Abstract
Hypoxia and post-hypoxic reoxygenation induces disruption of the blood–brain barrier (BBB). Alterations of the BBB function after hypoxia/reoxygenation (H/R) injury remain unclear. Cyclosporin A (CsA), a potent immunosuppressant, induces neurotoxic effects by entering the brain, although the transport of CsA across the BBB is restricted by P-glycoprotein (P-gp), a multidrug efflux pump, and tight junctions of the brain capillary endothelial cells. The aim of this study was to evaluate whether the BBB after H/R damage is vulnerable to CsA-induced BBB dysfunction. We attempted to establish a pathophysiological BBB model with immortalized mouse brain capillary endothelial (MBEC4) cells. The effects of CsA on permeability and P-gp activity of the MBEC4 cells were then examined. Exposure to hypoxia for 4 h and reoxygenation for 1 h (H/R (4 h/1 h)) produced a significant decrease in P-gp function of MBEC4 cells, without changing cell viability and permeability for sodium fluorescein and Evan’s blue-albumin at 7 days after H/R (4 h/1 h). CsA-induced hyperpermeability and P-gp dysfunction in MBEC4 monolayers at 7 days after H/R (4 h/1 h) were exacerbated. The possibility that CsA penetrates the BBB with incomplete functions in the vicinity of cerebral infarcts to induce neurotoxicity has to be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclosporin A (CsA) is widely used in organ transplantation. The immunosuppressant action of CsA is very effective, but CsA induces adverse neurologic effects in many patients (Gijitenbeek et al. 1999; Serkova et al. 2004). CsA neurotoxicity, including tremor, seizure, cortical blindness, and encephalopathy, is frequent in patients with high blood drug levels, although within the therapeutic range. CsA appears to produce convulsions by inhibiting γ-aminobutyric acid (GABA)-ergic neural activity, and the binding properties of the GABAA receptor (Shuto et al. 1999). Inhibition of GABAergic neurotransmission by CsA may lead to the activation of serotonergic neural activity, and consequently produce tremors (Shuto et al. 1998). The transport of CsA across the blood–brain barrier (BBB) is restricted by P-glycoprotein (P-gp), a multidrug efflux pump, and the tight junctions of the brain capillary endothelial cells. We previously reported that CsA inhibits the function and expression of P-gp, and increases brain endothelial permeability (Dohgu et al. 2000, 2004; Kochi et al. 1999; Takata et al. 2007). While this could be the mechanism by which CsA produce encephalopathy, it remains unclear what determines vulnerability to CsA neurotoxicity among different groups of patients.
Changes in BBB function have been described in several disorders, including hypertension, stroke, human immunodeficiency virus encephalitis, Alzheimer’s disease, multiple sclerosis, and virus infection (Hawkins and Davis 2005; Huber et al. 2001). There is clinical evidence suggesting that CsA neurotoxicity is associated with hypocholesterolemia (de Groen et al. 1987), hypomagnesia (Thompson et al. 1984), advanced liver failure, and hypertension (Erer et al. 1996). CsA-induced pathological changes observed using magnetic resonance imaging appear to be similar to hypoxic injury-related signs (Jansen et al. 1996). Patients with CsA neurotoxicity show abnormal images such as cerebral infarcts on computed tomography scanning (Hauben 1996).
In in vivo models, cerebral ischemia and reperfusion markedly increase permeability of the brain microvasculature, leading to the development of vasogenic edema (Dimitrijevic et al. 2007; Lenzser et al. 2005). Post-ischemic reperfusion in vivo is a significant determinant of pathological events. In in vitro BBB models, hypoxia and/or post-hypoxic reoxygenation stresses also produce hyperpermeability of brain endothelial cells (Nishioku et al. 2007; Utepbergenov et al. 1998, Hayashi et al. 2004). Post-hypoxic reoxygenation is considered to have two phases (acute and chronic). Hypoxia-increased BBB permeability in vitro is slightly reduced in the acute phase (2 h) of post-hypoxic reoxygenation (Mark and Davis 2002). BBB functional alterations in the chronic phase of post-hypoxic reoxygenation have not been fully evaluated. Among patients with ischemic infarction, technetium-labeled diethylenetriamine penta-acetic acid can penetrate the BBB (Gumerlock 1989), suggesting that patients previously suffering from stroke or transient ischemic attack show partially damaged function of the BBB. Recently, we demonstrated that CsA aggravates electric shock-induced convulsions in mice with cerebral infarction (Yamauchi et al. 2005). Considering these results together, we hypothesized that CsA transport into the brain might be increased due to the incomplete recovery of the BBB from ischemia and post-ischemic injury.
