Abstract
We previously reported that inhibition of Rho-kinase (ROCK) by hydroxyl fasudil improves cognitive deficit and neuronal damage in rats with chronic cerebral ischemia (Huang et al., Cell Mol Neurobiol 28:757–768, 2008). In this study, fasudil mesylate (FM) was investigated for its neuroprotective potential in rats with ischemia following middle cerebral artery occlusion (MCAO) and reperfusion. The effect of fasudil mesylate was also studied in rat brain cortical and hippocampal slices treated with oxygen-glucose deprivation (OGD) injury. Gross anatomy showed that cerebral infarct size, measured with 2,3,5-triphenyltetrazolium chloride (TTC) staining, was significantly smaller in the FM-treated than in the non-FM-treated ischemic rats. In the brain regions vulnerable to ischemia of ischemic rats, fasudil mesylate was also found to significantly restore the enzyme protein expression level of endothelial nitric oxide synthase (eNOS), which was decreased in ischemia. However, it remarkably reduced the protein synthesis of inducible nitric oxide synthase (iNOS) that was induced by ischemia and reperfusion. In rat brain slices treated with OGD injury, fasudil mesylate increased the neuronal cell viability by 40% for cortex and by 61% for hippocampus, respectively. Finally, in the presence of OGD and fasudil mesylate, superoxide dismutase (SOD) activity was increased by 50% for cortex and by 58% for hippocampus, compared to OGD only group. In conclusion, our in vivo study showed that fasudil mesylate not only decreased neurological deficit but also reduced cerebral infarct size, possibly and at least partially by augmenting eNOS protein expression and inhibiting iNOS protein expression after ischemia-reperfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke represents one of the leading causes of human disability and death across the world. Nearly one-third of patients with acute ischemic stroke develop early neurological deterioration, a situation associated with increased mortality and long-term functional disability (Davalos et al. 1999). Besides a great deal of measures being taken to prevent the occurrence of ischemic stroke such as controlling hypertension and diabetes mellitus, pharmacological effort has also been made to discover effective drugs that reduce neuronal damage.
Fasudil hydrochloride, a Rho kinase (ROCK) inhibitor, was reported to reduce cerebral infarct size and lessen ischemic neuronal damage in vitro and in vivo (Rikitake et al. 2005; Yamashita et al. 2007). Our laboratory recently reported that relative long-term inhibition of ROCK by hydroxyl fasudil improves cognitive deficit and neuronal damage in rats with chronic cerebral ischemia (Huang et al. 2008a). Fasudil mesylate (synthesized by Wuhan pharmaceutical Co Ltd., Wuhan, China) is derived from fasudil hydrochloride with a hydrochloride group replaced by mesylate. In our recent published studies, fasudil mesylate was demonstrated to dilate dog cerebral vasospasm induced by subarachnoid hemorrhage. It also decreased cerebrovascular resistance and increased cerebral blood flow (Li et al. 2007). In rats with ischemia created by middle cerebral artery occlusion (MCAO) and reperfusion, there are many neurological damages, which can be seen structurally and functionally. This in vivo animal model has been used for pharmacological studies in ischemic stroke (Longa et al. 1989). In these rats, the neurovascular unit is damaged primarily by reduction of blood flow and secondarily by ensuing inflammatory processes. In the present study, we will use MCAO and reperfusion techniques to create the in vivo model of rats with ischemia and evaluate the effect of fasudil mesylate in these rats by examining changes in neuronal cell morphology and in the activities of nitric oxide synthase (NOS).
Inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) are the two isoforms of NOS that play important but opposing roles in cerebral ischemia. iNOS has been implicated as an important mediator of inflammatory responses during ischemia and reperfusion (Samdani et al. 1997). Induction of iNOS results in delayed neuronal cell death (Dawson et al. 1994) but also exacerbates glutamate excitotoxicity (Hewett et al. 1994). Nitric oxide (NO) derived from iNOS and its oxidative by-product peroxynitrite are thought to contribute to neuronal death due to oxidation of structural neuronal proteins during ischemia (Vaughan and Delanty 1999). In contrast, NO produced by eNOS has a protective physiological role and orchestrates the paracrine homeostatic functions of the endothelium, which include inhibition of leukocyte and platelet adhesion, control of vascular tone, and maintenance of a thrombo-resistant interface between the bloodstream and the vessel wall (Vaughan and Delanty 1999). A relative compartmentalization of NOS isoform activity in the brain, with contrasting roles for eNOS and iNOS in cerebral ischemia and reperfusion, has been suggested. Therefore, we will examine the effect of fasudil mesylate on both of these enzymes in cerebral ischemic animal model by immunohistochemistry and Western blot to monitor any changes in enzyme protein expression.
