain-gut-microbiome axis to modulate symptoms of neuropsychiatric disease. The localization of TAAR1 expression in the gut implicates a mechanism by which trace amines, as well as other endogenous or exogenous TAAR1 ligands, are implicated in inflammatory bowel diseases and the reported comorbidities of neuropsychiatric disorders and gastrointestinal disorders.
Although we focused on reviewing tyramine and β-phenylethylamine, it is important to acknowledge that there are additional trace amines, e.g. tryptamine, which are known in the human metabolome(Jeffery et al. 2012), which have similar effects to tyramine and β-phenylethylamine on gut motility and neurons (Williams et al. 2014), promoting adherence of bacteria to epithelial cells (Luqman et al. 2018), with identified accumulation in both colon cancer and irritable bowel syndrome (Ahmed et al. 2016; Bearcroft et al. 1998; Hong et al. 2011; Ponnusamy et al. 2011). It is also important to recognize that the levels of trace amines and the expression patterns of TAAR1 are highly dynamic and can be affected by diet, drugs, disease and psychological state. Likewise, the variable levels of trace amines may augment the secretion of neuromodulators into circulation, thereby modulating the levels of neurotransmitters in the brain (Yano et al. 2015). Both direct actions of trace amines on TAAR1 in the cells of the intestine and brain, as well as the secondary effects on neurotransmitters (e.g. serotonin, norepinephrine) remains to be further explored.
Localization in the intestine and luminal apical localization (Fig. 2) of epithelial cells in the gut and other polarized epithelia (thyroid) (Szumska et al. 2015) demonstrate a potential yet unexplored significance of TAAR1 in the gut. The commensal microbes of the microbiome niches on luminal apical membranes of the intestine. Additional studies are needed to understand if the effects of tyramine, β-phenylethylamine and other trace amines that are seen in in vitro epithelial cell lines are mediated by TAAR1. Similarly, TAAR1 may have an unappreciated role in the regulation of homeostasis in the gut, as TAAR1 may serve as a microbial sensor in the gastrointestinal tract mediating differentiation of the lumen or polarization of epithelial cells. To understand the role of the microbiota in the regulation of TAAR1 expression and activation, it would be prudent to study TAAR1 expression in germ-free mice or specific pathogen free mice, as some datasets in NCBI GeoData suggest a low level of TAAR1 expression in germ-free and specific pathogen free mice, though the conflicting detection of TAAR1 in RNA-seq data may be confounding these effects.
There is an underappreciated function and role of the so-called ‘elusive trace amines’ and their role in normal human physiology. The emergence of fecal metabolomic studies has classified trace amine levels at micromolar concentrations in the body for the first time, suggesting trace amines may be physiologically active in the gut (Jacobs et al. 2016; Santoru et al. 2017; Smith and Macfarlane 1997; Vandenberg et al. 2003; Kisuse et al. 2018). With the identification of TAAR1 expression in myriad cells in the intestine, there presents a great opportunity to further study complex mechanisms of the brain-gut-microbiome axis as it relates to intestine development, immune cell maturation as it relates to the ‘hygiene hypothesis’ for allergies and immunological disorders. Further, TAAR1 may serve as a novel therapeutic drug target to be further investigated for the treatment comorbid gut and neuropsychiatric disorders.