In the present study, we attempted to establish a pathophysiological BBB model in the chronic stage of post-hypoxic reoxygenation using immortalized mouse brain capillary endothelial (MBEC4) cells, and then to investigate the effects of CsA on the BBB after hypoxia and reoxygenation (H/R) treatment.
Materials and Methods
Cell Culture
MBEC4 cells, which were isolated from BALB/c mouse brain cortex and immortalized by SV40 transformation (Tatsuta et al. 1992), were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, with a humidified atmosphere of 5% CO2/95% air. They were seeded on collagen-coated 12-well Transwell inserts (3.0 μm pore size; Corning, Acton, MA, USA), and 24-well culture plates (Corning) at a density of 42,000 and 21,000 cells/cm2, respectively. The cultures were used for experiments after they reached confluence (3 days).
Establishment of a Pathophysiological BBB Model After H/R Treatment
To induce hypoxic conditions, confluent monolayers of MBEC4 cells were washed three times with PBS. They were exposed to hypoxia and aglycemia by adding d-glucose-free Krebs–Ringer buffer (143 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1.2 mM MgCl2, 1.0 mM NaH2PO4, 10 mM HEPES, and 11 mM sucrose, pH 7.4) in a humidified atmosphere of 5% CO2/94% N2/1% O2 at 37°C, using a multi-gas incubator chamber (APM-30D; Astec, Fukuoka, Japan). d-Glucose-free Krebs–Ringer buffer was bubbled with argon for 20 min to remove dissolved oxygen in the buffer prior to use. The atmospheric oxygen reached 1% in the incubator chamber at 1 h after the onset of nitrogen injection. We defined this hypoxia + aglycemia treatment as ‘hypoxic conditions’. For controls, cultures were exposed to normoxic conditions by adding normal Krebs–Ringer buffer (143 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1.2 mM MgCl2, 1.0 mM NaH2PO4, 10 mM HEPES, and 11 mM d-glucose, pH 7.4) in a humidified atmosphere of 5% CO2/95% air at 37°C, for the same length of time as for hypoxic conditions. At the end of the hypoxia period, buffer was removed and pO2 levels were measured with the IRMA blood gas analyzer (Diametrics, St. Paul, MN, USA). Reoxygenation was initiated by adding serum-free DMEM. The cells were incubated in a humidified atmosphere of 5% CO2/95% air at 37°C for 1–24 h. After H/R treatment, cells were cultured in DMEM containing 10% FBS to recover MBEC4 monolayers from induced damage. They were incubated in a humidified atmosphere of 5% CO2/95% air at 37°C for 1–7 days, and culture medium was replaced every other day. We defined this treatment as ‘recovery conditions’.
When the effects of CsA on the function of MBEC4 cells were examined, CsA (kindly supplied by Novartis Pharma, Basel, Switzerland) was first dissolved in ethanol, and diluted with serum-free DMEM (0.1% final ethanol concentration). MBEC4 cells were washed three times with serum-free DMEM, and then exposed to CsA for 12 h.