The use of brain slices, as a model of ischemia in central nervous system, has been well described previously (Cohen et al. 1984). Brain slices are a preparation that offer many advantages over other in vivo and in vitro techniques in the study of brain ischemia. First of all, the extracellular compartment can be directly and immediately accessed in the slices due to the lack of a blood–brain barrier. Secondly, the environmental factors can be directly controlled. Lastly, the tissue morphology of the slices is relatively unchanged from the intact animals since intercellular connections are preserved (De et al. 1999). Thus, the brain tissue slices can be an excellent preparation for quantitative pharmacology. The cortical and hippocampal slices treated with oxygen-glucose deprivation (OGD) have been used as an in vitro model of stroke (Huang et al. 2008b). The present study will also investigate the effects of fasudil mesylate on neuronal cell damage utilizing freshly prepared rat cortical and hippocampal slices in vitro.
NO produced by eNOS plays an important role in the regulation of cell growth and apoptosis as well as vasodilation and antithrombotic actions. However, it is not clear whether NO-dependent antiinflammatory effect of fasudil could play a role in the neuroprotection. There is evidence that conditions leading to enhanced ROCK activity could downregulate eNOS expression, worsening the endothelial function, whereas ROCK inhibition mediated upregulation of eNOS activity could increase cerebral blood flow and thereby play a predominant role in neuroprotection, making ROCK inhibition a possible attractive therapeutic target for improving intracerebral ischemic conditions (Rikitake et al. 2005). Although inhibition of Rho kinase for induction of iNOS has been studied previously in various investigations, there are controversial reports concerning the regulation of iNOS. Effect of inhibition of Rho kinase on iNOS expression appears to vary from cell to cell but it is consistent that the inhibition of ROCK signal involves the regulation of iNOS expression (Dobashi et al. 2008; Johansson and Persson 2004). Additionally, NO production by lysophosphatidic acid (LPA)-treated, cytokine-stimulated cells was reduced, which were prevented by Rho kinase inhibition with Y-27632 (Kadowaki et al. 2004). Thus, the down-regulation by LPA of cytokine-induced increases in iNOS activity is likely to involve a Rho-dependent signaling pathway. Our study will use fasudil mesylate in intact animals with MCAO ischemia and rat brain tissue slices with OGD injury and measure the protein expression of eNOS and iNOS during brain ischemia-reperfusion injury.
Materials and Methods
Materials
Fasudil mesylate was from Wuhan pharmaceutical Co Ltd. (Wuhan, China). It was dissolved in normal saline (NS) and preserved away from light. All other reagents used were of analytical grade. 2,3,5-Triphenyltetrazolium chloride (TTC) and propidium iodide (PI) were purchased from Sigma (St Louis, MO, USA). Assay kits for superoxide dismutase (SOD), lactate dehydrogenase (LDH), iNOS and total protein quantitation were purchased from Nanjing Jiancheng Bio-Tek Co. (Nanjing, China). Rabbit anti-rat eNOS/iNOS monoclonal IgG was from Santa Cruz Biotechnology (Santa Cruz, USA).
Animals and Drug Application
Adult male Sprague–Dawley rats (200–250 g) were obtained from Experimental Animal Center of Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China). They were housed in a temperature and humidity-controlled room (temperature: 22 ± 1°C, humidity: 60%) with free access to food and water. The rats were kept on a 12-h light/dark cycle and adapted to these conditions for at least 7 days before experiments. Fasudil mesylate was dissolved in NS and administered intraperitoneally (i.p.). Rats were randomly divided into three groups, namely, sham, vehicle, and fasudil mesylate (FM)-treated groups (n = 10). For the sham group, the rats were only anesthetized and their carotid arteries were separated but not occluded. FM-treated group was administered with FM (10 mg/kg, i.p.) at 48, 24, and 2 h before MCAO, whereas the vehicle group was given the same amount of saline.
MCAO Surgery and Neurological Deficit Evaluation
All procedures used in this study comply with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). Rats were anesthetized using 10% chloral hydrate (350 mg/kg, i.p.). The middle cerebral artery was occluded with a 4-0 silicone-coated nylon suture by surgical operation (Longa et al. 1989). Reperfusion was induced after 2 h MCAO by filament withdrawal. Sham-operated animals were subjected to the same surgical procedure, but the suture was not advanced beyond the internal carotid bifurcation. The rat rectal temperature was maintained at 37°C throughout the anesthetic period. After revival from anesthesia, animals were housed back at room temperature 22 ± 1°C with free access to food and water.
