Abstract
Both sex and steroid hormones are important to consider in human ischemic stroke and its experimental models. Stroke initiates a cascade of changes that lead to neural cell death, but also activates endogenous protective processes that counter the deleterious consequences of ischemia. Steroids may be part of these cerebroprotective processes. One option to provide cerebroprotection is to reinforce these intrinsic protective mechanisms. In the current review, we first summarize studies describing sex differences and the influence of steroid hormones in stroke. We then present and discuss our recent results concerning differential changes in endogenous steroid levels in the brains of male and female mice and the importance of progesterone receptors (PR) during the early phase after stroke. In the third part, we give an overview of experimental studies, including ours, that provide evidence for the pleiotropic beneficial effects of progesterone and its promising cerebroprotective potential in stroke. We also highlight the key role of PR signaling as well as potential additional mechanisms by which progesterone may provide cerebroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is caused by the interruption of blood supply to brain tissue due to the occlusion of an artery by a thrombus, whereas the rupture of a brain artery leads to hemorrhagic stroke (Amarenco et al. 2009). Cerebral ischemia results in the rapid death of neural cells and in neurological deficits such as loss of some sensorimotor functions, paralysis, depression, and dementia. It is the leading cause of adult disability and the second leading cause of dementia and death in industrialized countries (Lo et al. 2003; Feigin et al. 2009).
Sex is a crucial parameter to consider when designing experiments to investigate the pathophysiology of stroke and to develop therapeutic pharmacological strategies. This aspect has been neglected for a long time, most studies having been carried out in male animals to avoid variability related to the female estrus cycle. However, recently, distinct pathophysiological mechanisms and different therapeutic responses according to sex have been described, notably those concerning brain functions (McCarthy et al. 2012). Incorporation of sex as a variable in the design of experiments is highly needed and recommended (http://www.nimh.nih.gov/researchfunding/scientific-meetings/2011/sex-differences-in-brain-behavior-mental-health-and-mental-disorders/index.shtml).
Several studies have shown that both sex and steroids are important factors to be taken into consideration when studying injury mechanisms and outcomes following brain ischemia (Cheng and Hurn 2010; Choleris et al. 2018; Herson and Hurn 2010). Recently, Liberale and colleagues have nicely reviewed and discussed the multiple sex differences in ischemic stroke (Liberale et al. 2018).
Sex Differences in Stroke
In humans, sex has an important impact on the etiology of stroke. Epidemiological studies have indeed revealed marked sex differences in stroke incidence, prevalence, ethiology, severity outcomes, and death (Reeves et al. 2008; Appelros et al. 2010; Haast et al. 2012; Wilson 2013; Gibson 2013). Stroke incidence and mortality rates are higher in men than in women, who are considered to be protected by their ovarian hormones (Petrea et al. 2009). Importantly, there exists an interaction between sex and age (Roy-O’Reilly and McCullough 2018). Whereas stroke rates increase with age in both sexes, starting after the age of 50, they become significantly higher in women when compared to men around the age of 80 (Petrea et al. 2009; Roger et al. 2011; Mozaffarian et al. 2016).
Experimental studies have demonstrated that sex is a key parameter for ischemic stroke outcomes. Transient or permanent middle cerebral artery occlusion (MCAO) with a filament is a commonly used model of ischemic stroke in rodents. Transient MCAO, followed by abrupt reperfusion, may be considered as a translational model of endovascular thrombectomy, which has become a reference therapy for patients with large vessel occlusion (Sutherland et al. 2016).
During their reproductive period, young female rats show smaller ischemic brain infarcts than aged-matched male rats after transient MCAO (Alkayed et al. 1998). However, this sex difference is no longer observed in females deprived of their ovarian steroids by ovariectomy and in aging senescent females (Alkayed et al. 1998, 2000). In another study, young female rats showed smaller infarcts and reduced sensory-motor deficits than young male rats or middle-aged female rats (Selvamani et al. 2014). Sex differences in the susceptibility to ischemia and its outcomes have been shown in different experimental models, of note even in the presence of comorbidities such as diabetes or hypertension (Toung et al. 2000; Carswell et al. 1999; Herson and Hurn 2010; Li et al. 2004).
Mechanisms underlying these sex differences include cell death pathways after ischemic stroke (Reeves et al. 2008; Yuan et al. 2009; Liu et al. 2011; Gibson 2013; Sohrabji et al. 2017; Choleris et al. 2018). In males, ischemic cell death is mainly the consequence of the activation of the poly (ADP ribose) polymerase 1 (PARP-1), a DNA repair enzyme involved in the caspase-independent pathway of apoptosis. The oxidative stress due to ischemia leads to the formation of single stranded DNA molecules, causing the activation of PARP-1. This activation induces cytosolic nicotinamide adenine dinucleotide (NAD) depletion, inhibition of glycolysis and depolarization of mitochondria and finally cell death (Alano et al. 2010). Conversely, in females, ischemic neural cell death mainly involves caspase-9 and caspase-3 activation. Thus, pharmacological inhibition of PARP-1 improved outcomes in males but not in females (Eliasson et al. 1997; McCullough et al. 2005), whereas caspase inhibition was beneficial in females but not in males (Liu et al. 2011). Interestingly, one study reported that deletion of PARP-1 increased ischemic damage in females but reduced infarct in males (Liu et al. 2011).
Sex Steroids and Stroke
In both women and men, age is the strongest risk factor for ischemic stroke, and the lifetime risk of stroke in both sexes shows a gradual increase from the age of 50 onwards (Petrea et al. 2009; Chen et al. 2010). Moreover, stroke mortality, morbidity and poor functional recovery are higher in the elderly. In women, stroke rates increase after menopause and become higher at an advanced age when compared with men (Petrea et al. 2009). The increase in stroke risk after menopause has been related to hormonal changes. Indeed, circulating levels of estradiol are reduced by more than 90% in women 5 years after menopause when compared to premenopausal women, and they even further decline thereafter (Rothman et al. 2011). However, it is important to note that postmenopausal women are not completely deprived of estrogens, as both estrone (about 40 pg/ml), its sulfated conjugate (about 250 pg/ml), testosterone (about 100 pg/ml), dehydroepiandrosterone (DHEA, about 2 ng/ml), and dehydroepiandrosterone sulfate (DHEAS, about 600 ng/ml) continue to circulate at significant levels in postmenopausal women as determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Rothman et al. 2011; Wang et al. 2015; Martel et al. 2016). Estrone can be converted to estradiol by the 17ß-hydroxysteroid dehydrogenase, and androgens to estrogens by the aromatase, an enzyme abundant in brain, adipose tissues, bone and liver (Arevalo et al. 2015). Circulating DHEA and DHEAS, provided by the postmenopausal ovaries (about 20%) and adrenal glands, are a major precursor for the synthesis of biologically active steroid hormones inside hormone-sensitive tissues (Labrie 2015).
Increased stroke risk in women related to menopause has also been associated with the age-dependent increase in risk factors, including abdominal adiposity, increased levels of triglycerides and cholesterol, enhanced insulin resistance and elevated blood pressure (Lisabeth and Bushnell 2012). Interestingly, many of the stroke risk factors, including metabolic dysfunctions and a generalized proinflammatory milieu, are consequences of decreased ovarian functions (Della Torre et al. 2014). Androgens may increase blood pressure which is a major risk factor for stroke in men. Thus, in addition to the loss of female sex hormones, the increasing levels of testosterone with aging may contribute to the increase in blood pressure and to the greater risk of stroke in women after menopause (Reckelhoff 2001).
Over the past two decades, obesity rates increased (Ford et al. 2014; Ogden et al. 2015) and as consequence, the related disorders are also increasing. Obesity is a risk factor of different diseases including hypertension, sleep disorders, atherosclerosis, cardiovascular diseases, stroke, and diabetes (Sharma et al. 2017). There is a sexual dimorphism with respect to adipose tissue deposition, distribution, and function; sex steroid hormones play a key role as endogenous regulators of adiposity (Palmer and Clegg 2015; White and Tchoukalova 2014). For example, estrogens protect against obesity by decreasing food intake and increasing energy expenditure in women. Men show more visceral adipose deposits which are positively correlated with growing cardiovascular risk. In contrast, females shows subcutaneous adipose deposits before menopause; this feature is associated with lower cardiovascular risk. After menopause, the decrease of estrogens leads to a shift in favor of the visceral fat, as seen in men, and an increase in cardiovascular risks and stroke (Palmer and Clegg 2015).
In addition to the systemic health factors, age-related changes in the brain contribute to its increased vulnerability to ischemic injury. They comprise multiple degenerative processes, structural changes in white matter, small-vessel diseases, reduced brain weight and intraneuronal inclusions of tau and α-synuclein (Chen et al. 2010).
In men, similar to women, the incidence of stroke gradually increases from the age of 50 onwards (Petrea et al. 2009). However, in contrasts to the ovaries, there is no abrupt decline in testicular activity at this age. Instead, testosterone levels gradually decrease in men from about 40 years onwards (Andersson et al. 2007; Huhtaniemi et al. 2012). However, it is important to note that individuals differ in terms of hormonal aging. Although the mean decrease in testosterone levels in men is gradual, the rate of decline is more important in some aging men than in others. The incidence of hypogonadal testosterone levels increases progressively: about 20% of men over 60 and about 50% of men over 80 years of age (Harman et al. 2001).
According to the prevailing consensus, ovarian estradiol and progesterone protect women against stroke, but the role of androgens in men is more controversial. As in the young adult population, men have a higher incidence of stroke and elevated levels of androgens have been suspected to represent a risk factor for stroke vulnerability. However, there is little evidence for this assumption, and the established link between anabolic steroid abuse and cardiovascular pathologies does not provide information on the role of endogenous androgens (Quillinan et al. 2014). On the other hand, the age-dependent increase in the incidence and severity of stoke in men suggests a protective effect of androgens (Quillinan et al. 2014). However, in spite of reported benefits of the therapeutic normalization of low testosterone levels (Sharma et al. 2015), concerns have been raised about the cardiovascular safety of testosterone therapy in aging men (Vigen et al. 2013). When discussing the usefulness of testosterone replacement, it is important to be aware that elevated levels of DHEA and DHEAS contribute to the pool of androgens and estrogens present in aging men, as they do in women (Labrie 2010).