Transcellular Transport of Sodium Fluorescein (Na-F) and Evan’s Blue-albumin (EBA)
MBEC4 monolayer permeability was evaluated using two permeability markers, Na-F (MW 376) and EBA (MW 67,000). To initiate the transport experiments, the medium was removed, and MBEC4 cells were washed three times with assay buffer (118 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1.2 mM MgCl2, 1.0 mM NaH2PO4, 25 mM NaHCO3, and 11 mM d-glucose, pH 7.4). This buffer (1.5 ml) was added to the outside of the insert (abluminal side). Assay buffer (0.5 ml) containing 100 μg/ml Na-F (Sigma, St. Louis, MO, USA) and 4% bovine serum albumin (Sigma) mixed with 0.67 mg/ml Evan’s blue dye (Sigma) was loaded on the luminal side of the insert. Samples (0.5 ml) were removed from the abluminal chamber at 30, 60, 90, and 120 min, and immediately replaced with fresh buffer. Aliquots (5 μl) of the abluminal medium were mixed with 200 μl assay buffer, and then the concentration of Na-F was determined with a CytoFluor Series 4000 fluorescence multiwell plate reader (PerSeptive Biosystems, Framingham, MA, USA) using a fluorescent filter pair [Ex(λ) 485 ± 10 nm; Em(λ) 530 ± 12.5 nm]. The EBA concentration in the abluminal chamber was measured by determining the absorbance of aliquots (150 μl) at 630 nm with a microplate reader (Opsys MR, DYNEX Technologies, Chantilly, VA, USA). The permeability coefficient and clearance were calculated according to the method described by Dehouck et al. (1992). Clearance was expressed as microliters of tracer diffusing from the luminal to abluminal chamber, and was calculated from the initial concentration of tracer in the luminal chamber and final concentration in the abluminal chamber: Clearance (μl) = [C]A × VA/[C]L, where [C]L is the initial luminal tracer concentration, [C]A is the abluminal tracer concentration, and VA is the volume of the abluminal chamber. During a 2-h period, the clearance volume increased linearly with time. The average volume cleared was plotted versus time, and the slope was estimated by linear regression analysis. The slope of clearance curves for the MBEC4 monolayer systems was denoted by PSapp, where PS is the permeability × surface area product (in μl/min). The slope of the clearance curve with a control membrane was denoted by PSmembrane. The real PS value for the MBEC4 monolayer system (PStrans) was calculated from 1/PSapp = 1/PSmembrane + 1/PStrans. The PStrans values were divided by the surface area of the Transwell inserts (1 cm2) to generate the permeability coefficient (Ptrans, in cm/min).
Functional Activity of P-gp
The functional activity of P-gp was determined by measuring cellular accumulation of rhodamine 123 (Sigma) according to the method of Fontaine et al. (1996). MBEC4 cells were washed three times with Krebs–Ringer buffer. MBEC4 cells were incubated with 0.5 ml Krebs–Ringer buffer containing 5 μM rhodamine 123 for 60 min. The solution was removed, and cells were washed three times with ice-cold PBS and solubilized in 1 M NaOH (0.2 ml). Aliquots (5 μl) of the cell solution were removed for measurement of cellular protein, using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The remaining solution was neutralized with 1 M HCl, and the rhodamine 123 content was determined with the CytoFluor Series 4000 using a fluorescent filter pair [Ex(λ) 485 ± 10 nm; Em(λ) 530 ± 12.5 nm].
Assessment of Cell Viability
The effect of hypoxia/reoxygenation injury on the viability of cells in the MBEC4 monolayer was assessed using a WST-8 assay (Cell Counting Kit-8; DOJINDO, Kumamoto, Japan). A highly water-soluble formazan dye (WST-8), reduced by mitochondrial dehydrogenase, was measured by determining the absorbance of each sample with a 450 nm test wavelength and a 700 nm reference wavelength, using a microplate reader (Opsys MR; DYNEX Technologies).
Statistical Analysis
Values are expressed as the mean ± SEM Statistical analysis was performed using Student’s t test to compare two groups. One and two way analysis of variance (ANOVA) followed by Tukey–Kramer’s test were used for multiple comparisons. The differences between means were considered to be significant at P < 0.05.