Neurological behavioral assessment was performed 2 h before MCAO and 6 h, and 26 h after ischemia, and scored on a 6-point scale: 0, no neurological deficit; 1, failure to extend left forepaw fully; 2, circling to the left; 3, inability to bear weight on the left; 4, no spontaneous walking with depressed level of consciousness; and 5, death.
Cerebral Infarct Size Measurement
Cerebral infarct size was assessed with TTC staining method. After 2 h MCAO and 24 h reperfusion, all animals were anesthetized and the brains quickly isolated and sectioned into consecutive 2-mm-thick coronal slices using a Vibratome (Campden Instruments, USA). Slices were immediately immersed in 2% TTC medium at 37°C for 30 min. Stained slices were washed in phosphate buffer saline (PBS) for 5 min and fixed in buffered formaldehyde solution for 24 h. After the end of staining and fixation, color image of these slices were captured using a video camera (Olympus, Japan). All brain slices of each experimental group were analyzed for the infarct size using the Image-Pro plus 5.0 analysis software (O’Donnell et al. 2004). Percentage infarct size was calculated as described (Swanson et al. 1990): [(V C − V L)/V C] × 100%, where V C is the volume of control hemisphere and V L the volume of non-infarcted tissue in the lesioned hemisphere.
Morphology
Rats were anesthetized with chloral hydrate (350 mg/kg, i.p.), and then perfused transcardially with normal saline followed by 4% paraformaldehyde. All brains were then fixed in the same fixative at 4°C, dehydrated and then embedded in paraffin blocks. Coronal sections of 5 μm were stained with hematoxylin-eosin (H & E).
Immunohistochemistry
Tissue sections were deparaffinized and wet through graded alcohol. Endogenous peroxidase activity was blocked by incubation in 10% hydrogen peroxide for 10 min. After rinsed with PBS three times, the sections were blocked with 1:10 normal goat serum to minimize nonspecific background staining. After rabbit anti-rat eNOS/iNOS antibody (Santa Cruz, USA) (dilution 1:100) were applied to the samples and incubated for 24 h at 4°C, the sections were incubated with biotinylated goat anti-rabbit IgG (1:100) for 1 h at 37°C, followed by the steps according to SABC kit protocol. The sections were subsequently incubated with diaminobenzidine 0.5 g/l and observed under light microscope.
Western-blot Analysis
After the rats were decapitated, the infarct side of cortex (1–5 mm posterior to the bregma and 1–5 mm beside the sagittal suture) was harvested for assay of protein expression of eNOS and iNOS. The brain tissue was homogenized in an ice-cold buffer (tris-(hydroxymethyl) aminomethane 50 mmol/l, pH 7.4, NaCl 150 mmol/l, 0.5% Triton X-100, edetic acid 1 mmol/l, phenylmethylsulfony fluoride 1 mol/l, and aprotinin 5 mg/l), centrifuged at 14,000g at 4°C for 30 min. The supernatants were then collected as total proteins, which were electrophoresed through an 8% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and electrically transferred to a nitrocellulose membrane. The membrane was then incubated at 4°C overnight in tris-(hydroxymethyl)-aminomethane buffered saline (TBS) containing 5% milk and the primary rabbit antibodies against eNOS and iNOS of rat (1:500 dilution, Santa Cruz, USA). After washing with TBS, the membranes were incubated with the secondary antibody (goat anti-rabbit IgG) at room temperature for 1 h. The protein band intensities were quantified using Scion Image (Scion, Maryland, USA), and the amount was normalized with β-actin values in the same lane.
Oxygen-Glucose Deprivation (OGD) Injury of Brain Slices
The slices were made as described (Huang et al. 2008b). The SD rats were decapitated and the whole brains quickly removed and immersed in 10 ml iced artificial cerebrospinal fluid (ACSF) containing (mM): NaCl 126, KCl 3.5, NaH2PO4 1.2, MgCl2 1.3, CaCl2 2.0, d-(+)-glucose 11, NaHCO3 25; 290 mOsm; gassed with 95% O2 and 5% CO2 (pH 7.4). Both cortical and hippocampal slices of the rat brain were dissected out and transverse sections (400 μm) were prepared using a Mcllwain tissue chopper (The Mickle Laboratory Engineering Co. LTD, USA). All the slices were maintained in ACSF bubbled with 95% O2 and 5% CO2 at room temperature for recovery. After the slices were incubated in oxygenated ACSF at 34°C for 30 min, the slices were transferred for OGD experiment. OGD was used as an in vitro model of ischemia. It was conducted by replacing 3 ml ACSF with ischemia medium (the same volume) (0 mM glucose, 10 mM cane sugar; other solutes as in ACSF) gassed with 95% N2 and 5% CO2 for 15 min. After OGD process, the slices were placed back to 3 ml ACSF and re-oxygenated for 120 min (Huang et al. 2008b).