Animal studies have provided strong evidence for cerebroprotective effects of steroid hormones after MCAO. We briefly review here the roles of estrogens and androgens. The effects of progesterone and its metabolites are discussed in detail in paragraph 4. The cerebroprotective effects of estrogens after an ischemic insult have been documented by a large number of studies and have been extensively reviewed (Wise et al. 2001; McCullough and Hurn 2003; Gibson et al. 2006; Herson et al. 2009; Lebesgue et al. 2009; Strom et al. 2009; Liu et al. 2010; Inagaki and Etgen 2013; Hurn and Macrae 2000). Treatment with either low or supra-physiological doses of estradiol has been shown to protect the female rat brain against stroke injury (Dubal et al. 1998; Suzuki et al. 2009; Carpenter et al. 2016). Importantly, protective effects of estrogen treatment are also observed in the presence of comorbidities such as diabetes (Toung et al. 2000).
A systematic meta-analysis has revealed that most experimental studies showing protective effects of estradiol after ischemic stroke used ovariectomized females. Some of the studies performed in gonadally intact young adult females failed to show beneficial effects of estradiol treatment, most likely because of the protective effects of endogenous ovarian hormones (Gibson et al. 2006). Cerebroprotective effects of endogenous ovarian hormones have been demonstrated by ovariectomy. Removing the ovaries of female rats indeed resulted in increased infarct volumes (Alkayed et al. 1998; Rusa et al. 1999; Inagaki and Etgen 2013). Larger infarcts were observed in cycling female rats in proestrus phase when their circulating levels of estrogens are low comparatively to metestrus (Carswell et al. 2000). Moreover, plasma estradiol levels have been shown to be inversely correlated with cortical infarct volume and neutrophil accumulation (Liao et al. 2001). These observations suggest an important neuroprotective role of endogenous estrogens. Interestingly, treatment of gonadally intact female mice with the intracellular estrogen receptor (ER) antagonist ICI182,780 increased ischemic infarct volumes, confirming the importance of endogenous estrogens and demonstrating the involvement of ER signaling (Sawada et al. 2000).
The ERα isoform, which is upregulated in response to MCAO, mediates the early cerebroprotective effects of estradiol. Thus, 24 h after cerebral ischemia, the neuroprotective effects of estradiol observed in wild-type mice were also observed in ERβ-KO mice but not in ERα-KO mice (Dubal et al. 2001). However, while ERα plays a critical role in the acute phase of stroke, both ERα and ERß are necessary for the stimulation of neurogenesis within the subventricular zone observed at 96 h after MCAO (Suzuki et al. 2007). Furthermore, a more recent study using a specific ERß agonist, reported a role of ERß in the recovery of sensorimotor functions at later time points (8 and 17 days post-ischemia) (Madinier et al. 2014).
Effects of estrogens on the brain are age-dependent. Treatment of middle-aged ovariectomized female rats with physiological doses of estradiol decreased infarct volume by about 50% as it did in young ovariectomised female rats (Dubal and Wise 2001; Wise et al. 2001). This was an unexpected finding as at the middle age, the ability of estradiol to regulate the hypothalamo-pituitary axis has already markedly decreased (Downs and Wise 2009). With age, the neuroprotective efficacy of estrogens may decrease and estrogens may even exert adverse effects. Thus, in acyclic reproductive senescent female rats, estrogen treatment increased infarct volumes (Selvamani and Sohrabji 2010). As estradiol exerts its neuroprotective effects in synergy with IGF-1, the age-dependent decrease in IGF-1 levels may explain why estradiol ceases to be neuroprotective in aged females (Arevalo et al. 2015).
Estradiol treatment is also protective in male rats exposed to transient or permanent MCAO (Hawk et al. 1998; Toung et al. 1998; Perez-Alvarez et al. 2012). Different estrogen-mediated cerebroprotective mechanisms have been reported, including anti-inflammatory effects, protection against apoptosis, enhanced angiogenesis and increased neurogenesis (McCullough and Hurn 2003; Suzuki et al. 2009; Petrone et al. 2014).
As for the influence of testosterone on stroke in men, animal studies have revealed a complex picture, with androgens having either deleterious or protective effects (Quillinan et al. 2014). Most studies showed that gonadectomy of young adult male rats reduced infarct volume suggesting that endogenous androgens exacerbate stroke injury in males (Quillinan et al. 2014). For example, castration of male rats decreased ischemic brain damage after transient MCAO, whereas replacement with testosterone increased infarct size. In contrast to testosterone, estradiol treatment was protective (Hawk et al. 1998). Likewise, treatment of castrated male rats with the 5α-dihydrotestosterone (5α-DHT), a metabolite of testosterone that is not converted to estrogens but binds with high affinity to the intracellular androgen receptor (AR), restored infarct volumes to those of uncastrated males (Cheng et al. 2007). However, it was then shown that the effects of testosterone and 5α-DHT are dose-dependent. Whereas castrated male mice treated with low doses of either androgen had smaller infarct volumes, those treated with higher doses had larger infarcts than non-treated castrated mice.
The testosterone effects were AR-dependent, as they could be blocked with flutamide (Uchida et al. 2009). Taken together, these results suggest that endogenous testicular androgens may increase the susceptibility of the brain to ischemic damage and that the adverse effects of androgens may involve AR signaling.
It is important to better define the doses–responses relationships of androgens in stroke, to know precisely what is the optimal dose that provide protective response and at which dose there is a transition to damage effect. In future studies, it will be important to determine the type of the doses–responses curves: sigmoidal, U-shaped or inverse U-shaped. Several drugs tested for stroke therapy showed U-shaped doses–reponses curves (Calabrese 2008).
As noted above, in contrast to testosterone, estradiol treatment was found to be protective against ischemic injury in male rats (Hawk et al. 1998; Toung et al. 1998; Perez-Alvarez et al. 2012). However, whereas chronic treatment with the ER antagonist ICI182,780 of female mice exacerbated ischemic damage after MCAO, this treatment had no effects in males (Sawada et al. 2000). This suggests that endogenous estradiol may play a significant role in the resistance of the brain to ischemic damage in females but not in males.
The absence of protective effects of elevated doses of testosterone and endogenous estrogens in males may come as a surprise. Indeed, conversion of testosterone to estradiol in the male brain by the aromatase enzyme is neuroprotective and plays a key role in the resistance of neural cells to a variety of insults (Garcia-Segura et al. 2003; Arevalo et al. 2015). However, we have to be aware that expression of the brain aromatase is upregulated in response to injury, mainly in astrocytes, which do not constitutively express the enzyme in adult rats (Garcia-Segura et al. 1999). The aromatase is also induced by MCAO in astrocytes of the peri-infarct area. This Increase was transient as it was observed at 24 h and 8 days, but neither at 2 h or at 30 days post-ischemia (Carswell et al. 2005). These observations suggest a delay in the actions of testosterone-derived estradiol, which may regulate neuroinflammation and play a role in regenerative processes. The importance of aromatase is also suggested by the observation that MCAO-induced neurogenesis is reduced in aromatase knockout mice (Li et al. 2011).
Sex-Dependent Changes in Endogenous Steroid Levels in Response to Stroke
Increase in steroid levels is part of endogenous mechanisms triggered after ischemic stroke. In a first study, we have shown that levels of progesterone and 5α-dihydroprogesterone (5α-DHP) were highly upregulated in the brain of male mice as early as 6 h after MCAO (Liu et al. 2012). We have then performed a detailed study to investigate the temporal changes of steroid levels in brain and plasma of both male and female mice at diestrus phase using gas chromatography-tandem mass spectrometry (GC-MS/MS) (Zhu et al. 2017). Our study revealed marked differences in brain steroid levels between males and females in the absence of injury and also sex-dependent changes in endogenous steroid levels after MCAO. Data are summarized in Fig. 1.
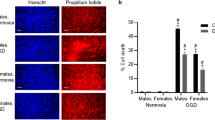
Brain levels of steroids in male and female mice intact and 6 h post-MCAO, as analyzed by gas chromatography tandem mass spectrometry (GC/MS/MS). Young male and female mice (diestrus) were subjected to 1 h MCAO and brain steroid levels were measured at 6-h post-MCAO. Data are expressed as means ± SEM (ng/g of tissue; n = 6–10 per group) and were analyzed by two-way ANOVA (surgery × sex) followed by Newman–Keuls multiple comparisons tests. ***p < 0.001, **p < 0.01, *p < 0.05 MCAO versus intact; $$$p < 0.001, $p < 0.05 MCAO females versus MCAO males as indicated. DHP dihydroprogesterone, THP tetrahydroprogesterone, DOC 11-deoxycorticosterone, DHDOC dihydro-11-deoxycorticosterone, THDOC tetrahydro-11-deoxycorticosterone, DHT dihydrotestosterone, THT tetrahydrotestosterone, 3α-HSOR 3α-hydroxysteroid oxidoreductase, P450aro P450 aromatase (data from Zhu et al. 2017)
Stroke Increased Brain Levels of Progesterone and its Neuroactive 5α-Reduced Metabolite in Male But Not in Female Mice
Surprisingly, levels of 5α-DHP, a natural PR agonist (Rupprecht et al. 1993), were higher in male than in female brain of intact mice. After MCAO, brain progesterone and 5α-DHP levels were rapidly upregulated in males, but not in females, reaching highest levels at 6-h post-MCAO (Zhu et al. 2017). Levels of the potent GABAA receptor active progesterone metabolite 3α,5α-tetrahydroprogesterone (3α,5α-THP, allopregnanolone) were also higher in the male brain, but its levels did not significantly change with time after MCAO (Fig. 1a) (Zhu et al. 2017).
