Abstract
1. The neuroprotective effect of cactus polysaccharide (CP) on oxygen and glucose deprivation (OGD) and reoxygenation (REO)-induced damage in the cortical and hippocampal slices of rat brain was investigated. 2. Cell viability was evaluated by using the 2, 3, 5-triphenyl tetrazolium chloride (TTC) method. The fluorescence of propidium iodide (PI) staining was used for quantification of cellular survival, and lactate dehydrogenase (LDH) activity in incubation medium was assessed by LDH assay to evaluate the degree of injury. 3. The OGD ischemic condition significantly decreased cellular viability and increased LDH release in the incubation medium. CP (0.2 mg/l∼2 mg/l) protected brain slices from OGD injury in a dosage dependent manner as demonstrated by increased A 490 value of TTC, decreased PI intensity and LDH release. At the above concentration, CP also prevented the increase of nitric oxide (NO) content and inducible nitric oxide synthase (iNOS) activity induced by OGD. 4. CP can protect the brain slices (cortical and hippocampus) against injury induced by OGD. Its neuroprotective effect may be partly mediated by the NO/iNOS system induced by OGD insult.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a common cause of death in many countries and especially in China. One-third of patients with acute ischemic stroke develop early neurological deterioration, a situation associated with increased mortality and long-term functional disability (Castillo et al. 1997; Dávalos et al. 1999).
A great deal of effort has been made to discover effective drugs that reduce neuronal damage insult. Opuntia dillenii Haw has been used clinically as a traditional medicine in China since Qing dynasty and Cactus polysaccharide (CP) is one of active components in Opuntia dillenii Haw. It exists in all types of cactus. The CP used in this study was extracted from Opuntia Milpa Alta cultivated in China. Opuntia Milpa Alta was introduced from Milpa region in Mexico by Ministry of Culture of China in 1997. It is an excellent breed selected for food via adaptable cultivating and breed screening. Its tender leaf can be used as both vegetable and medicine.
Pharmacologic studies have shown that CP has activities of anti-oxidation, anti-inflammatory, anti-tumor, anti-aging, and enhancing immune system (Chen et al. 1991, 1997; Ji et al. 2004; Yan and Liu 2003; Wang and Huang 2006). We have found that CP can protect rat brain slices from oxidative stress injury induced by H2O2 insult (Huang et al. 2007). The aim of the present study was to evaluate the effect of CP in rat brain slices (cortical and hippocampus) subjected to oxygen/glucose deprivation and reperfusion. Neuroprotective effects of CP were then examined by TTC and PI staining and LDH activity assay. Finally, we measured the release of nitric oxide (NO) and activity of inducible nitric oxide synthase (iNOS) from the brain slices in the presence of CP to further explore its mechanism of action.
Methods
Animals and Reagents
Male Sprague–Dawley rats, 200∼300 g, were obtained from the Laboratory Animal Center of Tongji Medical college of Huazhong University of Science and Technology, Wuhan, China. Cactus polysaccharide, a dried spiny crystal, was extracted from Opuntia Milpa Alta by Chemistry and Environment Engineering School of Yangtze University, China (Yan and Liu 2003; Jin et al. 2000). The content of total sugar in the extract was detected as 42.9% by the method of Phenol-sulfuric acid (Jin et al. 2000). Filter paper chromatography detection includes glucose, galactose, arabinose, beechwood sugar and rhamnose. CP was dissolved in artificial cerebrospinal fluid (ACSF ). TTC and PI were purchased from Sigma (St Louis, Missouri, USA). They were dissolved in ACSF and dimethyl sulfoxide (DMSO), respectively and kept in the dark to avoid the light.