References
Adriaenssens A, Lam BY, Billing L et al (2015) A transcriptome-led exploration of molecular mechanisms regulating somatostatin-producing D-cells in the gastric epithelium. Endocrinology 156(11):3924–3936. https://doi.org/10.1210/en.2015-1301
Ahmed I, Roy BC, Khan SA et al (2016) Microbiome, metabolome and inflammatory bowel disease. Microorganisms 4(2):20. https://doi.org/10.3390/microorganisms4020020
Anderson MC, Hasan F, McCrodden JM et al (1993) Monoamine oxidase inhibitors and the cheese effect. Neurochem Res 18(11):1145–1149
Babusyte A, Kotthoff M, Fiedler J et al (2013) Biogenic amines activate blood leukocytes via trace amine-associated receptors TAAR1 and TAAR2. J Leukoc Biol 93(3):387–394. https://doi.org/10.1189/jlb.0912433
Baker GB, Bornstein RA, Rouget AC et al (1991) Phenylethylaminergic mechanisms in attention-deficit disorder. Biol Psychiatry 29(1):15–22
Barbieri F, Montanari C, Gardini F et al (2019) Biogenic amine production by lactic acid bacteria: a review. Foods 8(1):17. https://doi.org/10.3390/foods8010017
Bargossi E, Tabanelli G, Montanari C et al (2015) Tyrosine decarboxylase activity of enterococci grown in media with different nutritional potential: tyramine and 2-phenylethylamine accumulation and TyrDC gene expression. Front Microbiol 6:259. https://doi.org/10.3389/fmicb.2015.00259
Bargossi E, Tabanelli G, Montanari C et al (2017) Growth, biogenic amine production and tyrdc transcription of Enterococcus faecalis in synthetic medium containing defined amino acid concentrations. J Appl Microbiol 122(4):1078–1091. https://doi.org/10.1111/jam.13406
Bearcroft CP, Perrett D, Farthing MJ (1998) Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 42(1):42–46. https://doi.org/10.1136/gut.42.1.42
Berry MD (2004) Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem 90:257–271. https://doi.org/10.1111/j.1471-4159.2004.02501.x
Berry MD, Shitut MR, Almousa A et al (2013) Membrane permeability of trace amines: evidence for a regulated, activity-dependent, nonexocytotic, synaptic release. Synapse 67(10):656–667. https://doi.org/10.1002/syn.21670
Berry MD, Gainetdinov RR, Hoener MC et al (2017) Pharmacology of human trace amine-associated receptors: therapeutic opportunities and challenges. Pharmacol Ther 180:161–180. https://doi.org/10.1016/j.pharmthera.2017.07.002
Blakeley AG, Nicol CJ (1978) Accumulation of amines by rabbit erythrocytes in vitro. J Physiol 277:77–90. https://doi.org/10.1113/jphysiol.1978.sp012261
Bonnin-Jusserand M, Grandvalet C, Rieu A et al (2012) Tyrosine-containing peptides are precursors of tyramine produced by Lactobacillus plantarum strain ir BL0076 isolated from wine. BMC Microbiol 12:199. https://doi.org/10.1186/1471-2180-12-199
Borowsky B et al (2001) Trace amines: identification of a family of mammalian G protein coupled receptors. Proc Natl Acad Sci USA 98:8966–8971. https://doi.org/10.1073/pnas.151105198
Borresen T, Klausen NK, Larsen LM et al (1989) Purification and characterisation of tyrosine decarboxylase and aromatic-l-amino-acid decarboxylase. Biochim Biophys Acta 993(1):108–115. https://doi.org/10.1016/0304-4165(89)90149-9
Boulton AA (1974) Letter: amines and theories in psychiatry. Lancet (Lond, Engl) 2(7871):52
Broadley KJ, Akhtar Anwar M, Herbert AA et al (2009) Effects of dietary amines on the gut and its vasculature. Br J Nutr 101(11):1645–1652. https://doi.org/10.1017/S0007114508123431
Bugda Gwilt K, Olliffe N, Hoffing R et al (2019a) Trace amine associated receptor 1 (TAAR1) expression and modulation of inflammatory cytokine production in mouse bone marrow-derived macrophages: a novel mechanism for inflammation in ulcerative colitis. Immunopharmacol Immunotoxicol 41(6):1–9. https://doi.org/10.1080/08923973.2019.1672178
Bugda Gwilt K, Schueler A, Miller G et al (2019b) P132 dextran sulfate sodium-induced colitis is attenuated in trace amine associated receptor 1 knockout mice. Inflamm Bowel Dis 25(Crohn’s and Colitis Congress Supplement_1):S63–S63. https://doi.org/10.1093/ibd/izy393.150