Stroke Increased Levels of Glucocorticoids in Both Male and Female Mice
Levels of steroids that are related to stress, namely, deoxycorticosterone (DOC), 5α-dihydrodeoxycorticosterone (5α-DHDOC), 3α,5α-tetrahydrodeoxycorticosterone (3α,5α-THDOC) and corticosterone were also upregulated in response to ischemia in both plasma and brain of males and females. However, in contrast to progesterone and its metabolites, levels of corticosterone remained lower in brain than in plasma between 1- and 4-h post-MCAO. Interestingly, a marked sex difference was observed at 6 h: in males, brain levels of corticosterone were highly increased, reaching plasma levels (about 200 ng/g tissue), whereas in females, brain levels remained significantly lower than in plasma (about 70 ng/g tissue vs 190 ng/ml in plasma) (Zhu et al. 2017) (Fig. 1b). Therefore, after ischemic injury, the male brain is transiently exposed to higher amounts of corticosterone than the female brain. Glucocorticoids and stress are known to aggravate ischemic brain damage (Sapolsky and Pulsinelli 1985; Sugo et al. 2002). It is thus conceivable that the increase in levels of progesterone and its 5α-reduced metabolite in the male brain may be a mechanism to protect neural cells against the damaging effects of elevated glucocorticoid levels. Consistent with this hypothesis, a recent study has shown that chronic stress exacerbated inflammation and neural loss in the hippocampus of male rats after global ischemia and that progesterone was efficient in decreasing the deleterious effects of stress (Espinosa-Garcia et al. 2017).
Stroke Decreased Levels of Androgens in Males and had no Significant Effects on Estradiol Levels
In contrast to progesterone and glucocorticoids, brain levels of testosterone and 5α-DHT decreased as early as 1-h post-MCAO in males. At 6 h, their brain levels were respectively 15- and 10-times lower than in intact male mice (Fig. 1c). The downregulation of androgens may be the consequence of a disruption of the hypothalamo-pituitary-gonadal axis, or of the stress caused by stroke. Indeed, stress, adrenal hyperactivity and high doses of corticosteroids impair different aspects of reproduction including steroidogenesis (Rivest and Rivier 1991; Tilbrook et al. 2000; Orr et al. 1994; Maric et al. 1996; Kostic et al. 1998). Another possibility may be a competitive inhibition of the P450c17 enzyme necessary for testosterone synthesis, by the increased levels of progesterone and 5α-DHP as they are also substrates of this enzyme (Shet et al. 1994; Auchus et al. 2003).
Brain levels of estradiol were low in intact mice and did not differ between males and females. In both sexes, no significant changes in brain estradiol were observed between 1 and 24 h after MCAO, although there was a tendency to decreased estradiol levels in females at 6-h post-MCAO (Zhu et al. 2017) (Fig. 1c). However, it is important to note that in our study steroid levels were measured in large brain samples (the whole ischemic hemisphere); therefore, localized changes in steroid levels in specific brain regions after MCAO cannot be excluded. For instance, levels of 17β-estradiol have been shown to increase after MCAO in the dialysate from parabrachial nucleus of male rats. This increase was transitory with a maximum at 10 min followed by a decrease to levels bellow baseline by 90-min post-MCAO (Saleh et al. 2004). Furthermore, the same group showed a continuous increase of 17β-estradiol in the dialysate from the central nucleus of the amygdala beginning at 30-min post-MCAO with maximal values measured at 4-h post-MCAO (Saleh et al. 2005).
Stroke Increased Levels of 5ß-Reduced Steroids in Female But Not in Male Mice
In contrast to what was observed in males (increase of 5α-reduced steroids following MCAO), an increase of 5β-reduced steroids was observed in the brain of female mice. In particular, brain levels of 3α,5β-THP, 5β-DHDOC, and 3α,5β-THDOC increased in females and were higher than in males at 6-h post-MCAO (Fig. 1a, b) (Zhu et al. 2017). This observation may be of importance, as 3α,5β-THP and 3α,5β-THDOC are, positive allosteric modulators of GABAA receptors. 5β-reduction of steroids may also be a mechanism to regulate their concentrations and their availability for receptors (Belelli et al. 1996; Chen and Penning 2014; Gunn et al. 2015).
Progesterone Receptor Signaling Mediates the Early Endogenous Cerebroprotection After Ischemic Stroke in Young and Aging Male and Female Mice
If the endogenous progesterone and 5α-DHP are important for the cerebroprotection at the acute phase of stroke, PR may be a key mediator. To test this hypothesis, we studied the response of PR Knockout mice to ischemia.
We first used available PR knockout mice (PRKO) lacking PR expression in all tissues (Ismail et al. 2002). We demonstrated in young adult male mice that lack of PR expression increased ischemic brain infarct and motor dysfunctions at 6 and 24 h, but not at 48 h post-MCAO (Liu et al. 2012). These observations highlight the importance of PR-dependent mechanisms in the protection of the brain at the acute phase after stroke. To go further, we generated a new transgenic mice line (PRNesCre) selectively lacking PR expression in neural cells, to evaluate the relative role of PR specifically expressed in the brain. At 6-h post-MCAO, both young and aging male and female PRNesCre mice showed exacerbated neurological deficits and increased infarct volumes comparatively to their control PRloxP/loxP littermates that express normal levels of PR (Fig. 2) (Zhu et al. 2017).
Specific deletion of PR in neural cells leads to increased neurological deficits (a) and infarct volumes (b) in both young and aging male and female mice. Mice were subjected to 1 h MCAO and neurological deficit scores (higher scores reflect higher disability) and total infarct volumes were analyzed at 6-h post-MCAO. PRloxP/loxP mice: transgenic mice in which exon 2 of PR was flanked by loxP sites; mice expressing normal levels of PR. PRNesCre mice: transgenic mice that selectively lack PR in the neural cells using the Cre-lox strategy (data from Zhu et al. 2017)
However, the invalidation of PR expression had more deleterious effects in young males than in young females. In particular, the exacerbation of tissue damage in PRNesCre mice was more pronounced in males than in females (Fig. 2b) (Zhu et al. 2017). This observation points to additional endogenous processes, independent from neural PR signaling, which contribute to the prevention of tissue loss in the female brain. These additional mechanisms may depend on ERα as its expression is up-regulated early after cerebral ischemia in females (Dubal et al. 2006) but not in males (Westberry et al. 2008).
Our findings demonstrate an early endogenous cerebroprotective mechanism depending on PR function in neural cells in young and aging males and females. This strongly suggests that selective ligands of PR may be useful for cerebroprotection after ischemic stroke. However, they may show different efficiencies in young males and females.
Progesterone is a Promising Pleiotropic Cerebroprotective Agent After Stroke
Neural cells are very sensitive to oxygen and glucose deprivation, and they rapidly start dying after ischemic stroke. The major problem is the progressive spreading of nervous tissue damage. There is thus an urgent need for cerebroprotective agents that limit the death of neurons in the peri-infarct area (Stankowski and Gupta 2011).
Experimental models of ischemic stroke have provided strong evidence for the cerebroprotective effects of progesterone (Gibson et al. 2009; Wong et al. 2013b). The majority of studies that evaluated the effects of progesterone treatment on infarct size and functional outcomes reported beneficial effects. Only few studies reported no effects and one study reported a deleterious effect (Gibson et al. 2009; Wong et al. 2013b). For the studies showing beneficial effects, progesterone was administrated at moderate doses early after ischemia. In contrast, administration of very high doses of progesterone before ischemia induction to ovariectomised females showed no effect on cortical infarct and even an increase of the sub-cortical infarct when the treatment was chronic (Murphy et al. 2000). The observed negative results may be due to the high doses of progesterone, the time of treatment initiation, the endocrine status of animals at the time of ischemia and/or the early time of analysis. Therefore, endogenous progesterone levels, the dose, time, and schedule of progesterone treatment are all very important to be taken into account when designing preclinical studies.
The systematic meta-analysis by Wong et al. showed that in mice and rats exposed to either transient or permanent MCAO, progesterone treatment at moderate doses reduced infarct volume and improved functional recovery, such as the ability to remain on the rotarod and the reduction of neurological score deficits (Wong et al. 2013b). Most studies were performed in young males, some in ovariectomized young females and few in aging animals. Unfortunately, no study has been performed in gonadally intact young females (Wong et al. 2013a). The few studies that have used aged males, reported positive neuroprotective effects of progesterone (Wang et al. 2010; Yousuf et al. 2014a, b; Wali et al. 2014, 2016). Only three studies evaluated the effect of progesterone treatment in aging females. While one study showed a reduction of cortical infarct volume in aging female rats with chronic pretreatment by progesterone implants for 1 week (Alkayed et al. 2000); a second study showed no effect on infarct volume by acute treatment of progesterone initiated just 0.5 h before MCAO (Toung et al. 2004). A third study showed a reduction of total infarct volume, but no effect on neurological scores when progesterone was administered at 1-, 6-, and 24-h post-MCAO (Gibson et al. 2011). Most studies except few ones used healthy mice and rats without any comorbidities or risk factors (Ankolekar et al. 2012). Studies using male hypertensive animals have shown beneficial effects of progesterone on infarct size and neurological outcomes at 7 and 14 days post-MCAO (Kumon et al. 2000; Wong et al. 2014; Yousuf et al. 2016). However, one study showed no effect of progesterone on lesion volume nor on neurological outcomes at 24-h post-MCAO in spontaneously hypertensive male rats (Spratt et al. 2014).
Treatment Schedule, Dose–Response, Time Window, and Mode of Progesterone Administration
The majority of experimental studies evaluating the effects of progesterone treatment in rodents used the dose of 8 mg/kg administered by subcutaneous and/or intraperitoneal injections (Wong et al. 2014; Gibson and Murphy 2004; Lee et al. 2015; Liu et al. 2012; Dang et al. 2011; Yousuf et al. 2016; Spratt et al. 2014; Sayeed et al. 2006, 2007; Ishrat et al. 2010, 2012; Wang et al. 2011; Gibson et al. 2011). The schedule of administration at 1-, 6-, and 24-h post-MCAO is the one that has been the most often used (Gibson et al. 2008; Wong et al. 2013b). Dose–response studies have also been performed (Chen et al. 1999; Wali et al. 2014, 2016; Yousuf et al. 2014a). The dose of progesterone with an optimal neuroprotective effect was 8 mg/kg. For instance, in a transient MCAO model, administration of progesterone at 2-h post-MCAO at the dose of 8 mg/kg reduced infarct size and improved functional outcomes, whereas treatment with 4-or 32-mg/kg had no effects (Chen et al. 1999). In a permanent stroke model, Wali and colleagues showed that moderate doses of progesterone (8- or 16-mg/kg) were efficient in reducing infarct volume and improving functional outcomes for up to 3 weeks of post-MCAO in aging rats. However, the dose of 8 mg/kg was more efficient in improving the spatial memory. Of note, progesterone treatment still provide neuroprotection when treatment was initiated at 6-h post-MCAO (Wali et al. 2014). In a more recent study, the same group showed that the beneficial effects of progesterone treatment on infarct size and neurological outcomes still be observed for up to 8 weeks post-MCAO (Wali et al. 2016).