Preparation and Incubation of Brain Slices
Rats were decapitated and the whole brains rapidly removed and then placed in cold oxygenated ACSF (126 mM NaCl, 3.5 mM KCl, 1.2 mM NaH2PO4, 1.3 mM MgCl2, 2.0 mM CaCl2, 11 mM D-(+)-glucose, 25 mM NaHCO3, saturated with 95% O2 and 5% CO2 and a final pH 7.4). Brains were cut coronal into 0.4 mm thickness sections with a Mcllwain tissue chopper (The Mickle Laboratory Engineering Co. LTD, USA). Cortical and hippocampus slices were quickly isolated from the appropriate sections. The slices were maintained in oxygenated pre-cold ACSF for 60 min to rom sallow maximal recovery flicing trauma. Brain slices were transferred to 2 ml of incubation tubes. Each tube contained four isolated slices. The slices were incubated in a water bath at 35°C and bubbled with 95% O2 and 5% CO2 (Gong et al. 2001).
Oxygen–Glucose Deprivation
Oxygen–glucose deprivation (OGD) was used as an in vitro model of ischemia. For OGD, the slices were placed into 2 ml of ischemic medium which did not contain glucose but substituted with 10 mM cane sugar instead, gassed with 95% N2 and 5% CO2 for 15 min. Before use, OGD medium was saturated by flushing a gas mixture of 95% N2 and 5% CO2. After OGD, the slices were placed back in their original culture conditions and reoxygenated (REO) for 2 h. The rat cerebral cortical and hippocampus slices were divided into 5 groups. Group 1 was injury group in which the slices were treated with OGD for 15 min followed by 30 min of normal incubation and then treated with REO with ACSF for 2 h. Group 2, 3, and 4 were CP treatment groups including the slices incubated with 0.2 mg/l, 1 mg/l and 2 mg/l CP for 30 min prior to and during OGD insult, respectively. The group 5 was normal group in which the slices were incubated with ACSF for 165 min.
One ml of the incubation medium collected at the end of ischemia or REO was stored at −70°C for quantification of LDH released from the slices. The brain slices were collected at the end of the experiment and stored at −70°C for analysis of NO, iNOS and tissue protein preparation of the slices.
TTC Staining
After 2 h of REO the slices were co-incubated with TTC at 35°C in the dark for 30 min. Then they were taken out of and rinsed by normal saline (NS). The wet slices were weighed after the water on the surface was blotted. Extracted fluid (ethanol: DMSO = 1:1) was added with a proportion of 20 ml to 1 g of slice tissue. After 24 h of protection from light, formanzan, the red crystal product was extracted, and the degree of injury was evaluated by measuring the absorbance at 490 nm (A 490) using an ELISA reader (TECAN A-5082 (megllan), AUSTRIA). Brain injury rate was calculated as tissue injury = (1-A 490 nm injury/A 490 nm control) × 100% (Preston and Webster 2000).
PI Staining
PI has been used for quantification of cellular survival as it permeates exclusively into cell membrane intact cells (Xiang and Bergold 2000), therefore, it could be an indicator of neuronal cell damage. As previously reported (Wang et al. 2000), we have added PI to the culture medium for 30 min at final concentrations of 2 M at the end of brain slice culture. The slices were bubbled with 95% O2 and 5% CO2 constantly, then washed with ACSF (35°C) at least 3 times (3 min/time) and placed in ACSF at room temperature in the sample cell of LCSM (Leica, TCS-SP, German). Both transmission and fluorescence images were captured by LCSM under the following working conditions: excitation wavelength: 490 nm; emission wavelength: 650 nm; power: 200; scanning section thickness: 4. The neuronal damage within each slice was calculated by the average intensities of PI fluorescence.
LDH Assay
LDH activity in the incubation medium was assayed by using a commercial kit from Jiancheng- Bioeng Institute (China). Fifty μl of incubation medium was mixed with 0.7 ml of reaction medium in a temperature controlled cuvette (37°C). Changes in the absorbance were read at 440 nm after 30 s then every minute during 2 min. LDH activity was calculated according to average absorbance change and then corrected with protein levels of the slices. The definition of one unit of LDH activity is every 1 μmol pyroracemic acid per 1,000 ml of sample interacted with ground plasm per 15 min. The ultimate element of LDH activity was expressed as nmol/min/g protein.