The rat cortical and hippocampal slices were randomly assigned to one of the following groups respectively: the slices in control group were incubated in oxygenated ACSF at 34°C for 165 min. The slices in OGD group were incubated first in ACSF for 30 min and then had OGD treatment for 15 min, followed by re-oxygenated in ACSF for 120 min. For the slices in OGD + FM group, fasudil mesylate (10, 100 μmol/l) was added at the beginning of the normal incubation for 30 min and the incubation continued during the OGD insult. The brain slices were then re-oxygenated in ACSF for 120 min without fasudil mesylate.
One milliliter of incubation medium collected at the end of the experiment was stored at −70°C for quantification of LDH activity of the slices. The brain slices collected at the end of the experiment were stored at −70°C for analysis of iNOS, SOD and tissue total protein of the slices.
Measurement of Brain Slices Viability In Vitro
At the end of incubation, all slices of each group were incubated in 2% TTC solution at 35°C in the dark for 30 min. After that, they were taken out and rinsed by normal saline. The wet slices of each group were weighed after the water on the surface was removed by filter paper. Extracted fluid (ethanol: dimethyl sulfoxide = 1:1) was added with a proportion of 20 ml to 1.0 g of slice tissue into each group according to the wet weight. After 24 h of immersion under the dark, formanzan, the red crystal product, was extracted, and the degree of injury was evaluated by measuring the absorbance of the red crystal product at 490 nm (OD490) using an ELISA reader (TECAN A-5082, megllan, Austria) (Huang et al. 2008b). LDH releases in the incubation medium were measured by spectrophotometry according to the method described previously (Huang et al. 2008a).
The fluorescence of propidium iodide (PI), shown to enter exclusively damaged cells, was used for quantification of cellular survival (Macklis and Madison 1990). The level of cellular damage in rat brain slices has been observed using a laser confocal scanning microscope (LCSM; Leica, TCS-SP, Germany) with PI labeling. Fluorescent probe propidium iodide (PI) was dissolved in dimethyl sulfoxide, and added into the incubating chamber in a final concentration of 2 μM bubbled with 95% O2 and 5% CO2 for 30 min at the end of incubation at 34°C, and washed away with ACSF for three times, 3 min each. The brain slices were then observed by LCSM in the sample pool with ACSF at room temperature. Both transmission and fluorescence images were captured by LCSM under the following working conditions including excitation wavelength: 490 nm; emission wavelength: 650 nm; scanning section thickness: 4 μm. The scanning from the cut surface of the slices was excluded. The cell damage of each slice was calculated by the average relative intensity of PI fluorescence (Huang et al. 2008b; Macklis and Madison 1990).
Assessment of iNOS and SOD In Vitro
The brain slices were homogenized with NS and centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was collected for assessment of iNOS and SOD. iNOS activities were determined according to manufacturers’ instructions (Huang et al. 2008b). The assay for SOD was based on its ability to inhibit the oxidation of oxymine by O −2 produced from the xanthine/xanthine oxidase system (Liao et al. 2004).
Statistical Analysis
Data are presented as mean ± SEM. Statistical comparisons were made using ANOVA followed by a Student’s t-test, Fisher’s PLSD test, Dunnett’s test, or the Mann–Whitney U-test. The statistical significance of P < 0.05, 0.01, or 0.001 is indicated in the figure legends.
Results
Effects of Fasudil Mesylate on Cerebral Infarct Size and Neurological Deficit Score In Vivo
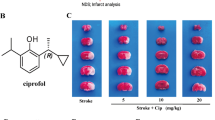
Representative consecutive 2-mm-thick coronal slices stained with 2% TTC from one sample of sham, vehicle and FM-treated groups, respectively, are presented in Fig. 1a. In sham group, the rats were only anesthetized and their carotid arteries were separated but not occluded. In FM-treated group, the rats were administered with FM (10 mg/kg, i.p.) at 48, 24 and 2 h before MCAO, whereas the vehicle group was given the same amount of saline. Fasudil mesylate (10 mg/kg i.p.) treatment resulted in a significant decrease in cerebral infarct size after 2 h MCAO and 24 h reperfusion (Fig. 1b) (P < 0.01). Neurological deficit score was measured at 6 and 26 h after MCAO, and inhibited at both time points when FM was used (Fig. 1c) (P < 0.01).