Buňková L, Buňka F, Hlobilová M et al (2009) Tyramine production of technological important strains of lactobacillus, lactococcus and streptococcus. Eur Food Res Technol 229(3):533–538. https://doi.org/10.1007/s00217-009-1075-3
Bunzow JR et al (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60:1181–1188
Chacko A, Cummings JH (1988) Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut 29(6):809–815. https://doi.org/10.1136/gut.29.6.809
Chiellini G, Erba P, Carnicelli V et al (2012) Distribution of exogenous [125I]-3-iodothyronamine in mouse in vivo: relationship with trace amine-associated receptors. J Endocrinol 213(3):223–230. https://doi.org/10.1530/JOE-12-0055
Christian SL, Berry MD (2018) Trace amine-associated receptors as novel therapeutic targets for immunomodulatory disorders. Front Pharmacol 9:680. https://doi.org/10.3389/fphar.2018.00680
Coton E, Coton M (2009) Evidence of horizontal transfer as origin of strain to strain variation of the tyramine production trait in lactobacillus brevis. Food Microbiol 26(1):52–57. https://doi.org/10.1016/j.fm.2008.07.009
Coton M, Coton E, Lucas P et al (2004) Identification of the gene encoding a putative tyrosine decarboxylase of Carnobacterium divergens 508. Development of molecular tools for the detection of tyramine-producing bacteria. Food Microbiol 21(2):125–130. https://doi.org/10.1016/j.fm.2003.10.004
Coton M, Fernandez M, Trip H et al (2011) Characterization of the tyramine-producing pathway in Sporolactobacillus sp. P3j. Microbiology 157(Pt 6):1841–1849. https://doi.org/10.1099/mic.0.046367-0
D’Andrea G, Pizzolato G, Gucciardi A et al (2019) Different circulating trace amine profiles in de novo and treated Parkinson’s disease patients. Sci Rep 9(1):6151. https://doi.org/10.1038/s41598-019-42535-w
De Angelis M, Vannini L, Di Cagno R et al (2016) Salivary and fecal microbiota and metabolome of celiac children under gluten-free diet. Int J Food Microbiol 239:125–132. https://doi.org/10.1016/j.ijfoodmicro.2016.07.025
de Las Rivas B, Ruiz-Capillas C, Carrascosa AV et al (2008) Biogenic amine production by gram-positive bacteria isolated from spanish dry-cured “chorizo” sausage treated with high pressure and kept in chilled storage. Meat Sci 80(2):272–277. https://doi.org/10.1016/j.meatsci.2007.12.001
Del Rio B, Redruello B, Linares DM et al (2017) The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem 218:249–255. https://doi.org/10.1016/j.foodchem.2016.09.046
Di Cagno R, De Angelis M, De Pasquale I et al (2011) Duodenal and faecal microbiota of celiac children: molecular, phenotype and metabolome characterization. BMC Microbiol 11:219. https://doi.org/10.1186/1471-2180-11-219
Espinoza S, Ghisi V, Emanuele M et al (2015) Postsynaptic D2 dopamine receptor supersensitivity in the striatum of mice lacking TAAR1. Neuropharmacology 93:308–313. https://doi.org/10.1016/j.neuropharm.2015.02.010
Felice VD, O’Mahony SM (2017) The microbiome and disorders of the central nervous system. Pharmacol Biochem Behav 160:1–13. https://doi.org/10.1016/j.pbb.2017.06.016
Fernandez de Palencia P, Fernandez M, Mohedano ML et al (2011) Role of tyramine synthesis by food-borne Enterococcus durans in adaptation to the gastrointestinal tract environment. Appl Environ Microbiol 77(2):699–702. https://doi.org/10.1128/AEM.01411-10
Fischer W, Neubert RH, Brandsch M (2010) Transport of phenylethylamine at intestinal epithelial (CACO-2) cells: mechanism and substrate specificity. Eur J Pharm Biopharm 74(2):281–289. https://doi.org/10.1016/j.ejpb.2009.11.014
Forsythe P, Bienenstock J, Junze WA (2014) Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol 817:115. https://doi.org/10.1007/978-1-4939-0897-4_5
Gainetdinov RR, Hoener MC, Berry MD (2018) Trace amines and their receptors. Pharmacol Rev 70(3):549–620. https://doi.org/10.1124/pr.117.015305
Goedert JJ, Sampson JN, Moore SC et al (2014) Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis 35(9):2089–2096. https://doi.org/10.1093/carcin/bgu131