Results
Pathophysiological BBB Model with MBEC4 Cells Exposed to H/R (4 h/1–24 h)
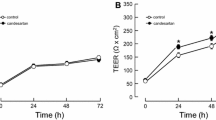
Reoxygenation (1, 3, 6, 12, and 24 h) after 4 h hypoxia significantly increased (6.8–18.6-fold) the permeability coefficients of MBEC4 cells for Na-F and EBA, compared with those for control normoxic conditions (data not shown). For the permeability to Na-F and EBA, two-way ANOVA showed significant effects for the factor condition (normoxia and hypoxia) [Na-F, F(1, 67) = 72.42, P < 0.001; EBA, F(1, 67) = 72.33, P < 0.001], but not reoxygenation time and interaction (condition × reoxygenation time). Then, a 1 h period of reoxygenation was employed in the following experiments. As shown in Fig. 1 (panel A inset), viability of MBEC4 cells was slightly decreased to 95% of the controls just after exposure to 4 h hypoxia followed by 1 h reoxygenation (H/R (4 h/1 h)) (day 0), and recovered to the control levels after 1 day. However, two-way ANOVA showed no significant effects for the factor condition (control and H/R), recovery time, and interaction (condition × recovery time). Just after exposure to normal Krebs–Ringer buffer and DMEM without FBS for 5 h (4 + 1 h) (day 0), the control MBEC4 monolayers showed about 6- to 8-fold increase in Na-F and EBA permeability, respectively, compared with those at 1–7 days after H/R (Fig. 1). These increased permeabilities recovered to normal levels after MBEC4 cells were incubated with DMEM containing 10% FBS for 1 day. The increased permeability coefficients of Na-F and EBA just after H/R treatment were gradually reduced to control levels within days after H/R treatment (Fig. 1). In the control MBEC4 monolayers, Na-F and EBA permeability reached the plateau phase much earlier than for MBEC4 monolayers with H/R-induced damage (Fig. 1). For Na-F and EBA permeability, two-way ANOVA showed significant effects for the factor condition [Na-F, F(1, 112) = 42.48, P < 0.0001; EBA, F(1, 112) = 53.04, P < 0.0001], recovery time [Na-F, F(4, 112) = 40.57, P < 0.0001; EBA, F(4, 112) = 47.71, P < 0.0001], and interaction [Na-F, F(4, 112) = 16.00, P < 0.0001; EBA, F(4, 112) = 22.14, P < 0.0001]. At 7 days after H/R (4 h/1 h), the permeability coefficients of Na-F in MBEC4 monolayers returned to control levels (1.77 ± 0.25 × 10−4 cm/min for control and 2.08 ± 0.22 × 10−4 cm/min for the H/R group in Fig. 1A). Similarly, those of EBA were recovered from H/R damage after 7 days (1.02 ± 0.19 × 10−4 cm/min for control and 1.19 ± 0.16 × 10−4 cm/min for H/R group in Fig. 1B). The recovery period of H/R-damaged MBEC4 cells was determined as 7 days.
Time course of Na-F (A) and EBA (B) permeability in MBEC4 monolayers after exposure to H/R (4 h/1 h). Permeability studies were performed at 0, 1, 3, 5, and 7 days after H/R (4 h/1 h). Values are means ± SEM (n = 11–15). *P < 0.05, **P < 0.01, significantly different from controls. The inset in panel A shows the time course of cell viability in MBEC4 monolayers after H/R (4 h/1 h). Results are expressed as percentage of cell viability in the control monolayer. Values are means ± SEM (n = 9–15)
Functional Activity of P-gp in MBEC4 Monolayers After H/R Treatment
Intracellular accumulation of rhodamine 123 was significantly increased by 21.9% in MBEC4 monolayers at 7 days after H/R (4 h/1 h) compared to controls (P < 0.01) (Fig. 2).
Rhodamine 123 accumulation in MBEC4 cells after H/R damage. P-gp function was evaluated at 7 days after H/R (4 h/1 h). Results are expressed as percentage of controls (0.67 ± 0.18 nmol/mg protein). Protein concentrations of the control and H/R-treated MBEC4 cells were 0.34 ± 0.02 mg and 0.30 ± 0.02 mg, respectively. Values are means ± SEM (n = 12). **P < 0.01, significantly different from control MBEC4 cells
Effects of CsA on BBB Function of MBEC4 Monolayers After H/R Treatment
At 7 days after control or H/R (4 h /1 h) treatment, MBEC4 monolayers were exposed to 5 μM CsA for 12 h. CsA significantly increased the permeability coefficients of Na-F and EBA to 107.8 ± 1.2% and 111.8 ± 1.5% of vehicle, respectively, in the control MBEC4 monolayers, and 114.4 ± 1.1% and 124.4 ± 1.5% of vehicle, respectively, in the recovered MBEC4 monolayers after H/R treatment (Fig. 3). In the H/R-treated MBEC4 monolayers, CsA-induced hyperpermeability of Na-F and EBA was exacerbated. For Na-F and EBA permeability, two-way ANOVA showed significant effects for the factor condition (control and H/R-treated cells) [Na-F, F(1, 43) = 5.87, P < 0.05; EBA, F(1, 43) = 18.69, P < 0.0001], treatment (vehicle and CsA) [Na-F, F(1, 43) = 65.63, P < 0.0001; EBA, F(1, 43) = 154.22, P < 0.0001], and interaction (condition × treatment) [Na-F, F(1, 43) = 5.87, P < 0.05; EBA, F(1, 43) = 18.69, P < 0.0001]. Tukey–Kramer post hoc tests indicated that CsA significantly increased permeability of the H/R-treated MBEC4 monolayers to Na-F (P < 0.01) and EBA (P < 0.01), compared with that of control monolayers.