One of the STAIR’s recommendations is to test different modes of administration of therapeutic drugs in preclinical studies. We are currently investigating the cerebroprotective potential of intranasal administration of progesterone. We have shown that progesterone dissolved in oleogel and administrated intranasally penetrated efficiently into the brain, and is cerebroprotective in male mice subjected to MCAO. Furthermore, brain levels of corticosterone were lower in progesterone-treated mice than in vehicle mice, suggesting that this mode of administration is a non-stressful route of progesterone delivery to brain that warrant evaluation in future experimental studies (Frechou et al. 2015; Guennoun et al. 2018).
Pleiotropic Effects of Progesterone Treatment After Ischemic Injury
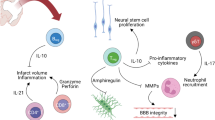
As presented above, several experimental studies have shown that progesterone treatment decreases the extent of ischemic infarction and improves functional outcomes. The underlying mechanisms of progesterone effects are beginning to be unraveled. Thus, progesterone treatment has been shown to regulate different cellular and functional events important for cerebroprotection, including edema formation, neurotoxicity, blood–brain barrier (BBB) disruption, apoptosis, inflammatory responses and mitochondrial functions.
Progesterone Treatment Decreases Blood–Brain Barrier Disruption and Edema
One of the deleterious consequences of cerebral ischemia is the dysfunction of the blood–brain barrier (BBB) (Jiang et al. 2018). Excessive production of free radicals that cause oxidative stress activate matrix metalloproteases (MMPs). This activation leads to the degradation of the basal lamina as well as intercellular junctions of the BBB (Gidday et al. 2005; Yang et al. 2007). Infiltration of leukocytes contributes to the alteration of the BBB (Jiang et al. 2018; Kebir et al. 2007; McColl et al. 2007). BBB leakage results in edema formation, hemorrhagic transformation, and increased inflammation.
Different studies showed that progesterone limits BBB leakage after stroke. Thus, we have recently shown that intranasal administration of progesterone at the time of reperfusion attenuates BBB opening at 4-h post-MCAO (Frechou et al. 2015). This effect on the BBB during the early phase after stroke may contribute to the beneficial effects of progesterone on neuronal survival and on functional outcome observed at later time points. Likewise, Ishrat and colleagues demonstrated that progesterone treatment decreased the permeability of the BBB barrier at 72 h after ischemia by acting on the expression of MMPs, the pro-inflammatory molecules TNF-α and interleukin-6, and the tight junction proteins occludin 1 and claudin 5 (Ishrat et al. 2010). The effects of progesterone on BBB permeability and on the expression of the tight junction proteins were also demonstrated in vitro using mouse brain endothelial cells treated with thrombin (Hun Lee et al. 2015). Furthermore, progesterone decreased the hemorrhagic transformation, brain swelling, BBB leakage, and the induction of MMP-9 and VEGF expression observed in rats treated with tissue plasminogen activator (tPA) at 4.5-h post-MCAO (Won et al. 2014).
Cerebral edema is a major complication of ischemic stroke that contributes to increased mortality. Progesterone treatment has been found to be efficient in reducing brain edema after ischemic stroke (Grossman et al. 2004; Gibson et al. 2005; Liu et al. 2012; Jiang et al. 2016). Progesterone was also able to counter the increased edema formation induced by t-PA treatment after transient MCAO (Won et al. 2014).
Progesterone Treatment Decreases the Inflammatory Response
After cerebral ischemia, there is an acute and prolonged inflammatory response consisting of the early activation of microglia and astrocytes, the synthesis and release of pro-inflammatory cytokines and chemokines and the infiltration into the brain parenchyme of neutrophils, T cells, and macrophages. This cascade of events participates in brain tissue loss (Jin et al. 2010; Iadecola and Anrather 2011). There is a double function of microglia after stroke. Activated microglia can exert either beneficial or detrimental effects, depending on their phenotype (Hu et al. 2015; Ransohoff 2016; Ma et al. 2017).
Progesterone treatment has been shown to regulate the density and polarisation of microglia and to reduce pro-inflammatory cytokines and nitric oxide synthase-2 (Grossman et al. 2004; Habib and Beyer 2015; Ishrat et al. 2010; Jiang et al. 2009; Habib et al. 2014a, b; Coughlan et al. 2005; Won et al. 2015; Allen et al. 2016; Lammerding et al. 2016; Yousuf et al. 2016). Recently, Espinosa-Garcia et al. evaluated the anti-inflammatory potential of progesterone in the hippocampus of mice exposed to stress followed by global ischemia. They showed that stress exacerbated the inflammatory response by increasing the activation of microglia, affecting their phenotype, enhancing the expression of inflammatory cytokines, and reducing the expression of protective factors. Progesterone treatment counteracted the effects of stress and ischemia by mitigating the inflammatory response and regulating the polarization of microglia (Espinosa-Garcia et al. 2017). Inflammasomes are multiprotein complexes that play a key role in central nervous system inflammation and their activation represents a critical step in the neuro-inflammatory responses (Singhal et al. 2014). Recent studies showed that the anti-inflammatory effects of progesterone involve interactions between inflammasomes activation and their related regulatory miRNAs (Slowik and Beyer 2015).
Progesterone Treatment Reduces Brain Mitochondrial Dysfunction and Oxidative Damage
Mitochondria are the site of energy production and are major regulators of oxidative stress (Gaignard et al. 2018). Due to the high metabolic rate and the low energy storage capacity in neurons, mitochondria play a key role in brain function. We have recently investigated the role of endogenous steroids in the brain mitochondria function (Gaignard et al. 2015). In particular, we have shown that mitochondrial respiration is higher, while oxidative stress is lower in the brain of young adult females as compared to young adult males. These differences were not observed in ovariectomised mice and in aged senescent mice (Gaignard et al. 2015). Our findings suggest that endogenous ovarian steroids may influence brain mitochondrial functions under physiological conditions.
With regard to stroke, mitochondria play a key role since they regulate energy production, oxidative stress, and cell death (Kalogeris et al. 2014; Gaignard et al. 2018). The drop in blood supply causes a decrease in ATP synthesis and the lack of oxygen causes a depolarisation of the inner mitochondrial membrane, leading to the production of high levels of reactive oxygen species (ROS). Energy drop and oxidative stress result in disturbance of ionic pumps and excitotoxicity, leading to cell death (Sims and Muyderman 2010; Abramov et al. 2007; Manzanero et al. 2013; Dirnagl et al. 1999). Mitochondria are thus promising therapeutic targets for promoting recovery from stroke (Jin et al. 2016).
Treatment with progesterone increased the level of the antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and catalase, restored levels of total glutathione, and attenuated lipid peroxidase (Aggarwal et al. 2008; Ozacmak and Sayan 2009). Progesterone was also shown to inhibit the translocation of the apoptotic factor, cytochrome c from mitochondria to cytosol (Sayeed et al. 2009).
In a recent study, we have investigated the effects of progesterone on the brain mitochondrial respiratory chain and oxidative damage at 6-h post-MCAO (Gaignard et al. 2016). We observed a sex difference in stroke effects on the brain mitochondrial respiratory chain. The reduced flavin adenine dinucleotide (FADH2)-linked respiration and the activity of complex II (CII) decreased in females but not in males. The reduced nicotinamide adenine dinucleotide (NADH)-linked respiration decreased in both males and females. The mitochondrial pool of reduced glutathione (GSH) is the main anti-oxidant factor and its levels regulate neuronal cell death (Wullner et al. 1999). As demonstrated by others (Anderson and Sims 2002), we showed that levels of mitochondrial GSH decreased after MCAO. We demonstrated that progesterone treatment is efficient in preserving mitochondrial functions that are altered by ischemia. Our findings identify the mitochondria as target of progesterone action after stroke and suggest that the effects of progesterone on mitochondrial function may be one of the mechanisms by which progesterone provide neuroprotection (Gaignard et al. 2016). Recently, Andarabi and colleagues provided further evidence for the beneficial effects of progesterone on brain mitochondrial function after ischemic injury (Andrabi et al. 2017). They indeed showed that progesterone (1) restored the function of mitochondrial respiratory chain by increasing the activities of complex I and complex II and the levels of complex V; (2) modulated different oxidative stress parameters such as lipid peroxidation, ROS production, and mitochondrial GSH; and (3) reduced the swelling of mitochondria, and the release of cytochrome c from mitochondria in the cytosol (Andrabi et al. 2017).
Progesterone Treatment Regulates Levels of Serotonin and Dopamine and Some Markers of Neurotoxicity and Neuroprotection
Neurotransmitter imbalance causes dysregulation of brain functions and may lead to secondary neuronal damage (Chen et al. 2014). Levels of dopamine and serotonin increased in the frontal cortex after ischemia and progesterone treatment counteracted this increase (Andrabi et al. 2017). Likewise, similar effects of ischemia and progesterone were observed for the activities of the monoamine oxidase and the acetylcholine esterase enzymes. Furthermore, progesterone was efficient in re-establishing the activity of the Na+, K+-ATPase that was decreased by ischemia thereby attenuating the mitochondrial damage (Andrabi et al. 2017).
Brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) regulate neuronal survival, neurogenesis and also vascular remodeling, angiogenesis, and brain plasticity (Greenberg et al. 2009; Jin et al. 2002; Ruan et al. 2015). BDNF is neuroprotective after cerebral ischemia (Chen et al. 2013), while VEGF may play a dual role. For instance, a delayed treatment with VEGF increased angiogenesis and improved functional outcomes. In contrast, treatment at the first hour following stroke leads to increased BBB permeability, hemorrhagic transformation and tissue damage (Zhang et al. 2000). Progesterone administration increased the levels of BDNF and VEGF in the peri-infarct at 72 h and decreased them at 14 days post-ischemia (Ishrat et al. 2012). In this study, progesterone was also shown to reduce apoptosis and its related proteins. Similarly, a recent study showed that progesterone decreased VEGF and increased BDNF levels in the cortex at day 3 post-MCAO. Progesterone increased neurogenesis in the sub-ventricular zone and the density of the newly generated neurons in the peri-infarct at day 7 post-MCAO. These effects could partially underlie the improvement of neurologic functions observed on days 7 and 14 post-MCAO (Jiang et al. 2016).
Modes of Action of Progesterone After Stroke: A Key Role of PR
The classical mechanism of action of progesterone is the regulation of gene transcription after binding to its intracellular receptors PR. Progesterone actions may also be mediated by specific membrane receptors, either the progesterone receptor membrane component 1 (PGRMC1) or the seven-transmembrane G protein-coupled progesterone receptors (mPRs). Progesterone is also a competitive inhibitor of sigma-1 receptors. Finally, progesterone may be converted to allopregnanolone, a potent modulator of GABAA receptors. All these mechanisms may contribute to the cerebroprotective actions of progesterone as all these receptors are largely distributed in the brain and as progesterone is actively metabolized to allopregnanolone in the brain (Schumacher et al. 2007; Guennoun et al. 2015).
As discussed in paragraph 3, our recent studies demonstrated a key role of PR in the endogenous cerebroprotection at 6- and 24-h post-MCAO (Liu et al. 2012; Zhu et al. 2017). PR is a limiting factor, as even heterozygous PR+/− mice showed larger ischemic infarcts comparatively to wild-type PR+/+ mice (Liu et al. 2012). The key role of PR was also demonstrated after progesterone treatment. Indeed, progesterone treatment was efficient for decreasing infarct volume, neurological and motor deficits in wild-type PR+/+ mice, but not in PR−/− knockout mice (Liu et al. 2012). Another study confirmed the importance of PR in stroke as progesterone decreased the infarct volume at 48-h post-MCAO in wild-type PR+/+ but not in heterozygous PR+/− mice (Lee et al. 2015). To know if the activation of PR is sufficient to provide cerebroprotection, we used Nestorone: a potent and selective PR agonist with no unwanted interaction with other receptors (Kumar et al. 2000) and which is not converted to GABAA receptor-active metabolites (Kumar et al. 2017). We showed that Nestorone at a very low dose (100-times lower than progesterone) decreased infarct volume and motor deficits (Liu et al. 2012). We have recently extended our analysis concerning the role of PR. We have shown in particular that progesterone treatment (1) increased the density of neurons, of oligodendrocytes and of their precursors; (2) decreased the density of activated microglia and of astrocytes and of aquaporin 4 expression; and (3) that the selective invalidation of PR expression in neural cells blocked all these effects (Zhu et al. 2018).
Treatment with allopregnanolone has also been shown to be neuroprotective after MCAO. In particular, administration of allopregnanolone has been shown to decrease infarct volume, edema, motor deficits, BBB dysfunctions, neuroinflammation and the activation of the mitochondrial permeability transition pore (Sayeed et al. 2006, 2009; Ishrat et al. 2010; Liu et al. 2012). Although both progesterone and allopregnanolone are neuroprotective when administered after ischemic injury, their mechanisms of action are different. Allopregnanolone exerts cerebroprotective effects via PR-independent signaling pathway as it has no affinity for PR and its effects have also been demonstrated in PR knockout mice (Liu et al. 2012). As a positive modulator of GABAA receptor, allopregnanolone may counteract excitotoxic mechanisms by potentiating GABAA receptor-dependent decrease in neuronal excitability. Intracellular PR play a key role in mediating the effects of progesterone. Indeed, neuroprotective effects of progesterone are no longer observed in PR knockout mice and Nestorone, the selective PR agonist, is sufficient to provide efficient cerebroprotection at a very low dose. These findings also indicated that the in vivo bioconversion of progesterone to allopregnanolone is not the mechanism through which progesterone provides cerebroprotection, otherwise progesterone treatment would have been protective in PR knockout mice.
Although there is strong evidence for a key role of PR-depending signals in the mediation of the cerebroprotective effects progesterone, these findings do not exclude the involvement of additional progesterone signaling mechanisms, depending on the dose and timing of progesterone administration, and on the time and type of outcome measures. Cai et al. investigated the potential mechanisms underlying the neuroprotective effects of progesterone. They showed in particular that PR, via activation of the Src-ERK1/2 cascade, mediated the cerebroprotective effects of progesterone observed in the hippocampus at 48-h post-MCAO. In contrast, the acute protective effects observed at 1 h involved the antagonistic actions of progesterone on sigma-1 receptors, resulting in an attenuation of the NMDA-induced increase in intracellular calcium concentrations (Cai et al. 2008).
The phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) cascade is a signal transduction pathway that regulates inflammation, cell survival in response to growth factors and is involved in cerebroprotection after ischemic stroke (Brazil et al. 2004; Zhao et al. 2006; Xu et al. 2008; Wang et al. 2009). The hypothesis that this pathway may mediate some of the beneficial effects of progesterone has been tested (Ishrat et al. 2012). Inhibiting the PI3K/Akt pathway with Wortmannin decreased the beneficial effects of progesterone on infarct size, edema, apoptosis and VEGF levels observed at 24-h post-MCAO (Ishrat et al. 2012).
Summary and Conclusions
Understanding the significance of sex differences in response to ischemic stroke injury and in cerebroprotection is fundamental for developing and refining effective treatment strategies that are beneficial for both men and women. Rather than ignoring sex and steroid hormones as variables, as done in almost all preclinical studies, there is much to be gained by taken them into account for studying pathophysiological and therapeutic mechanisms.
Advances in the understanding the mechanisms of action of steroids in stroke will permit to envisage therapeutic approaches based on a combination of steroids or new molecules modulating their synthesis, their receptors or their signaling pathways. In particular, cerebroprotection by progesterone or molecules targeting PR signaling offer great promises for the treatment of stroke patients. Taking into account the steroid status of patients and reinforcing progesterone signaling should be exploited in therapeutic strategies.
References
Abramov AY, Scorziello A, Duchen MR (2007) Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27(5):1129–1138. https://doi.org/10.1523/JNEUROSCI.4468-06.2007
Aggarwal R, Medhi B, Pathak A, Dhawan V, Chakrabarti A (2008) Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J Pharm Pharmacol 60(6):731–737. https://doi.org/10.1211/jpp.60.6.0008
Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA (2010) NAD + depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci 30(8):2967–2978. https://doi.org/10.1523/JNEUROSCI.5552-09.2010
Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD (1998) Gender-linked brain injury in experimental stroke. Stroke 29(1):159–165 (discussion 166)
Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM (2000) Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke 31(1):161–168
Allen RS, Sayeed I, Oumarbaeva Y, Morrison KC, Choi PH, Pardue MT, Stein DG (2016) Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: progesterone receptor levels may play an important role. Restor Neurol Neurosci 34(6):947–963. https://doi.org/10.3233/RNN-160672
Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG (2009) Classification of stroke subtypes. Cerebrovasc Dis 27(5):493–501. https://doi.org/10.1159/000210432
Anderson MF, Sims NR (2002) The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J Neurochem 81(3):541–549
Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE (2007) Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab 92(12):4696–4705. https://doi.org/10.1210/jc.2006-2633
Andrabi SS, Parvez S, Tabassum H (2017) Progesterone induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in rats. Dis Model Mech 10(6):787–796. https://doi.org/10.1242/dmm.025692
Ankolekar S, Rewell S, Howells DW, Bath PM (2012) The influence of stroke risk factors and comorbidities on assessment of stroke therapies in humans and animals. Int J Stroke 7(5):386–397. https://doi.org/10.1111/j.1747-4949.2012.00802.x
Appelros P, Stegmayr B, Terent A (2010) A review on sex differences in stroke treatment and outcome. Acta Neurol Scand 121(6):359–369. https://doi.org/10.1111/j.1600-0404.2009.01258.x
Arevalo MA, Azcoitia I, Garcia-Segura LM (2015) The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 16(1):17–29. https://doi.org/10.1038/nrn3856
Auchus RJ, Sampath Kumar A, Andrew Boswell C, Gupta MK, Bruce K, Rath NP, Covey DF (2003) The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21. Arch Biochem Biophys 409(1):134–144
Belelli D, Lambert JJ, Peters JA, Gee KW, Lan NC (1996) Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology 35(9–10):1223–1231
Brazil DP, Yang ZZ, Hemmings BA (2004) Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci 29(5):233–242. https://doi.org/10.1016/j.tibs.2004.03.006
Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L (2008) Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology 55(2):127–138. https://doi.org/10.1016/j.neuropharm.2008.04.023