Grandy DK, Miller GM, Li JX (2016) “TAARgeting addiction”–the alamo bears witness to another revolution: an overview of the plenary symposium of the 2015 behavior, biology and chemistry conference. Drug Alcohol Depend 159:9–16. https://doi.org/10.1016/j.drugalcdep.2015.11.014
Gupta S, Masand PS, Kaplan D et al (1997) The relationship between schizophrenia and irritable bowel syndrome (IBS). Schizophr Res 23(3):265–268
Gwilt K, Hoffing RNO et al (2018) Abstract: nutritional regulation of gastrointestinal inflammatory responses: a case for biogenic amines; RISE Research Innovation and Scholarship Expo: 2018. Northeastern University. https://www.northeastern.edu/rise/presentations/nutritional-regulation-of-gastrointestinal-inflammatory-responses-a-case-for-biogenic-amines/. Accessed 25 Sept 2019
Harmeier A, Obermueller S, Meyer CA et al (2015) Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur Neuropsychopharmacol 25(11):2049–2061. https://doi.org/10.1016/j.euroneuro.2015.08.011
Hemmings G (2004) Schizophrenia. Lancet 364(9442):1312–1313. https://doi.org/10.1016/S0140-6736(04)17181-X
Hillman ET, Lu H, Yao T et al (2017) Microbial ecology along the gastrointestinal tract. Microb Environ 32(4):300. https://doi.org/10.1264/jsme2.ME17017
Hoefig CS, Wuensch T, Rijntjes E et al (2015) Biosynthesis of 3-iodothyronamine from T4 in murine intestinal tissue. Endocrinology 156(11):4356–4364. https://doi.org/10.1210/en.2014-1499
Hong YS, Hong KS, Park MH et al (2011) Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol 45(5):415–425. https://doi.org/10.1097/MCG.0b013e318207f76c
Ito J, Ito M, Nambu H et al (2009) Anatomical and histological profiling of orphan g-protein-coupled receptor expression in gastrointestinal tract of c57bl/6j mice. Cell Tissue Res 338(2):257–269. https://doi.org/10.1007/s00441-009-0859-x
Jacobs JP, Goudarzi M, Singh N et al (2016) A disease-associated microbial and metabolomics state in relatives of pediatric inflammatory bowel disease patients. Cell Mol Gastroenterol Hepatol 2(6):750–766. https://doi.org/10.1016/j.jcmgh.2016.06.004
Jeffery IB, O’Toole PW, Ohman L et al (2012) An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61(7):997–1006. https://doi.org/10.1136/gutjnl-2011-301501
Karoum F, Linnoila M, Potter WZ et al (1982) Fluctuating high urinary phenylethylamine excretion rates in some bipolar affective disorder patients. Psychiatry Res 6(2):215–222
Kidd M, Modlin IM, Gustafsson BI et al (2008) Luminal regulation of normal and neoplastic human ec cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 295(2):G260. https://doi.org/10.1152/ajpgi.00056.2008
Kisuse J, La-Ongkham O, Nakphaichit M et al (2018) Urban diets linked to gut microbiome and metabolome alterations in children: a comparative cross-sectional study in thailand. Front Microbiol 9:1345. https://doi.org/10.3389/fmicb.2018.01345
Kolho KL, Pessia A, Jaakkola T et al (2017) Faecal and serum metabolomics in paediatric inflammatory bowel disease. J Crohns Colitis 11(3):321–334. https://doi.org/10.1093/ecco-jcc/jjw158
Kurina LM, Goldacre MJ, Yeates D et al (2001) Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health 55(10):716–720. https://doi.org/10.1136/jech.55.10.716
La Gioia F, Rizzotti L, Rossi F et al (2011) Identification of a tyrosine decarboxylase gene (tdca) in streptococcus thermophilus 1tt45 and analysis of its expression and tyramine production in milk. Appl Environ Microbiol 77(3):1140–1144. https://doi.org/10.1128/AEM.01928-10
Ladero V, Fernandez M, Calles-Enriquez M et al (2012) Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol 30(1):132–138. https://doi.org/10.1016/j.fm.2011.12.016
Ladero V, Linares DM, Del Rio B et al (2013) Draft genome sequence of the tyramine producer enterococcus durans strain ipla 655. Genome Announc 1(3):e00265. https://doi.org/10.1128/genomeA.00265-13
Landete JM, Ferrer S, Pardo I (2005) Which lactic acid bacteria are responsible for histamine production in wine? J Appl Microbiol 99(3):580–586. https://doi.org/10.1111/j.1365-2672.2005.02633.x