Effects of CsA on Na-F (A) and EBA (B) permeability in MBEC4 monolayers after H/R damage. MBEC4 cells were cultured with normal medium for 7 days after H/R (4 h/1 h). Permeability studies were performed after exposure to CsA (5 μM) for 12 h. Permeability coefficients of Na-F in each vehicle-treated group were 1.91 ± 0.10 × 10−4 and 1.94 ± 0.11 × 10−4 cm/min for control and H/R-treated MBEC4 cells, respectively. Permeability coefficients of EBA for each corresponding vehicle-treated group were 0.50 ± 0.03 × 10−4 and 0.52 ± 0.04 × 10−4 cm/min for control and H/R-treated MBEC4 cells, respectively. Results are expressed as percentage of each corresponding vehicle-treated group. Values are means ± SEM (n = 11–12). **P < 0.01, significantly different from each corresponding vehicle group. (##P < 0.01; significant difference between control and H/R-treated MBEC4 cells, when exposed to CsA
As shown in Fig. 4, the cellular accumulation of rhodamine 123 was significantly increased in the H/R-treated MBEC4 monolayers after an exposure to CsA (2 and 5 μM) for 12 h. Two-way ANOVA showed significant effects for the factor condition (control and H/R-treated cells) [F(1, 88) = 17.98, P < 0.0001], treatment (vehicle, 2 and 5 μM of CsA) [F(2, 88) = 8.42, P < 0.001], and interaction [F(2, 88) = 4.50, P < 0.05]. Tukey–Kramer post hoc tests indicated that CsA at 2 (P < 0.01) and 5 μM (P < 0.01) significantly increased the intracellular accumulation of rhodamine 123 in the recovered MBEC4 monolayers after H/R treatment, compared with that in control monolayers.
Effect of CsA on rhodamine 123 accumulation in MBEC4 cells after H/R damage. MBEC4 cells were cultured with normal medium for 7 days after H/R (4 h/1 h). P-gp function was evaluated after exposure to CsA (2 and 5 μM) for 12 h. Results are expressed as percentage of each corresponding vehicle (control MBEC4 cells, 2.72 ± 0.38 nmol/mg protein and H/R-treated MBEC4 cells, 2.41 ± 0.34 nmol/mg protein). Protein concentrations of the control MBEC4 cells after treatment with vehicle, CsA 2 μM and 5 μM were 0.05 ± 0.01 mg, 0.07 ± 0.01 mg, and 0.07 ± 0.01 mg, respectively. Protein concentrations of the H/R treated MBEC4 cells after treatment with vehicle, CsA 2 μM and 5 μM were 0.07 ± 0.01 mg, 0.07 ± 0.01 mg, and 0.06 ± 0.01 mg, respectively. Values are means ± SEM (n = 14–16). *P < 0.05, **P < 0.01, significant difference from each corresponding vehicle. (##P < 0.01; significant difference between control and H/R-treated MBEC4 cells, when exposed to CsA (2 μM). ††P < 0.01; significant difference between control and H/R-treated MBEC4 cells when exposed to CsA (5 μM)
Discussion
The first aim of the present study was to establish a pathophysiological BBB model by exposing MBEC4 cells to three consecutive conditions: (1) hypoxia (glucose-free Krebs–Ringer buffer, 5% CO2/94% N2/1% O2); (2) reoxygenation (serum-free DMEM, 5% CO2/95% air); and (3) recovery (10% FBS/DMEM, 5% CO2/95% air). Hypoxic conditions here included glucose deprivation, which is known to accelerate hypoxic injury and BBB disruption (Abbruscato and Davis 1999; Brillault et al. 2002). The serum-free medium was employed for reoxygenation conditions to exclude the effects of serum during reoxygenation. The exposure times to hypoxia and reoxygenation were determined as 4 and 1 h, respectively, to produce damage and/or dysfunction of the MBEC4 cells. The damaged cells were then cultured for 7 days with the medium containing 10% FBS under normal conditions until cell viability and permeability of Na-F and EBA were recovered.