NO and iNOS Assay
The brain slices were homogenized with NS (1:9) and centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was collected and splitted into two parts. One was subjected to nitrite analysis and the other subjected to iNOS assay. NO content and iNOS activity in the brain slices were determined by using commercial kits from Jiancheng- Bioeng Institute (China). NO formation was measured indirectly by qualification of nitrite and nitrate, the stable products of NO oxidation (Schulz et al. 1999). An assay based on the conversion of nitrate to nitrite by the enzyme nitrate reductase in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) was used in this study. Nitrate concentration was determined by using an aqueous solution of sodium nitrate and a standard curve. Absorbance was measured at 550 nm. There are mainly two types of NOS (cNOS and iNOS). The iNOS bind calmodulin tightly and their activity is essentially Ca2+-independent. L-Arg and dioxygen react by catalysis of NOS and form into NO. NO reacts with cryophilic material and their product can be detected at 530 nm.
Total Protein Assay
After homogenization of the slices, 50 μl of supernatant collected from the homogenate were applied for the measurement of tissue protein levels using a commercial kit from Jiancheng-Bioeng Institute (China). The protein levels were calculated by measuring at the wavelength of 595 nm. Protein standards (0.615 g/l) were processed in the same way as the tissue samples. All experimental results were normalized according to total protein levels of the slices.
Statistical Analysis
All data in the text are described as mean ± SEM. The differences between the results were tested by Tukey–Kramer multiple comparison test or Student’s t-test. A probability of P < 0.05 was considered significant.
Results
Effect of CP on TTC Staining of OGD Injured Brain Slices
Different concentrations of CP (0.2 mg/l, 1 mg/l and 2 mg/l) were co-incubated with cerebral cortical and hippocampus slices of rat brain respectively for 3 h. They had very little effect on TTC staining of the brain slices. No significant differences were observed in A 490 values of TTC between any of the two groups (P > 0.05).
As shown in Fig. 1, after being exposed to OGD insult for 15 min followed by 2 h of post-incubation, the activities of the brain slices were reduced significantly with injury rate of cortical slices being 36.36 ± 4.16 % and that of hippocampus slices being 52.38 ± 6.73 %. There was a statistically significant difference between the OGD and control group (P < 0.01). CP can prevent the decreases in tissue activity induced by OGD insult in a dose dependent manner. Among the three doses used in the study, 2 mg/l of CP had the greatest neuroprotective effect on brain slices.
Effect of cactus polysaccharide(0.2∼2 mg/l) on TTC staining of OGD injured rat cerebral cortical and hippocampus slices (n = 10). TTC assay was performed in brain slices submitted to OGD and REO (Injury groups). Results are determined as percentage of the non-OGD control group. The control was made by co-incubation with ACSF for 165 min. The Injury was made by OGD15 min after 30 min of normal incubation, and then the slices were re-incubated with normal ACSF for 2 h. cactus polysaccharide 0.2 mg/l, 1 mg/l and 2 mg/l were added at the beginning of the normal incubation for 30 min and during insult. TTC: 2, 3, 5-triphenyl tetrazolium chloride; \( \ifmmode\expandafter\bar\else\expandafter\=\fi{x} \) ± s, *P < 0.05, **P < 0.01, compared with Injury. □ Cortical slices; ■ Hippocampus slices
Effect of CP on PI Fluorescence of OGD Injured Brain Slices
Rat brain slices showed strong red fluorescence after PI staining under LCSM. As shown in Fig. 2, after OGD insults, the average intensities of PI fluorescence were 323.89 ± 35.69 for hippocampus slices and 189.76 ± 20.06 for cortical slices, respectively. They were significantly increased as compared to control groups (P < 0.01) in both hippocampus and cortical slices. CP at 1 mg/l and 2 mg/l could significantly inhibit the increase of PI staining of OGD injured brain slices.