Leisner JJ, Laursen BG, Prevost H et al (2007) Carnobacterium: positive and negative effects in the environment and in foods. FEMS Microbiol Rev 31(5):592–613. https://doi.org/10.1111/j.1574-6976.2007.00080.x
Linares DM, Martin MC, Ladero V et al (2011) Biogenic amines in dairy products. Crit Rev Food Sci Nutr 51(7):691–703. https://doi.org/10.1080/10408398.2011.582813
Luqman A, Nega M, Nguyen MT et al (2018) SADA-expressing staphylococci in the human gut show increased cell adherence and internalization. Cell Rep 22(2):535–545. https://doi.org/10.1016/j.celrep.2017.12.058
Lynnes T, Horne SM, Pruss BM (2014) B-phenylethylamine as a novel nutrient treatment to reduce bacterial contamination due to Escherichia coli O157:H7 on beef meat. Meat Sci 96(1):165–171. https://doi.org/10.1016/j.meatsci.2013.06.030
Maifreni M, Frigo F, Bartolomeoli I et al (2013) Identification of the enterobacteriaceae in montasio cheese and assessment of their amino acid decarboxylase activity. J Dairy Res 80(1):122–127. https://doi.org/10.1017/S002202991200074X
Marcobal A, de Las Rivas B, Munoz R (2006) First genetic characterization of a bacterial beta-phenylethylamine biosynthetic enzyme in enterococcus faecium rm58. FEMS Microbiol Lett 258(1):144–149. https://doi.org/10.1111/j.1574-6968.2006.00206.x
Marcobal A, de Las Rivas B, Landete JM et al (2012) Tyramine and phenylethylamine biosynthesis by food bacteria. Crit Rev Food Sci Nutr 52(5):448–467. https://doi.org/10.1080/10408398.2010.500545
Metchnikoff E, Mitchell PC (1908) The prolongation of life: optimistic studies. G.P. Putnam’s Sons, New York
Min J-S, Lee S-O, Jang A et al (2004) Production of biogenic amines by microflora inoculated in meats. Asian Australas J Anim Sci 17(10):1472–1478. https://doi.org/10.5713/ajas.2004.1472
Moreno-Arribas V, Lonvaud-Funel A (2001) Purification and characterization of tyrosine decarboxylase of lactobacillus brevis ioeb 9809 isolated from wine. FEMS Microbiol Lett 195(1):103–107. https://doi.org/10.1111/j.1574-6968.2001.tb10505.x
Nagao-Kitamoto H, Shreiner AB, Gillilland MG et al (2016) Functional characterization of inflammatory bowel disease-associated gut dysbiosis in gnotobiotic mice. Cell Mol Gastroenterol Hepatol 2(4):468–481. https://doi.org/10.1016/j.jcmgh.2016.02.003
Ohta H, Takebe Y, Murakami Y et al (2017) Tyramine and beta-phenylethylamine, from fermented food products, as agonists for the human trace amine-associated receptor 1 (hTAAR1) in the stomach. Biosci Biotechnol Biochem 81(5):1002–1006. https://doi.org/10.1080/09168451.2016.1274640
O’Reilly R, Davis BA, Durden DA et al (1991) Plasma phenylethylamine in schizophrenic patients. Biol Psychiatry 30(2):145–150
Paley EL (2019) Diet-related metabolic perturbations of gut microbial shikimate pathway-tryptamine-trna aminoacylation-protein synthesis in human health and disease. Int J Tryptophan Res 12:1178646919834550. https://doi.org/10.1177/1178646919834550
Panas MW, Xie Z, Panas HN et al (2012) Trace amine associated receptor 1 signaling in activated lymphocytes. J Neuroimmune Pharmacol 7(4):866–876. https://doi.org/10.1007/s11481-011-9321-4
Park AJ, Collins J, Blennerhassett PA et al (2013) Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil 25(9):733. https://doi.org/10.1111/nmo.12153
Perin LM, Belviso S, Bello BD, Nero LA, Cocolin L (2017) Technological properties and biogenic amines production by bacteriocinogenic lactococci and enterococci strains isolated from raw goat’s milk. J Food Prot 80:151–157. https://doi.org/10.4315/0362-028X.JFP-16-267
Pessione E, Mazzoli R, Giuffrida MG et al (2005) A proteomic approach to studying biogenic amine producing lactic acid bacteria. Proteomics 5(3):687–698. https://doi.org/10.1002/pmic.200401116
Pessione E, Pessione A, Lamberti C et al (2009) First evidence of a membrane-bound, tyramine and beta-phenylethylamine producing, tyrosine decarboxylase in Enterococcus faecalis: a two-dimensional electrophoresis proteomic study. Proteomics 9(10):2695–2710. https://doi.org/10.1002/pmic.200800780