In vitro studies have demonstrated that hypoxia followed by reoxygenation increases endothelial cell permeability (Nishioku et al. 2007; Utepbergenov et al. 1998), while there is evidence that post-hypoxic reperfusion attenuates hypoxia-increased BBB permeability (Fleegal et al. 2005; Mark and Davis 2002). Transient and moderate brain ischemia increases BBB permeability during H/R, this phenomenon being recovered after ischemia (Dobbin et al. 1989). In the present study, H/R-induced hyperpermeability of MBEC4 cells to Na-F and EBA was gradually attenuated by culturing with normal medium, reaching control levels at 7 days after H/R (Fig. 1). The viability of MBEC4 cells with H/R damage was slightly reduced just after H/R and returned to control levels at 1 day after H/R (Fig. 1). These results suggest that H/R-induced hyperpermeability to Na-F and EBA is not due to cytotoxicity. This hyperpermeability may be interpreted as occurring due to alterations in the expression and localization of tight junction-associated proteins (Mark and Davis 2002), and/or increases in the albumin transport pathway (transcellular route) (Plateel et al. 1997).
We designed this study to determine whether endothelial cells after H/R damage work as a barrier, in a manner similar to the normal BBB, against infiltration of CsA which is transported from the brain to the blood by P-gp (Tsuji et al. 1993). At 7 days after H/R, accumulation of rhodamine 123, a P-gp substrate, in the recovered MBEC4 cells was significantly increased (Fig. 2). This result indicated that the function of P-gp in recovered endothelial cells after H/R damage is lowered, but paracellular (Na-F) and transcellular (EBA) permeability is not. P-gp expression in brain capillaries temporally disappears after focal ischemia (Samoto et al. 1994). Reactive oxygen species generated during reoxygenation may be attributed to this decreased P-gp expression (Wachtel et al. 2002; Wartenberg et al. 2001). Considering these results together, it is conceivable that the BBB after H/R damage allows CsA to enter the brain through a transcellular pathway.
We evaluated the effects of CsA on the permeability and P-gp function in H/R-treated MBEC4 monolayers. CsA increased Na-F and EBA permeability, with this effect in the H/R-treated monolayers being more apparent than in the control monolayers (Fig. 3). In addition, CsA markedly increased accumulation of rhodamine 123 in H/R-treated MBEC4 cells (Fig. 4). These results indicate that the BBB after H/R damage is more vulnerable to CsA than is the normal BBB. Our previous findings showed that CsA disrupts the BBB function by stimulating nitric oxide (NO) formation in brain endothelial cells and astrocytes, and by inhibiting transforming growth factor (TGF)-β1 in brain pericytes (Dohgu et al. 2004; Yamauchi et al. 2007; Takata et al. 2007). One possible mechanism by which CsA can induce NO production is by the inhibition of calcineurin due to CsA binding to cyclophilin, a cytosolic protein (Ikesue et al. 2000). Therefore, vulnerability of the BBB to CsA after H/R damage may be attributed to the sequential aggravating pathway: (1) lowered P-gp activity of the recovered brain endothelial cells after H/R damage; (2) increased intracellular CsA in the brain endothelial cells, astrocytes, and pericytes; (3) increased NO and decreased TGF-β1 production, and (4) aggravation of the lowered barrier function of the BBB.
In the present study, these data indicated that P-gp functional activity was decreased and CsA-induced BBB dysfunction aggravated in the BBB after H/R damage. Therefore, infiltration of CsA into the brain may be increased in patients, who have previously suffered from stroke or a transient ischemic attack. Lacunar infarction, which is caused by the occlusion of small cerebral arteries, is presumed to be clinically silent in many cases. Patients with lacunar stroke often have lesions in the white matter (Wardlaw et al. 2003), in which CsA frequently induces neuroimaging abnormalities (Gijitenbeek et al. 1999). Taken together with these findings, the present study suggests that clinically silent brain infarcts may be included in the risk factors for CsA neurotoxicity.