Calabrese EJ (2008) Drug therapies for stroke and traumatic brain injury often display U-shaped dose responses: occurrence, mechanisms, and clinical implications. Crit Rev Toxicol 38(6):557–577. https://doi.org/10.1080/10408440802014287
Carpenter RS, Iwuchukwu I, Hinkson CL, Reitz S, Lee W, Kukino A, Zhang A, Pike MM, Ardelt AA (2016) High-dose estrogen treatment at reperfusion reduces lesion volume and accelerates recovery of sensorimotor function after experimental ischemic stroke. Brain Res 1639:200–213. https://doi.org/10.1016/j.brainres.2016.01.058
Carswell HV, Anderson NH, Clark JS, Graham D, Jeffs B, Dominiczak AF, Macrae IM (1999) Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension 33(2):681–685
Carswell HV, Dominiczak AF, Macrae IM (2000) Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 278(1):H290–H294. https://doi.org/10.1152/ajpheart.2000.278.1.H290
Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM (2005) Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol 96(1):89–91. https://doi.org/10.1016/j.jsbmb.2005.02.016
Chen M, Penning TM (2014) 5beta-reduced steroids and human delta(4)-3-ketosteroid 5beta-reductase (AKR1D1). Steroids 83:17–26. https://doi.org/10.1016/j.steroids.2014.01.013
Chen J, Chopp M, Li Y (1999) Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J Neurol Sci 171(1):24–30
Chen RL, Balami JS, Esiri MM, Chen LK, Buchan AM (2010) Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol 6(5):256–265. https://doi.org/10.1038/nrneurol.2010.36
Chen A, Xiong LJ, Tong Y, Mao M (2013) The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep 1(2):167–176. https://doi.org/10.3892/br.2012.48
Chen Y, Garcia GE, Huang W, Constantini S (2014) The involvement of secondary neuronal damage in the development of neuropsychiatric disorders following brain insults. Front Neurol 5:22. https://doi.org/10.3389/fneur.2014.00022
Cheng J, Hurn PD (2010) Sex shapes experimental ischemic brain injury. Steroids 75(11):754–759. https://doi.org/10.1016/j.steroids.2009.10.014
Cheng J, Alkayed NJ, Hurn PD (2007) Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab 27(9):1553–1562. https://doi.org/10.1038/sj.jcbfm.9600457
Choleris E, Galea LAM, Sohrabji F, Frick KM (2018) Sex differences in the brain: implications for behavioral and biomedical research. Neurosci Biobehav Rev 85:126–145. https://doi.org/10.1016/j.neubiorev.2017.07.005
Coughlan T, Gibson C, Murphy S (2005) Modulatory effects of progesterone on inducible nitric oxide synthase expression in vivo and in vitro. J Neurochem 93(4):932–942. https://doi.org/10.1111/j.1471-4159.2005.03068.x
Dang J, Mitkari B, Kipp M, Beyer C (2011) Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav Immun 25(4):715–726. https://doi.org/10.1016/j.bbi.2011.01.013
Della Torre S, Benedusi V, Fontana R, Maggi A (2014) Energy metabolism and fertility: a balance preserved for female health. Nat Rev Endocrinol 10(1):13–23. https://doi.org/10.1038/nrendo.2013.203
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22(9):391–397
Downs JL, Wise PM (2009) The role of the brain in female reproductive aging. Mol Cell Endocrinol 299(1):32–38. https://doi.org/10.1016/j.mce.2008.11.012
Dubal DB, Wise PM (2001) Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology 142(1):43–48. https://doi.org/10.1210/endo.142.1.7911
Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM (1998) Estradiol protects against ischemic injury. J Cereb Blood Flow Metab 18(11):1253–1258. https://doi.org/10.1097/00004647-199811000-00012
Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM (2001) Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA 98(4):1952–1957. https://doi.org/10.1073/pnas.041483198
Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM (2006) Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology 147(6):3076–3084. https://doi.org/10.1210/en.2005-1177
Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL (1997) Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 3(10):1089–1095
Espinosa-Garcia C, Sayeed I, Yousuf S, Atif F, Sergeeva EG, Neigh GN, Stein DG (2017) Stress primes microglial polarization after global ischemia: Therapeutic potential of progesterone. Brain Behav Immun 66:177–192. https://doi.org/10.1016/j.bbi.2017.06.012
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8(4):355–369. https://doi.org/10.1016/S1474-4422(09)70025-0
Ford ES, Maynard LM, Li C (2014) Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA 312(11):1151–1153. https://doi.org/10.1001/jama.2014.8362
Frechou M, Zhang S, Liere P, Delespierre B, Soyed N, Pianos A, Schumacher M, Mattern C, Guennoun R (2015) Intranasal delivery of progesterone after transient ischemic stroke decreases mortality and provides neuroprotection. Neuropharmacology 97:394–403. https://doi.org/10.1016/j.neuropharm.2015.06.002
Gaignard P, Savouroux S, Liere P, Pianos A, Therond P, Schumacher M, Slama A, Guennoun R (2015) Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology 156(8):2893–2904. https://doi.org/10.1210/en.2014-1913
Gaignard P, Frechou M, Schumacher M, Therond P, Mattern C, Slama A, Guennoun R (2016) Progesterone reduces brain mitochondrial dysfunction after transient focal ischemia in male and female mice. J Cereb Blood Flow Metab 36(3):562–568. https://doi.org/10.1177/0271678X15610338
Gaignard P, Frechou M, Liere P, Therond P, Schumacher M, Slama A, Guennoun R (2018) Sex differences in brain mitochondrial metabolism: influence of endogenous steroids and stroke. J Neuroendocrinol. https://doi.org/10.1111/jne.12497
Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB (1999) Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience 89(2):567–578
Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I (2003) Aromatase: a neuroprotective enzyme. Prog Neurobiol 71(1):31–41
Gibson CL (2013) Cerebral ischemic stroke: is gender important? J Cereb Blood Flow Metab 33(9):1355–1361. https://doi.org/10.1038/jcbfm.2013.102
Gibson CL, Murphy SP (2004) Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab 24(7):805–813. https://doi.org/10.1097/01.WCB.0000125365.83980.00
Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP (2005) Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol 193(2):522–530. https://doi.org/10.1016/j.expneurol.2005.01.009
Gibson CL, Gray LJ, Murphy SP, Bath PM (2006) Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab 26(9):1103–1113. https://doi.org/10.1038/sj.jcbfm.9600270
Gibson CL, Gray LJ, Bath PM, Murphy SP (2008) Progesterone for the treatment of experimental brain injury; a systematic review. Brain 131(Pt 2):318–328. https://doi.org/10.1093/brain/awm183
Gibson CL, Coomber B, Rathbone J (2009) Is progesterone a candidate neuroprotective factor for treatment following ischemic stroke? Neuroscientist 15(4):324–332. https://doi.org/10.1177/1073858409333069
Gibson CL, Coomber B, Murphy SP (2011) Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain 134(Pt 7):2125–2133. https://doi.org/10.1093/brain/awr132
Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS (2005) Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol 289(2):H558–H568. https://doi.org/10.1152/ajpheart.01275.2004
Greenberg ME, Xu B, Lu B, Hempstead BL (2009) New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci 29(41):12764–12767. https://doi.org/10.1523/JNEUROSCI.3566-09.2009
Grossman KJ, Goss CW, Stein DG (2004) Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res 1008(1):29–39. https://doi.org/10.1016/j.brainres.2004.02.022
Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M (2015) Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol 146:48–61. https://doi.org/10.1016/j.jsbmb.2014.09.001
Guennoun R, Frechou M, Gaignard P, Liere P, Slama A, Schumacher M, Denier C, Mattern C (2018) Intranasal administration of progesterone: a potential efficient route of delivery for cerebroprotection after acute brain injuries. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2018.06.006
Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D (2015) GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol 36:28–48. https://doi.org/10.1016/j.yfrne.2014.06.001
Haast RA, Gustafson DR, Kiliaan AJ (2012) Sex differences in stroke. J Cereb Blood Flow Metab 32(12):2100–2107. https://doi.org/10.1038/jcbfm.2012.141
Habib P, Beyer C (2015) Regulation of brain microglia by female gonadal steroids. J Steroid Biochem Mol Biol 146:3–14. https://doi.org/10.1016/j.jsbmb.2014.02.018
Habib P, Dang J, Slowik A, Victor M, Beyer C (2014a) Hypoxia-induced gene expression of aquaporin-4, cyclooxygenase-2 and hypoxia-inducible factor 1alpha in rat cortical astroglia is inhibited by 17beta-estradiol and progesterone. Neuroendocrinology 99(3–4):156–167. https://doi.org/10.1159/000362279
Habib P, Slowik A, Zendedel A, Johann S, Dang J, Beyer C (2014b) Regulation of hypoxia-induced inflammatory responses and M1–M2 phenotype switch of primary rat microglia by sex steroids. J Mol Neurosci 52(2):277–285. https://doi.org/10.1007/s12031-013-0137-y
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab 86(2):724–731. https://doi.org/10.1210/jcem.86.2.7219
Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW (1998) Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res 796(1–2):296–298
Herson PS, Hurn PD (2010) Gender and the injured brain. Prog Brain Res 186:177–187. https://doi.org/10.1016/B978-0-444-53630-3.00012-9
Herson PS, Koerner IP, Hurn PD (2009) Sex, sex steroids, and brain injury. Semin Reprod Med 27(3):229–239. https://doi.org/10.1055/s-0029-1216276
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11(1):56–64. https://doi.org/10.1038/nrneurol.2014.207
Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Silman AJ, Vanderschueren D, Forti G, Wu FC (2012) Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol 166(6):983–991. https://doi.org/10.1530/eje-11-1051
Hun Lee J, Won S, Stein DG (2015) Progesterone attenuates thrombin-induced endothelial barrier disruption in the brain endothelial cell line bEnd.3: the role of tight junction proteins and the endothelial protein C receptor. Brain Res 1613:73–80. https://doi.org/10.1016/j.brainres.2015.04.002
Hurn PD, Macrae IM (2000) Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab 20(4):631–652. https://doi.org/10.1097/00004647-200004000-00001
Iadecola C, Anrather J (2011) Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 14(11):1363–1368. https://doi.org/10.1038/nn.2953
Inagaki T, Etgen AM (2013) Neuroprotective action of acute estrogens: animal models of brain ischemia and clinical implications. Steroids 78(6):597–606. https://doi.org/10.1016/j.steroids.2012.12.015