Pircher A, Bauer F, Paulsen P (2007) Formation of cadaverine, histamine, putrescine and tyramine by bacteria isolated from meat, fermented sausages and cheeses. Zeitschrift für Lebensmittel- Untersuchung und -Forschung A 226(1):225–231. https://doi.org/10.1007/s00217-006-0530-7
Ponnusamy K, Choi JN, Kim J et al (2011) Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol 60(Pt 6):817–827. https://doi.org/10.1099/jmm.0.028126-0
Potkin SG, Karoum F, Chuang LW et al (1979) Phenylethylamine in paranoid chronic schizophrenia. Science 206(4417):470–471. https://doi.org/10.1126/science.504988
Press AG, Hauptmann IA, Hauptmann L et al (1998) Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol Ther 12(7):673–678. https://doi.org/10.1046/j.1365-2036.1998.00358.x
Price K, Smith SE (1971) Cheese reaction and tyramine. Lancet 1(7690):130–131
Pugin B, Barcik W, Westermann P et al (2017) A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb Ecol Health Dis 28(1):1353881. https://doi.org/10.1080/16512235.2017.1353881
Qin J, Li R, Raes J et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. https://doi.org/10.1038/nature08821
Rao JN, Wang JY (2010) Intestinal architecture and development. In: Neil Granger D, Granger JP (eds) Regulation of gastrointestinal mucosal growth. Integrated systems physiology: from molecule to function to disease. Morgan & Claypool Life Sciences, San Rafael, CA
Raab S, Wang H, Uhles S et al (2015) Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol Metab 5(1):47–56. https://doi.org/10.1016/j.molmet.2015.09.015
Rasnik KS, Hsin-Wen C, Di Y et al (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15(1):1–17. https://doi.org/10.1186/s12967-017-1175-y
Ray KJ, Cotter SY, Arzika AM et al (2019) High-throughput sequencing of pooled samples to determine community-level microbiome diversity. Ann Epidemiol. https://doi.org/10.1016/j.annepidem.2019.09.002
Revel FG, Moreau JL, Pouzet B et al (2013) A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18(5):543–556. https://doi.org/10.1038/mp.2012.57
Sabelli HC, Javaid JI (1995) Phenylethylamine modulation of affect: therapeutic and diagnostic implications. J Neuropsychiatry Clin Neurosci 7(1):6–14. https://doi.org/10.1176/jnp.7.1.6
Sandgren AM, Brummer RJM (2018) Adhd-originating in the gut? The emergence of a new explanatory model. Med Hypotheses 120:135–145. https://doi.org/10.1016/j.mehy.2018.08.022
Santoru ML, Piras C, Murgia A et al (2017) Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci Rep 7(1):9523. https://doi.org/10.1038/s41598-017-10034-5
Schwartz MD, Canales JJ, Zucchi R et al (2018) Trace amine-associated receptor 1: a multimodal therapeutic target for neuropsychiatric diseases. Expert Opin Ther Targ 22(6):513–526. https://doi.org/10.1080/14728222.2018.1480723
Severance EG, Prandovszky E, Castiglione J et al (2015) Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep 17(5):27. https://doi.org/10.1007/s11920-015-0574-0
Severance EG, Gressitt KL, Stallings CR et al (2017) Probiotic normalization of candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun 62:41–45. https://doi.org/10.1016/j.bbi.2016.11.019
Shalaby AR (1996) Significance of biogenic amines to food safety and human health. Food Res Int 29(7):675–690. https://doi.org/10.1016/S0963-9969(96)00066-X
Shirkande S, O’Reilly R, Davis B et al (1995) Plasma phenylethylamine levels of schizophrenic patients. Can J Psychiatry 40(4):221. https://doi.org/10.1177/070674379504000417
Sinha R, Ahn J, Sampson JN et al (2016) Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. PLoS ONE 11(3):e0152126. https://doi.org/10.1371/journal.pone.0152126
Smith EA, Macfarlane GT (1997) Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 29:327–337. https://doi.org/10.1006/anae.1997.0121
Sotnikova TD et al (2010) The dopamine metabolite 3-methoxytyramine is a neuromodulator. PLoS ONE 5:e13452. https://doi.org/10.1371/journal.pone.0013452