Effects of fasudil mesylate (FM) on cerebral infarct size and neurological score after 2 h MCAO and 24 h reperfusion (n = 10). (a) Representative coronal brain sections stained with 2% TTC from sham, vehicle and FM-treated groups, respectively. (b) Quantitative analysis of cerebral infarct size from vehicle and FM-treated groups (## P < 0.01 vs. vehicle group). (c) Neurological deficit score was measured at 6 and 26 h after MCAO (## P < 0.01 vs. vehicle group). Sham group includes rats without MCAO, vehicle group is NS-treated rats with MCAO and FM-treated group FM-treated (10 mg/kg i.p.) rats with MCAO
Effects of Fasudil Mesylate on Morphological Changes of Rat Cortex In Vivo
H & E was used to stain the brain tissues described in the method to observe pathological alterations in ischemic regions of rats with MCAO and reperfusion. In the frontal cortex, marked morphological changes were visualized in ischemic region of vehicle group: neuronal cell loss, nuclei shrinkage, and dark staining of neurons. Fasudil mesylate treatment (10 mg/kg i.p.) markedly attenuated these pathological changes (Fig. 2).
Effect of fasudil mesylate (FM) on morphologic changes in ischemic regions of rat cortex induced by 2 h MCAO and 24 h reperfusion. The different treatments of animals are shown in (a) the cortex tissues from the rats without MCAO (sham group); (b) the tissues from NS-treated rats with MCAO (vehicle group); (c) the tissues from FM-treated rats with MCAO (FM-treated group). Fasudil mesylate treatment (10 mg/kg i.p.) markedly attenuated these pathological and morphological changes as compared with vehicle group
Effects of Fasudil Mesylate on Expression of eNOS and iNOS
The eNOS and iNOS protein expression were examined by Western blot and immunohistochemistry. The photomicrographs of immunohistochemical localization of iNOS and eNOS in ischemic brain tissues are illustrated in Fig. 3. The rats with MCAO and reperfusion (vehicle group) exhibited a remarkable decrease in eNOS reactivity and a significant increase in iNOS reactivity. However, FM treatment at 10 mg/kg attenuated these changes in cortex (Fig. 3), indicating that the FM-treated group altered eNOS and iNOS protein expression caused by MCAO in vehicle group.
Immunohistochemistry graphs show the expression of eNOS (upper panel) and iNOS (lower panel) in ischemic regions of rat cortex after 2 h MCAO and 24 h reperfusion. In the figures for both eNOS and iNOS immunoreactivities, (a) is sham group for rats without MCAO; (b) is vehicle group treated with NS in rats with MCAO; and (c) is FM-treated rats with MCAO (FM10 mg/kg i.p.). Arrows represent eNOS and iNOS positive cells, respectively (×400)
In Western blot, compared with sham group, there was a marked decrease in eNOS expression and increase in iNOS expression in vehicle group (P < 0.05) (Fig. 4b). Fasudil mesylate treatment (10 mg/kg i.p.) significantly increased eNOS expression but decreased iNOS expression in cortex of rats with MCAO and reperfusion (P < 0.05) (Fig. 4b). A substantial decrease in eNOS immunoreactivity but increase in iNOS immunoreactivity was noted in ischemic regions of cortex after ischemia-reperfusion. These data are consistent with the results of immunohistochemistry, showing that the fasudil mesylate largely reversed the change in the protein expression of both enzymes by intracranial ischemia in vehicle group.
Western blot analysis demonstrates the expression of eNOS (upper panel in a) and iNOS (lower panel in a) in ischemic regions of rat cortex after 2 h MCAO and 24 h reperfusion. (a) Western blot analysis showing protein bands of rat cortex from each group; the corresponding β-actin bands as controls shown in the same blot (upper lane). (b) After densitometric quantification, the data are expressed as mean ± SEM (* P < 0.05 vs. sham; # P < 0.05 vs. vehicle). Each group designated as sham, vehicle and FM-treated, was described as in Fig. 3
Effects of Fasudil Mesylate on Neuronal Cell Viability in Brain Slices After OGD Insult In Vitro
Fasudil mesylate (10, 100 μmol/l) was co-incubated with cerebral cortical and hippocampal slices respectively for 3 h. There was little effect of fasudil mesylate on TTC staining of brain slices. No significant difference was observed between fasudil mesylate-treated group and control group (perfused with ACSF-only medium) (P > 0.05).
After the slices were subjected to 15 min of OGD and 2 h of re-oxygenation (REO), the viability of slices were reduced both in cortical and hippocampal slices, compared with control group (n = 8, P < 0.001). Different concentrations of fasudil mesylate, as indicated in Fig. 5, were added at the beginning of the normal incubation for 30 min and during OGD insult for 15 min, respectively. Fasudil mesylate dose-dependently increased OD490 of TTC staining and decreased LDH efflux as shown in Fig. 5A and B (P < 0.05).