Effect of cactus polysaccharide (1∼2 mg/l) on PI staining of OGD-induced injury brain slices (n = 5). (A): Control; (B): Injury; (C): CP 1 mg/l; (D): CP 2 mg/l of Hippocampus slices; (E): Control; (F): Injury; (G): CP 1 mg/l; (H): CP 2 mg/l of Cortical slices. The average intensities of PI fluorescence were captured by using LCSM. The control was made by co-incubation with ACSF for 165 min. The Injury was made by OGD15 min after 30 min of normal incubation, then the slices were re-incubated with normal ACSF for 2 h. Cactus polysaccharide 1 mg/l and 2 mg/l were added at the beginning of the normal incubation for 30 min and during insult. PI: propidium iodide; \( \ifmmode\expandafter\bar\else\expandafter\=\fi{x} \) ± s, *P < 0.05, **P < 0.01, compared with Injury. □ Cortical slices; ■ Hippocampus slices
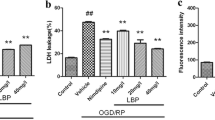
Effect of CP on LDH Release of OGD Injured Brain Slices
The release of LDH increased slightly after 15 min of OGD, but there is no statistical significance when compared to control (P > 0.05). However, it was significantly increased after 2 h of REO compared to control (P < 0.01). CP (0.2 mg/l, 1 mg/l and 2 mg/l) used in the study could decrease LDH release during REO of injured brain slices in comparison with that of the same samples without using CP (P < 0.05) (Fig. 3).
Effect of cactus polysaccharide (0.2∼2 mg/l) on LDH release of OGD injured rat cerebral cortical and hippocampus slices (n = 10). LDH assay was performed in incubation medium submitted to OGD and REO (Injury groups). The control was made by co-incubation with ACSF for 165 min. The Injury was made by OGD for 15 min after 30 min of normal incubation, and then the slices were re-incubated with normal ACSF for 2 h. Cactus polysaccharide 0.2 mg/l, 1 mg/l and 2 mg/l were added at the beginning of the normal incubation for 30 min and during insult. LDH: lactate dehydrogenase; \( \ifmmode\expandafter\bar\else\expandafter\=\fi{x} \) ± s, *P < 0.05, ** P < 0.01, compared with Injury. □ Cortical slices; ■ Hippocampus slices
Effect of CP on NO/iNOS System of OGD Injured Brain Slices
Figure 4 shows that NO contents and iNOS activities increased significantly in rat cortical and hippocampus slices after OGD-induced injury, compared with control, P < 0.01. However, CP (0.2 mg/l, 1 mg/l and 2 mg/l) could decrease the releases of NO and the activities of iNOS of OGD injured brain slices in a dose dependent manner as compared with injury group, P < 0.05.
Effect of cactus polysaccharide (0.2∼2 mg/l) on NO content and iNOS activity of OGD injured brain slices (n = 10). NO and iNOS assay was performed in incubation medium submitted to OGD and REO (Injury groups). The control was made by co-incubation with ACSF for 165 min. The Injury was made by OGD for 15 min after 30 min of normal incubation, and then the slices were re-incubated with normal ACSF for 2 h. Catcus polysaccharide 0.2 mg/l, 1 mg/l and 2 mg/l were added at the beginning of the normal incubation for 30 min and during OGD insult. NO: nitric oxide; iNOS: inducible nitric oxide synthase; \( \ifmmode\expandafter\bar\else\expandafter\=\fi{x} \) ± s, **P<0.05, **P<0.01, compared with Injury
Discussion
Several methods have been used for determination of OGD and/or REO induced tissue damage in in vitro studies. In our study, ischemia decreased the A 490 values of TTC staining and PI fluorescence as well as increased the release of LDH. It should be noted that OGD ischemia and REO caused a greater repression of TTC staining and PI fluorescence in hippocampus slices than that in cortical slices. Similarly, basal or REO-induced LDH leakage was also higher in hippocampus slices than in cortical slices (P < 0.05), which indicates that hippocampus slices are more vulnerable to OGD insult.