Sriram U, Cenna JM, Haldar B et al (2016) Methamphetamine induces trace amine-associated receptor 1 (TAAR1) expression in human T lymphocytes: role in immunomodulation. J Leukoc Biol 99(1):213–223. https://doi.org/10.1189/jlb.4A0814-395RR
Stavrou S, Gratz M, Tremmel E et al (2018) TAAR1 induces a disturbed GSK3beta phosphorylation in recurrent miscarriages through the odc. Endocr Connect 7(2):372–384. https://doi.org/10.1530/EC-17-0272
Stratton J, Hutkins RW, Taylor S (1991) Biogenic-amines in cheese and other fermented foods—a review. J Food Prot. https://doi.org/10.4315/0362-028X-54.6.460
Szabo A, Billett E, Turner J (2001) Phenylethylamine, a possible link to the antidepressant effects of exercise? Br J Sports Med 35(5):342–343. https://doi.org/10.1136/bjsm.35.5.342
Szumska J, Qatato M, Rehders M et al (2015) Trace amine-associated receptor 1 localization at the apical plasma membrane domain of fisher rat thyroid epithelial cells is confined to cilia. Eur Thyroid J 4(Suppl 1):30–41. https://doi.org/10.1159/000434717
Tchercansky DM, Acevedo C, Rubio MC (1994) Studies of tyramine transfer and metabolism using an in vitro intestinal preparation. J Pharm Sci 83(4):549–552
Turroni S, Fiori J, Rampelli S et al (2016) Fecal metabolome of the Hadza hunter-gatherers: a host-microbiome integrative view. Sci Rep 6:32826. https://doi.org/10.1038/srep32826
Urs NM, Bido S, Peterson SM et al (2015) Targeting beta-arrestin2 in the treatment of L-dopa-induced dyskinesia in Parkinson’s disease. Proc Natl Acad Sci 112(19):E2517–E2526. https://doi.org/10.1073/pnas.1502740112
Usdin E, Sandler M (1976) Trace amines and the brain. In: Annual Meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, 1976. Marcel Dekker
van Kessel SP, Frye AK, El-Gendy AO et al (2019) Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of parkinson’s disease. Nat Commun 10(1):310. https://doi.org/10.1038/s41467-019-08294-y
Vandenberg CM, Blob LF, Kemper EM et al (2003) Tyramine pharmacokinetics and reduced bioavailability with food. J Clin Pharmacol 43(6):604–609. https://doi.org/10.1177/0091270003253425
Wasik AM, Millan MJ, Scanlan T et al (2012) Evidence for functional trace amine associated receptor-1 in normal and malignant B cells. Leuk Res 36(2):245–249. https://doi.org/10.1016/j.leukres.2011.10.002
Williams BB, Van Benschoten AH, Cimermancic P et al (2014) Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16(4):495–503. https://doi.org/10.1016/j.chom.2014.09.001
Wolf ME, Mosnaim AD (1983) Phenylethylamine in neuropsychiatric disorders. Gen Pharmacol 14(4):385–390
Wolken WA, Lucas PM, Lonvaud-Funel A et al (2006) The mechanism of the tyrosine transporter TyrP supports a proton motive tyrosine decarboxylation pathway in lactobacillus brevis. J Bacteriol 188(6):2198–2206. https://doi.org/10.1128/JB.188.6.2198-2206.2006
Xie Z et al. (2007) Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther 321:116–127. https://doi.org/10.1124/jpet.106.116863
Xie Z, Miller GM (2008) Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: implication for modulatory roles of trace amines in brain. J Pharmacol Exp Ther 325(2):617–628. https://doi.org/10.1124/jpet.107.134247
Yano JM, Yu K, Donaldson GP et al (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161(2):264–276. https://doi.org/10.1016/j.cell.2015.02.047
Yuan BF, Zhu QF, Guo N et al (2018) Comprehensive profiling of fecal metabolome of mice by integrated chemical isotope labeling-mass spectrometry analysis. Anal Chem 90(5):3512–3520. https://doi.org/10.1021/acs.analchem.7b05355
Zhu H, Xu G, Zhang K et al (2016) Crystal structure of tyrosine decarboxylase and identification of key residues involved in conformational swing and substrate binding. Sci Rep 6:27779. https://doi.org/10.1038/srep27779
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bugda Gwilt, K., González, D.P., Olliffe, N. et al. Actions of Trace Amines in the Brain-Gut-Microbiome Axis via Trace Amine-Associated Receptor-1 (TAAR1). Cell Mol Neurobiol 40, 191–201 (2020). https://doi.org/10.1007/s10571-019-00772-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-019-00772-7