Effects of fasudil mesylate (FM) on the viability of rat brain cortical and hippocampal slices after OGD insult in vitro. OD490 value of TTC staining (A) was decreased but LDH efflux (B) increased in OGD group, whereas fasudil mesylate (10, 100 μmol/l) significantly attenuated these changes (P < 0.05). The data are expressed as mean ± SEM (n = 8). Compared with the control group, fluorescent intensity of PI staining (C) was significantly increased in OGD group, whereas fasudil mesylate at both 10 and 100 μmol/l decreased PI fluorescent intensity of cortical and hippocampal slices after OGD insult (P < 0.05). The data are expressed as mean ± SEM. (n = 5). For (A–C), *** P < 0.001 vs. control group; # P < 0.05, ## P < 0.01, and ### P < 0.001 vs. OGD group. In the bottom are representative images of PI staining (D) for cortical and hippocampal slices (×10). These graphs are (a and e) control slices without OGD; (b and f) OGD slices; (c and g) OGD slices + FM 10 μM; (d and h) OGD slices + FM 100 μM
With OGD insult, rat cortical and hippocampal slices showed strong red fluorescence after PI staining under the LCSM. OGD insult resulted in a significant increase in PI fluorescence intensity compared with control group (n = 5, P < 0.001). Fasudil mesylate at 10 and 100 μmol/l could significantly decrease PI staining (P < 0.05) (Fig. 5C). As shown in Fig. 5D, fasudil mesylate at 10 and 100 μmol/l significantly decreased the PI staining, which was remarkably increased by OGD treatment.
Effects of Fasudil Mesylate on iNOS and SOD Activity of OGD Injured Brain Slices In Vitro
The iNOS activity (Fig. 6a) was significantly increased both in rat cortical and hippocampal slices after OGD insult, compared with control group (P < 0.001). On the contrary, the SOD activity (Fig. 6b) was largely decreased compared with OGD group (P < 0.001 vs. control slices). However, the increased activity of iNOS could be reversed to some degree by fasudil mesylate, whereas the decreased SOD activity could be attenuated by fasudil mesylate in a dose-dependent manner (10, 100 μmol/l).
Effects of fasudil mesylate (FM) on iNOS and SOD activity of rat cortical and hippocampal slices after OGD insult (n = 8). The data are expressed as mean ± SEM (P < 0.05). *** P < 0.001 vs. control group; # P < 0.05, ## P < 0.01, and ### P < 0.001 vs. OGD group. Each group was treated as described in the method section
Discussion
The present study has demonstrated the neuroprotective effect of fasudil mesylate in rat brain slices with acute OGD/REO insult and in rats with cerebral ischemia and reperfusion injury. At the time of completion of this study, a report was published that describes the roles of fasudil in protecting neurons in early stage of ischemic infarction (Yamashita et al. 2007). However, in this published paper, the approaches were largely different from ours in that they examined the effects of fasudil and hydroxyfasudil (a main metabolite of fasudil) on oxygen-glucose deprivation (OGD)-induced PC12 cell death in vitro and on glutamate-induced neurotoxicity in primary cerebral neuronal culture. In addition, they measured neither eNOS protein expression nor iNOS expression and activities in their studies as we did in our study. In fact, data derived from their study and ours have no overlaps and can serve to support each other’s view regarding the neuroprotective roles of fasudil.
We observed that after 2 h MCAO and 24 h reperfusion, the rats developed marked neurological deficit along with brain infarction. The significant neuronal loss, nuclei condensation, and inflammation responses were also shown in frontal cortex. In our experiments, fasudil mesylate at 10 mg/kg significantly decreased cerebral infarct size and neurological deficit after focal cerebral ischemia and reperfusion injury, compared to the vehicle group or the group of ischemic rats without fasudil mesylate treatment (P < 0.01) (Fig. 1). As shown in Fig. 2, the histology pictures of the rat brains exhibited the attenuation of the neuronal loss and nuclei shrinkage after fasudil mesylate was given to the ischemic rats. Our study has demonstrated that fasudil mesylate improves neurological deficit and decreases infarct size in cerebral ischemia and reperfusion in vivo. These data provide further evidence supporting that fasudil has a potential for improving the impaired cognition in ischemic rats as we previously described (Huang et al. 2008a).