Ishrat T, Sayeed I, Atif F, Hua F, Stein DG (2010) Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol 226(1):183–190. https://doi.org/10.1016/j.expneurol.2010.08.023
Ishrat T, Sayeed I, Atif F, Hua F, Stein DG (2012) Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience 210:442–450. https://doi.org/10.1016/j.neuroscience.2012.03.008
Ismail PM, Li J, DeMayo FJ, O’Malley BW, Lydon JP (2002) A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16(11):2475–2489. https://doi.org/10.1210/me.2002-0169
Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y (2009) Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm Res 58(9):619–624. https://doi.org/10.1007/s00011-009-0032-8
Jiang C, Zuo F, Wang Y, Lu H, Yang Q, Wang J (2016) Progesterone changes VEGF and BDNF expression and promotes neurogenesis after ischemic stroke. Mol Neurobiol 54:571–581
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y (2018) Blood–brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163–164:144–171. https://doi.org/10.1016/j.pneurobio.2017.10.001
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99(18):11946–11950. https://doi.org/10.1073/pnas.182296499
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87(5):779–789. https://doi.org/10.1189/jlb.1109766
Jin Z, Wu J, Yan LJ (2016) Chemical conditioning as an Approach to ischemic stroke tolerance: mitochondria as the target. Int J Mol Sci 17(3):351. https://doi.org/10.3390/ijms17030351
Kalogeris T, Bao Y, Korthuis RJ (2014) Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2:702–714. https://doi.org/10.1016/j.redox.2014.05.006
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood–brain barrier disruption and central nervous system inflammation. Nat Med 13(10):1173–1175. https://doi.org/10.1038/nm1651
Kostic T, Andric S, Maric D, Kovacevic R (1998) The effect of acute stress and opioid antagonist on the activity of NADPH-P450 reductase in rat Leydig cells. J Steroid Biochem Mol Biol 66(1–2):51–54
Kumar N, Koide SS, Tsong Y, Sundaram K (2000) Nestorone: a progestin with a unique pharmacological profile. Steroids 65(10–11):629–636
Kumar N, Fagart J, Liere P, Mitchell SJ, Knibb AR, Petit-Topin I, Rame M, El-Etr M, Schumacher M, Lambert JJ, Rafestin-Oblin ME, Sitruk-Ware R (2017) Nestorone(R) as a novel progestin for nonoral contraception: structure-activity relationships and brain metabolism studies. Endocrinology 158(1):170–182. https://doi.org/10.1210/en.2016-1426
Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM (2000) Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg 92(5):848–852. https://doi.org/10.3171/jns.2000.92.5.0848
Labrie F (2010) DHEA, important source of sex steroids in men and even more in women. Prog Brain Res 182:97–148. https://doi.org/10.1016/s0079-6123(10)82004-7
Labrie F (2015) All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol 145:133–138. https://doi.org/10.1016/j.jsbmb.2014.06.001
Lammerding L, Slowik A, Johann S, Beyer C, Zendedel A (2016) Poststroke inflammasome expression and regulation in the peri-infarct area by gonadal steroids after transient focal ischemia in the rat brain. Neuroendocrinology 103(5):460–475. https://doi.org/10.1159/000439435
Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM (2009) Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids 74(7):555–561. https://doi.org/10.1016/j.steroids.2009.01.003
Lee RJ, Kim JK, Chao D, Kuo L, Mally A, McClean ME, Pemberton HE, Wilmington AR, Wong J, Murphy SP (2015) Progesterone and allopregnanolone improves stroke outcome in male mice via distinct mechanisms but neither promotes neurogenesis. J Neurochem 132(1):32–37. https://doi.org/10.1111/jnc.12990
Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD (2004) Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol 187(1):94–104. https://doi.org/10.1016/j.expneurol.2004.01.004
Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, Hurn PD, McCullough LD (2011) Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab 31(2):413–425. https://doi.org/10.1038/jcbfm.2010.181
Liao S, Chen W, Kuo J, Chen C (2001) Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci Lett 297(3):159–162
Liberale L, Carbone F, Montecucco F, Gebhard C, Luscher TF, Wegener S, Camici GG (2018) Ischemic stroke across sexes: what is the status quo? Front Neuroendocrinol. https://doi.org/10.1016/j.yfrne.2018.05.001
Lisabeth L, Bushnell C (2012) Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol 11(1):82–91. https://doi.org/10.1016/S1474-4422(11)70269-1
Liu M, Kelley MH, Herson PS, Hurn PD (2010) Neuroprotection of sex steroids. Minerva Endocrinol 35(2):127–143
Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD (2011) Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke 42(4):1090–1096. https://doi.org/10.1161/STROKEAHA.110.594861
Liu A, Margaill I, Zhang S, Labombarda F, Coqueran B, Delespierre B, Liere P, Marchand-Leroux C, O’Malley BW, Lydon JP, De Nicola AF, Sitruk-Ware R, Mattern C, Plotkine M, Schumacher M, Guennoun R (2012) Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology 153(8):3747–3757. https://doi.org/10.1210/en.2012-1138
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4(5):399–415. https://doi.org/10.1038/nrn1106
Ma Y, Wang J, Wang Y, Yang GY (2017) The biphasic function of microglia in ischemic stroke. Prog Neurobiol 157:247–272. https://doi.org/10.1016/j.pneurobio.2016.01.005
Madinier A, Wieloch T, Olsson R, Ruscher K (2014) Impact of estrogen receptor beta activation on functional recovery after experimental stroke. Behav Brain Res 261:282–288. https://doi.org/10.1016/j.bbr.2013.12.046
Manzanero S, Santro T, Arumugam TV (2013) Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int 62(5):712–718. https://doi.org/10.1016/j.neuint.2012.11.009
Maric D, Kostic T, Kovacevic R (1996) Effects of acute and chronic immobilization stress on rat Leydig cell steroidogenesis. J Steroid Biochem Mol Biol 58(3):351–355
Martel C, Labrie F, Archer DF, Ke Y, Gonthier R, Simard JN, Lavoie L, Vaillancourt M, Montesino M, Balser J, Moyneur E (2016) Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol 159:142–153. https://doi.org/10.1016/j.jsbmb.2016.03.016
McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ (2012) Sex differences in the brain: the not so inconvenient truth. J Neurosci 32(7):2241–2247. https://doi.org/10.1523/JNEUROSCI.5372-11.2012
McColl BW, Allan SM, Rothwell NJ (2007) Systemic inflammation and stroke: aetiology, pathology and targets for therapy. Biochem Soc Trans 35(Pt 5):1163–1165. https://doi.org/10.1042/BST0351163
McCullough LD, Hurn PD (2003) Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab 14(5):228–235
McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD (2005) Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 25(4):502–512. https://doi.org/10.1038/sj.jcbfm.9600059
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB (2016) Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133(4):447–454. https://doi.org/10.1161/cir.0000000000000366
Murphy SJ, Traystman RJ, Hurn PD, Duckles SP (2000) Progesterone exacerbates striatal stroke injury in progesterone-deficient female animals. Stroke 31(5):1173–1178
Ogden CL, Carroll MD, Fryar CD, Flegal KM (2015) Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 219:1–8
Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR (1994) Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17 alpha-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl 15(4):302–308
Ozacmak VH, Sayan H (2009) The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol Res 58(6):909–912
Palmer BF, Clegg DJ (2015) The sexual dimorphism of obesity. Mol Cell Endocrinol 402:113–119. https://doi.org/10.1016/j.mce.2014.11.029
Perez-Alvarez MJ, Maza Mdel C, Anton M, Ordonez L, Wandosell F (2012) Post-ischemic estradiol treatment reduced glial response and triggers distinct cortical and hippocampal signaling in a rat model of cerebral ischemia. J Neuroinflammation 9:157. https://doi.org/10.1186/1742-2094-9-157
Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA (2009) Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 40(4):1032–1037. https://doi.org/10.1161/STROKEAHA.108.542894
Petrone AB, Simpkins JW, Barr TL (2014) 17beta-estradiol and inflammation: implications for ischemic stroke. Aging Dis 5(5):340–345. https://doi.org/10.14336/AD.2014.0500340
Quillinan N, Deng G, Grewal H, Herson PS (2014) Androgens and stroke: good, bad or indifferent? Exp Neurol 259:10–15. https://doi.org/10.1016/j.expneurol.2014.02.004
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19(8):987–991. https://doi.org/10.1038/nn.4338
Reckelhoff JF (2001) Gender differences in the regulation of blood pressure. Hypertension 37(5):1199–1208
Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L (2008) Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7(10):915–926. https://doi.org/10.1016/S1474-4422(08)70193-5
Rivest S, Rivier C (1991) Influence of the paraventricular nucleus of the hypothalamus in the alteration of neuroendocrine functions induced by intermittent footshock or interleukin. Endocrinology 129(4):2049–2057. https://doi.org/10.1210/endo-129-4-2049
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics C, Stroke Statistics S (2011) Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209. https://doi.org/10.1161/CIR.0b013e3182009701
Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VH, Ridgway EC, Wierman ME (2011) Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids 76(1–2):177–182. https://doi.org/10.1016/j.steroids.2010.10.010
Roy-O’Reilly M, McCullough LD (2018) Age and sex are critical factors in ischemic stroke pathology. Endocrinology. https://doi.org/10.1210/en.2018-00465
Ruan L, Wang B, ZhuGe Q, Jin K (2015) Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res 1623:166–173. https://doi.org/10.1016/j.brainres.2015.02.042
Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F (1993) Progesterone receptor-mediated effects of neuroactive steroids. Neuron 11(3):523–530
Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD (1999) 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke 30(8):1665–1670
Saleh TM, Connell BJ, Legge C, Cribb AE (2004) Stroke-induced changes in estrogen release and neuronal activity in the parabrachial nucleus of the male rat. J Stroke Cerebrovasc Dis 13(1):24–34. https://doi.org/10.1016/j.jstrokecerebrovasdis.2004.01.003
Saleh TM, Connell BJ, Legge C, Cribb AE (2005) Estrogen synthesis in the central nucleus of the amygdala following middle cerebral artery occlusion: role in modulating neurotransmission. Neuroscience 135(4):1141–1153. https://doi.org/10.1016/j.neuroscience.2005.06.061
Sapolsky RM, Pulsinelli WA (1985) Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science 229(4720):1397–1400
Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD (2000) Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab 20(1):112–118. https://doi.org/10.1097/00004647-200001000-00015
Sayeed I, Guo Q, Hoffman SW, Stein DG (2006) Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med 47(4):381–389. https://doi.org/10.1016/j.annemergmed.2005.12.011
Sayeed I, Wali B, Stein DG (2007) Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci 25(2):151–159
Sayeed I, Parvez S, Wali B, Siemen D, Stein DG (2009) Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res 1263:165–173. https://doi.org/10.1016/j.brainres.2009.01.045
Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE (2007) Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28(4):387–439. https://doi.org/10.1210/er.2006-0050
Selvamani A, Sohrabji F (2010) Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging 31(9):1618–1628. https://doi.org/10.1016/j.neurobiolaging.2008.08.014
Selvamani A, Williams MH, Miranda RC, Sohrabji F (2014) Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin Sci 127(2):77–89. https://doi.org/10.1042/CS20130565
Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Ambrose JA, Barua RS (2015) Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J 36(40):2706–2715. https://doi.org/10.1093/eurheartj/ehv346
Sharma N, Lee J, Youssef I, Salifu MO, McFarlane SI (2017) Obesity, cardiovascular disease and sleep disorders: insights into the rising epidemic. J Sleep Disord Ther. https://doi.org/10.4172/2167-0277.1000260
Shet MS, Fisher CW, Arlotto MP, Shackleton CH, Holmans PL, Martin-Wixtrom CA, Saeki Y, Estabrook RW (1994) Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine P450 17A and rat NADPH-P450 reductase. Arch Biochem Biophys 311(2):402–417
Sims NR, Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 1802(1):80–91. https://doi.org/10.1016/j.bbadis.2009.09.003
Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 8:315. https://doi.org/10.3389/fnins.2014.00315
Slowik A, Beyer C (2015) Inflammasomes are neuroprotective targets for sex steroids. J Steroid Biochem Mol Biol 153:135–143. https://doi.org/10.1016/j.jsbmb.2015.02.013
Sohrabji F, Park MJ, Mahnke AH (2017) Sex differences in stroke therapies. J Neurosci Res 95(1–2):681–691. https://doi.org/10.1002/jnr.23855
Spratt NJ, Tomkins AJ, Pepperall D, McLeod DD, Calford MB (2014) Allopregnanolone and its precursor progesterone do not reduce injury after experimental stroke in hypertensive rats—role of postoperative temperature regulation? PLoS ONE 9(9):e107752. https://doi.org/10.1371/journal.pone.0107752
Stankowski JN, Gupta R (2011) Therapeutic targets for neuroprotection in acute ischemic stroke: lost in translation? Antioxid Redox Signal 14(10):1841–1851. https://doi.org/10.1089/ars.2010.3292
Strom JO, Theodorsson A, Theodorsson E (2009) Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: a systematic analysis. J Cereb Blood Flow Metab 29(8):1359–1372. https://doi.org/10.1038/jcbfm.2009.66
Sugo N, Hurn PD, Morahan MB, Hattori K, Traystman RJ, DeVries AC (2002) Social stress exacerbates focal cerebral ischemia in mice. Stroke 33(6):1660–1664
Sutherland BA, Neuhaus AA, Couch Y, Balami JS, DeLuca GC, Hadley G, Harris SL, Grey AN, Buchan AM (2016) The transient intraluminal filament middle cerebral artery occlusion model as a model of endovascular thrombectomy in stroke. J Cereb Blood Flow Metab 36(2):363–369. https://doi.org/10.1177/0271678x15606722
Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM (2007) Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol 500(6):1064–1075. https://doi.org/10.1002/cne.21240
Suzuki S, Brown CM, Wise PM (2009) Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol 30(2):201–211. https://doi.org/10.1016/j.yfrne.2009.04.007
Tilbrook AJ, Turner AI, Clarke IJ (2000) Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod 5(2):105–113
Toung TJ, Traystman RJ, Hurn PD (1998) Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 29(8):1666–1670
Toung TK, Hurn PD, Traystman RJ, Sieber FE (2000) Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke 31(11):2701–2706
Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ (2004) Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab 24(10):1160–1166. https://doi.org/10.1097/01.WCB.0000135594.13576.D2
Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD (2009) Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab 29(8):1454–1462. https://doi.org/10.1038/jcbfm.2009.60
Vigen R, O’Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM (2013) Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310(17):1829–1836. https://doi.org/10.1001/jama.2013.280386
Wali B, Ishrat T, Won S, Stein DG, Sayeed I (2014) Progesterone in experimental permanent stroke: a dose-response and therapeutic time-window study. Brain 137(Pt 2):486–502. https://doi.org/10.1093/brain/awt319
Wali B, Ishrat T, Stein DG, Sayeed I (2016) Progesterone improves long-term functional and histological outcomes after permanent stroke in older rats. Behav Brain Res 305:46–56. https://doi.org/10.1016/j.bbr.2016.02.024
Wang HY, Wang GL, Yu YH, Wang Y (2009) The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res 1297:177–184. https://doi.org/10.1016/j.brainres.2009.08.054
Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y (2010) Neuroprotective effects of progesterone following stroke in aged rats. Behav Brain Res 209(1):119–122. https://doi.org/10.1016/j.bbr.2010.01.026
Wang J, Zhao Y, Liu C, Jiang C, Zhao C, Zhu Z (2011) Progesterone inhibits inflammatory response pathways after permanent middle cerebral artery occlusion in rats. Mol Med Rep 4(2):319–324. https://doi.org/10.3892/mmr.2011.418
Wang Q, Bottalico L, Mesaros C, Blair IA (2015) Analysis of estrogens and androgens in postmenopausal serum and plasma by liquid chromatography-mass spectrometry. Steroids 99(Pt A):76–83. https://doi.org/10.1016/j.steroids.2014.08.012
Westberry JM, Prewitt AK, Wilson ME (2008) Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience 152(4):982–989. https://doi.org/10.1016/j.neuroscience.2008.01.048
White UA, Tchoukalova YD (2014) Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta 1842(3):377–392. https://doi.org/10.1016/j.bbadis.2013.05.006
Wilson ME (2013) Stroke: understanding the differences between males and females. Pflugers Arch 465(5):595–600. https://doi.org/10.1007/s00424-013-1260-x
Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M, Rosewell KL (2001) Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Rev 37(1–3):313–319
Won S, Lee JH, Wali B, Stein DG, Sayeed I (2014) Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway. J Cereb Blood Flow Metab 34(1):72–80. https://doi.org/10.1038/jcbfm.2013.163
Won S, Lee JK, Stein DG (2015) Recombinant tissue plasminogen activator promotes, and progesterone attenuates, microglia/macrophage M1 polarization and recruitment of microglia after MCAO stroke in rats. Brain Behav Immun 49:267–279. https://doi.org/10.1016/j.bbi.2015.06.007
Wong R, Bath PM, Kendall D, Gibson CL (2013a) Progesterone and cerebral ischaemia: the relevance of ageing. J Neuroendocrinol 25(11):1088–1094. https://doi.org/10.1111/jne.12042
Wong R, Renton C, Gibson CL, Murphy SJ, Kendall DA, Bath PM, Progesterone Pre-Clinical Stroke Pooling Project C (2013b) Progesterone treatment for experimental stroke: an individual animal meta-analysis. J Cereb Blood Flow Metab 33(9):1362–1372. https://doi.org/10.1038/jcbfm.2013.120
Wong R, Gibson CL, Kendall DA, Bath PM (2014) Evaluating the translational potential of progesterone treatment following transient cerebral ischaemia in male mice. BMC Neurosci 15:131. https://doi.org/10.1186/s12868-014-0131-5
Wullner U, Seyfried J, Groscurth P, Beinroth S, Winter S, Gleichmann M, Heneka M, Loschmann P, Schulz JB, Weller M, Klockgether T (1999) Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain Res 826(1):53–62
Xu X, Chua CC, Gao J, Chua KW, Wang H, Hamdy RC, Chua BH (2008) Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res 1227:12–18. https://doi.org/10.1016/j.brainres.2008.06.018
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27(4):697–709. https://doi.org/10.1038/sj.jcbfm.9600375
Yousuf S, Atif F, Sayeed I, Tang H, Stein DG (2014a) Progesterone in transient ischemic stroke: a dose-response study. Psychopharmacology 231(17):3313–3323. https://doi.org/10.1007/s00213-014-3556-8
Yousuf S, Sayeed I, Atif F, Tang H, Wang J, Stein DG (2014b) Delayed progesterone treatment reduces brain infarction and improves functional outcomes after ischemic stroke: a time-window study in middle-aged rats. J Cereb Blood Flow Metab 34(2):297–306. https://doi.org/10.1038/jcbfm.2013.198
Yousuf S, Atif F, Sayeed I, Wang J, Stein DG (2016) Neuroprotection by progesterone after transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Horm Behav 84:29–40. https://doi.org/10.1016/j.yhbeh.2016.06.002
Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD (2009) Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol 217(1):210–218. https://doi.org/10.1016/j.expneurol.2009.02.012
Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106(7):829–838. https://doi.org/10.1172/JCI9369
Zhao H, Sapolsky RM, Steinberg GK (2006) Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol 34(3):249–270. https://doi.org/10.1385/MN:34:3:249
Zhu X, Frechou M, Liere P, Zhang S, Pianos A, Fernandez N, Denier C, Mattern C, Schumacher M, Guennoun R (2017) A role of endogenous progesterone in stroke cerebroprotection revealed by the neural-specific deletion of its intracellular receptors. J Neurosci 37(45):10998–11020. https://doi.org/10.1523/JNEUROSCI.3874-16.2017
Zhu X, Frechou M, Schumacher M, Guennoun R (2018) Cerebroprotection by progesterone following ischemic stroke: Multiple effects and role of the neural progesterone receptors. J Steroid Biochem Mol Biol. https://doi.org/10.1016/j.jsbmb.2018.07.014
Author information
Authors and Affiliations
Contributions
All authors contributed in this work. RG designed, wrote the first draft of the manuscript, revised and edited the manuscript. XZ prepared the figures and revised critically the manuscript. MF revised critically the manuscript. PG revised critically the manuscript. AS revised critically the manuscript. PL revised critically the manuscript. MS revised critically the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Guennoun, R., Zhu, X., Fréchou, M. et al. Steroids in Stroke with Special Reference to Progesterone. Cell Mol Neurobiol 39, 551–568 (2019). https://doi.org/10.1007/s10571-018-0627-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-018-0627-0