Different dosages of CP (0.2 mg/l, 1 mg/l and 2 mg/l) did not affect the cellular viability of normal slices if co-incubated with brain slices. On the other hand, incubation of rat brain slices with CP at various concentrations (0.2 mg/l, 1 mg/l and 2 mg/l) before and during OGD periods significantly increased cellular viability, as assessed by a decline in TTC activity and PI fluorescence and decreased LDH release during REO of injury brain slices in a concentration-dependent manner. Taken together, these results indicate a neuroprotective effect of the extract.
The neuroprotective effects of CP (0.2–2 mg/l) are significant but relatively minor. One has to bear in mind that the extract is still a complex mixture of compounds, of which only one or a few may be relevant for the observed activity. Therefore, effective doses of active compound(s) can be expected to be lower than those of the extract here studied.
There is evidence that NO is involved in the mechanisms of neurotoxicity after cerebral ischemia. NO production is enhanced at all stages of cerebral ischemia and this increase is accompanied by up regulation of both nitric oxide synthase (NOS) activity and NOS gene expression (Iadecola 1997). While NO has been reported to be toxic to cells through several different mechanisms (Xie and Nathan 1994; Dawson et al. 1993; Huang et al. 1994; Buisson et al. 1992.), we here demonstrate that NO triggered by iNOS induction may contribute to an enhancement of OGD-induced neuronal death. The iNOS can be induced in brain slices after OGD insult in vitro. Furthermore, this inducement occurs in short period of time (2–3 h after ischemia), suggesting that NO can play an important pathogenic role in cell damage that occurs in early stages of cerebral ischemia (Moro et al. 1998; De Alba et al. 1999).
NO neurotoxicity is likely mediated by its free radical character, which makes NO react with certain proteins containing heme-iron prosthetic groups, iron-sulfur clusters, or reactive thiols. In addition to directly reacting with protein prosthetic groups, NO also reacts readily with superoxide (O −2 ) to produce peroxynitrite (ONOO−), which may mediate much of the NO neurotoxicity (Karen et al. 1997). Our studies previously showed that CP in a dose dependent manner can decrease the delivery of NO and the activity of iNOS in OGD injured rat brain slices. Moreover, we have found that CP can protect rat cerebral cortical and hippocampus slices from injury induced by H2O2 (Huang et al. 2007), which were consistent with the protective effect of CP which may be related to its antioxidant properties.
It has been reported that CP has anti-oxidation and anti-inflammatory activity (Chen et al. 1991, 1997; Ji et al. 2004; Yan et al. 2003; Wang and Huang 2006). There is substantial evidence that inflammation and oxidation contributes to secondary brain injury after ischemia and reperfusion, and that pharmacological anti-inflammatory and anti-oxidation treatments are beneficial in ischemia models. Hence, we cannot exclude the possibility that the neuroprotection of CP against ischemia-induced neuronal damage may partially depend on its anti-inflammatory and anti-oxidation activity.
Despite the incomplete understanding of molecular mechanisms involved with the proposed neuroprotective action of CP, we suggest that its inhibition of NO/iNOS system plays a significant role in reducing OGD-induced brain slices damage. The detailed mechanism by which CP decreased NO/iNOS system remains a subject for further investigation.
This study offers evidence of, and a possible explanation for, neuroprotective properties of CP. It is clear that additional work is required before a comprehensive knowledge of CP neuroprotection potential and mechanisms of action can be achieved. Other in vitro models such as neuron culture as well as other in vivo ischemia models (currently being implemented in our laboratory) should be used to confirm and better characterize CP neuroprotective properties.
Nevertheless, effects of CP that were reported here are considered new compared with its traditional usage. Considering the activities already identified and the traditional use of this medicinal species, data presented in this report may be valuable to the development of this plant-based drug.