It was reported that increased eNOS activity by estrogen and corticosteroid leads to decreased leukocyte adherence after ischemia/reperfusion injury (Hafezi-Moghadam et al. 2002; Simoncini et al. 2000). NO produced by eNOS may have a protective physiological role by orchestrating the paracrine homeostatic functions of the endothelium. These functions include inhibition of leukocyte and platelet adhesion, control of vascular tone, and maintenance of a thromboresistant interface between the bloodstream and the vessel wall (Vaughan and Delanty 1999). Our present study showed a substantial decrease in eNOS immunoreactivity in ischemic brain tissues after 2 h MCAO and 24 h reperfusion. Fasudil mesylate at 10 mg/kg significantly increased eNOS immunoreactivity (Fig. 3). Moreover, Western-blot analysis showed that the decreased eNOS protein expression by ischemia and reperfusion injury was up-regulated via fasudil mesylate administration (P < 0.05) (Fig. 4b). The augmentation or preservation of eNOS activity by fasudil mesylate treatment, therefore, could lead to beneficial anti-inflammatory effects in the vascular wall. These effects of fasudil mesylate may be achieved by a decrease in vascular tone, enhancement of cerebral blood flow and inhibition of leukocyte-mediated damage through up-regulating eNOS expression.
Inflammatory immune reaction is one of the most relevant processes in the pathogenesis of cerebral ischemia, especially in the period of reperfusion (Samdani et al. 1997). Induction of iNOS results in delayed neuronal cell death (Dawson et al. 1994) but also exacerbates glutamate excitotoxicity (Hewett et al. 1994). Our immunohistochemical study showed a substantial increase in iNOS immunoreactivity in ischemic brain tissues after 2 h MCAO and 24 h reperfusion in vivo injury. Fasudil mesylate significantly decreased iNOS immunoreactivity (Fig. 3) and iNOS protein expression (P < 0.05) (Fig. 4b). This result indicated that the in vivo neuroprotective effect of fasudil mesylate may be mediated, at least in part, by inhibition of inflammatory immune reaction injury through down-regulating iNOS activity.
In our study, the incubation of fasudil mesylate at 10 and 100 μmol/L with the cortical and hippocampal slices after OGD insult increases OD490 value of TTC staining compared to OGD slices without fasudil mesylate (P < 0.05) (Fig. 5A). LDH efflux is also markedly decreased in both cortical and hippocampal slices at the end of cultures in the presence of fasudil mesylate (P < 0.05) (Fig. 5B). Fluorescent probes labeled living cells and living tissue sections have been used widely in biomedical research (Pellmar 1995). Rat cortical and hippocampal slices labeled by fluorescent probe PI showed clear red fluorescent images under the LCSM. Because PI enters the damaged cells exclusively (Macklis and Madison 1990), we could evaluate the injury degree of slices by PI fluorescent intensity. Fasudil mesylate could decrease PI fluorescent intensity significantly (P < 0.05) (Fig. 5C), which was greatly augmented in damaged neuronal cells. These data provide direct evidence that fasudil mesylate reduces the neuronal cell damage in OGD slices in vitro.
There is evidence that iNOS can be induced in brain slices after OGD insult in vitro. Furthermore, this induction occurs in short period of time (2–3 h after ischemia), suggesting that NO derived from iNOS could play an important pathogenic role in cell damage that occurs in early stages of cerebral ischemia (Huang et al. 2008b; Pellmar 1995). Our study showed that fasudil mesylate, in a dose-dependent manner, could reduce the activity of iNOS in OGD injured rat brain slices (P < 0.05) (Fig. 6a).
We also examined antioxidant SOD, which serves as oxidative indices in brain ischemia. A decrease in the levels of SOD was noted in “ischemic” brain slices, indicating the participation of superoxide radical known to produce highly toxic hydroxyl radical through its reaction with H2O2 (Haber–Weiss reaction) (Lipski et al. 2007; Mukherjee et al. 2007). In this study, fasudil mesylate was found to elevate SOD activity in brain slices with OGD-induced injury (P < 0.05) (Fig. 6b). Therefore, the effect of fasudil mesylate on oxidative stress may also play a role in its neuroprotection.
In summary, fasudil mesylate decreased cerebral infarct size and neurological deficit, which may be related to its ability to restore eNOS activity and to inhibit iNOS activity in ischemic regions of rat cortex after 2 h MCAO and 24 h reperfusion in vivo. Fasudil mesylate also presents with the neuroprotective effect on neuronal cell damage in vitro and such an effect is possibly mediated by inhibition of iNOS activity and oxidative stress. The results from this laboratory suggest that the emerging of fasudil mesylate as a treatment in acute ischemia phase may provide a useful therapeutic strategy for ischemia stroke. Our preliminary data merit further studies to elucidate the underlying mechanisms of action for fasudil mesylate in ischemia.
References
Cohen MM, Pettegrew JW, Kopp SJ, Minshew N, Glonek T (1984) P-31 nuclear magnetic resonance analysis of brain: normoxic and anoxic brain slices. Neurochem Res 9:785–801. doi:10.1007/BF00965666
Davalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J (1999) Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke 30:2631–2636
Dawson VL, Brahmbhatt HP, Mong JA, Dawson TM (1994) Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal-glial cortical cultures. Neuropharmacology 33:1425–1430. doi:10.1016/0028-3908(94)90045-0
De AJ, Cardenas A, Moro MA, Leza JC, Lorenzo P, Lizasoain I (1999) Use of brain slices in the study of pathogenic role of inducible nitric oxide synthase in cerebral ischemia-reperfusion. Gen Pharmacol 32:577–581. doi:10.1016/S0306-3623(98)00280-8
Dobashi K, Araki S, Kubo K, Kawagoe R, Yamamoto Y, Shirahata A (2008) Hydroxymethylglutaryl-CoA reductase inhibitor inhibits induction of nitric oxide synthase in 3T3-L1 preadipocytes. Life Sci 82:85–90. doi:10.1016/j.lfs.2007.10.013
Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC et al (2002) Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med 8:473–479. doi:10.1038/nm0502-473
Hewett SJ, Csernansky CA, Choi DW (1994) Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron 13:487–494. doi:10.1016/0896-6273(94)90362-X
Huang L, He Z, Guo L, Wang H (2008a) Improvement of cognitive deficit and neuronal damage in rats with chronic cerebral ischemia via relative long-term inhibition of rho-kinase. Cell Mol Neurobiol 28:757–768. doi:10.1007/s10571-007-9157-x
Huang X, Li Q, Zhang Y, Lu Q, Guo L, Huang L et al (2008b) Neuroprotective effects of cactus polysaccharide on oxygen and glucose deprivation induced damage in rat brain slices. Cell Mol Neurobiol 28:559–568. doi:10.1007/s10571-007-9184-7
Johansson R, Persson K (2004) Phenotypic modulation of cultured bladder smooth muscle cells and the expression of inducible nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 286:R642–R648. doi:10.1152/ajpregu.00443.2003
Kadowaki S, Chikumi H, Yamamoto H, Yoneda K, Yamasaki A, Sato K et al (2004) Down-regulation of inducible nitric oxide synthase by lysophosphatidic acid in human respiratory epithelial cells. Mol Cell Biochem 262:51–59. doi:10.1023/B:MCBI.0000038215.89821.7f
Li Q, Huang L, Liu D, Wang W, Chen W, Guo L (2007) Effects of Ethanesulfonic fasudil on cerebral vasospasm in dogs. Chin J Hosp Pharm 27:746–749
Liao Y, Wang R, Tang XC (2004) Centrophenoxine improves chronic cerebral ischemia induced cognitive deficit and neuronal degeneration in rats. Acta Pharmacol Sin 25:1590–1596
Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D (2007) Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience 146:617–629. doi:10.1016/j.neuroscience.2007.02.003
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Macklis JD, Madison RD (1990) Progressive incorporation of propidium iodide in cultured mouse neurons correlates with declining electrophysiological status: a fluorescence scale of membrane integrity. J Neurosci Methods 31:43–46. doi:10.1016/0165-0270(90)90007-3
Mukherjee PK, Ahamed KF, Kumar V, Mukherjee K, Houghton PJ (2007) Protective effect of biflavones from Araucaria bidwillii Hook in rat cerebral ischemia/reperfusion induced oxidative stress. Behav Brain Res 178:221–228. doi:10.1016/j.bbr.2006.12.025
O’Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE (2004) Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab 24:1046–1056. doi:10.1097/01.WCB.0000130867.32663.90
Pellmar TC (1995) Use of brain slices in the study of free-radical actions. J Neurosci Methods 59:93–98. doi:10.1016/0165-0270(94)00198-P
Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T et al (2005) Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 36:2251–2257. doi:10.1161/01.STR.0000181077.84981.11
Samdani AF, Dawson TM, Dawson VL (1997) Nitric oxide synthase in models of focal ischemia. Stroke 28:1283–1288
Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK (2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538–541. doi:10.1038/35035131
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293
Vaughan CJ, Delanty N (1999) Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke 30:1969–1973
Yamashita K, Kotani Y, Nakajima Y, Shimazawa M, Yoshimura S, Nakashima S et al (2007) Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res 1154:215–224. doi:10.1016/j.brainres.2007.04.013
Acknowledgments
This research was supported by the National Foundation of Nature and Science of China (No. 30772559).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xian-Ju Huang contributed equally to this article.
Rights and permissions
About this article
Cite this article
Li, Q., Huang, XJ., He, W. et al. Neuroprotective Potential of Fasudil Mesylate in Brain Ischemia-Reperfusion Injury of Rats. Cell Mol Neurobiol 29, 169–180 (2009). https://doi.org/10.1007/s10571-008-9308-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-008-9308-8