References
Buisson A, Plotkine M, Boulu RG (1992) The neuroprotective effect of nitric oxide inhibitors in a rat focal model of cerebral ischaemia. Br J Pharmacol 106:766–767
Castillo J, Dávalos A, Noya M (1997) Progression of ischemic stroke and excitotoxic amino acids. Lancet 349:79–83
Chen S, Meng H (1997) Study on anti-lipid perioxidation of Opuntia dillemii Haw. Pharmacol Clin Chin Materia Medica 13(3):36–37
Chen S, Tang Y, Meng H (1991) Study on anti-inflammatory effect of Opuntia dillemii Haw. Pharmacol Clin Chin Materia Medica 7(6):33
Dávalos A, Toni D, Iweins F, Lesaffre E, Bastanello S, Castillo J (1999) Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I. Stroke 30:2631–2636
Dawson VL, Dawson TM, Bartley DA, Uhl GR, Snyder SH (1993) Mechanisms of nitric oxide mediated neurotoxicity in primary brain cultures. J Neurosci 13:261–2661
De Alba J, Cárdenas A, Moro MA, Leza JC, Lorenzo P, Lizasoain I (1999) Use of brain slices in the study of pathogenic role of inducible nitric oxide synthase in cerebral ischemia-reperfusion. Gen Pharmacol 32:577–581
Gong CX, Lidsky T, Wegiel J et al (2001) Metabolically active rat brain slices as a model to study the regulation of protein phosphorylation in mammalian brain. Br Res Protocol 6:134–140
Huang X-J, Guo L-J, Qu L, Lü Q, Xu X-L (2007) Protection of cactus polysaccharides against oxidative stress injury of rat brain slices. Chin J Pharmacol Toxicol 21(1):1–6
Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA (1994) Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 265:1883–1885
Iadecola C (1997) Bright and dark sides of nitric oxide in ischemic injury. Trends Neurosci 20:132–139
Ji C-F, Zhou X, Gao S-Y, Ji Y-B (2004). Study on anti-tumor effect of three kinds of cactus polysaccharide. J Harbin Univ Comm Nat Sci Ed 20(2):127–130
Jin D-H, Ji Y-H, Cui Y-H, Li S-H (2000) Extraction and assaying of crude Polysaccharides from Cactus Stem. Lishizhen Med Materia Medica Res 11(3):199–200
Karen SC, David SB (1997) Nitric oxide in excitable tissues: physiological roles and disease. Christopherson Bredt 100(10):2424–2429
Moro MA, De Alba J, Leza JC, Lorenzo P, Fernández AP, Bentura ML, Boscá L, Rodrigo J, Lizasoain I (1998) Neuronal expression of inducible nitric oxide synthase after oxygen and glucose depression in rat forebrain slices. Eur J Neurosci 10:445–446
Preston E, Webster J (2000) Spectrophotometric measurement of experimental brain injury. J Neurosci Methods 94(2):187–192
Schulz K, Kerber S, Kelm M (1999) Reevaluation of the Griess method for determining NO/NO2-in aqueous and protein-containing samples. Nitric Oxide 3(3):225–234
Wang S-F, Huang J (2006) Development of research on opuntia polysaccharides. Chin J Biochem Pharm 27(3):186–188
Wang X, Xing H, He Q, Xu J, Wu B (2000) Using laser confocal scanning microscope to study ischemia-hypoxia injury in rat brain slice. Chin Sci Bull 45(1):49–51
Xiang Z, Bergold PJ (2000) Synaptic depression and neuronal loss in transiently acidic hippocampal slice cultures. Brain Res 881(1):77–87
Xie Q-W, Nathan C (1994) The high-output nitric oxide pathway: role and regulation. J Leukoc Biol 56:576–582
Yan Y-G, Li C-Y, Cui M-Z, Dong F-L, Sun W-G (2003) Effect of cactus powder on blood sugar in diabetic rats. J Harbin Med Univ 37(1):23–24
Yan Z-K, Liu C-Y (2003) Extraction technology of polysaccharides from Opuntia Milpa Alta. J Hubei Agric Coll 23(4):277–280
Author information
Authors and Affiliations
Corresponding author
Additional information
Xianju Huang and Qin Li have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Huang, X., Li, Q., Zhang, Y. et al. Neuroprotective Effects of Cactus Polysaccharide on Oxygen and Glucose Deprivation Induced Damage in Rat Brain Slices. Cell Mol Neurobiol 28, 559–568 (2008). https://doi.org/10.1007/s10571-007-9184-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9184-7