References
Abbruscato TJ, Davis TP (1999) Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther 289:668–675
Brillault J, Berezowski V, Cecchelli R, Dehouck MP (2002) Intercommunications between brain capillary endothelial cells and glial cells increase the transcellular permeability of the blood-brain barrier during ischemia. J Neurochem 83:807–817
de Groen PC, Aksamit AJ, Rakela J, Forbes GS, Krom RAF (1987) Central nerves system toxicity after liver transplantation. N Engl J Med 317:861–866
Dehouck MP, Jolliet-Riant P, Brée F, Fruchart JC, Cecchelli R, Tillement J-P (1992) Drug transfer across the blood-brain barrier: correlation between in vitro and in vivo models. J Neurochem 58:1790–1797
Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV (2007) Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 38:1345–1353
Dobbin J, Crockard HA, Ross-Russell R (1989) Transient blood-brain barrier permeability following profound temporary global ischaemia: an experimental study using 14C-AIB. J Cereb Blood Flow Metab 9:71–78
Dohgu S, Yamauchi A, Nakagawa S, Takata F, Kai M, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y (2004) Nitric oxide mediates cyclosporine-induced impairment of the blood-brain barrier in cocultures of mouse brain endothelial cells and rat astrocytes. Eur J Pharmacol 505:51–59
Dohgu S, Kataoka Y, Ikesue H, Naito M, Tsuruo T, Oishi R, Sawada Y (2000) Involvement of glial cells in cyclosporine-increased permeability of brain endothelial cells. Cell Mol Neurobiol 20:781–786
Erer B, Polchi P, Lucarelli G, Angelucci E, Baronciani D, Galimberti M, Giardini C, Gaziev D, Maiello A (1996) CsA-associatied neurotoxicity and ineffective prophylaxis with clonazepam in patients transplanted for thalassemia major: analysis of risk factors. Bone Marrow Transpl 18:157–162
Fleegal MA, Hom S, Borg LK, Davis TP (2005) Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am J Physiol Heart Circ Physiol 289:H2012–H2019
Fontaine M, Elmquist WF, Miller DW (1996) Use of rhodamine 123 to examine the functional activity of P-glycoprotein in primary cultured brain microvessel endothelial cell monolayers. Life Sci 59:1521–1531
Gijitenbeek JMM, van den Bent MJ, Vecht ChJ (1999) Cyclosporine neurotoxicity: a review. J Neurol 246:339–346
Gumerlock MK (1989) Cerebrovascular disease and the blood-brain barrier. In: Neuwelt EA (ed) The clinical impact of the blood-brain barrier and its manipulation. Plenum Press, New York, pp 495–565
Hauben M (1996) Cyclosporine neurotoxicity. Pharmacotherapy 16:576–583
Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M (2004) Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept 123:77–83
Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev 57:173–185
Huber JD, Egleton RD, Davis TP (2001) Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci 24:719–725
Ikesue H, Kataoka Y, Kawachi R, Dohgu S, Shuto H, Oishi R (2000) Cyclosporine enhances α1-adrenoceptor-mediated nitric oxide production in C6 glioma cells. Eur J Pharmacol 407:221–226
Jansen O, Krieger D, Krieger S, Sartor K (1996) Cortical hyperintensity on proton density-weighted images: an MR sign of cyclosporine-related encephalopathy. Am J Neuroradiol 17:337–344
Kochi S, Takanaga H, Matsuo H, Naito M, Tsuruo T, Sawada Y (1999) Effect of cyclosporine A or tacrolimus on the function of blood-brain barrier cells. Eur J Pharmacol 372:287–295
Lenzser G, Kis B, Bari F, Busija DW (2005) Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res 1051:72–80
Mark KS, Davis TP (2002) Cerebral microvascular changes in permeability and tight junctions induced by hypxia-reoxygenation. Am J Physiol Heart Circ Physiol 282:H1485–H1495
Nishioku T, Takata F, Yamauchi A, Sumi N, Yamamoto I, Fujino A, Naito M, Tsuruo T, Shuto H, Kataoka Y (2007) Protective action of indapamide, a thiazide-like diuretic, on ischemia-induced injury and barrier dysfunction in mouse brain microvascular endothelial cells. J Pharmacol Sci 103:323–327
Plateel M, Teisseir E, Cecchelli R (1997) Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem 68:874–877
Samoto K, Ikezaki K, Yokoyama N, Fukui M (1994) P-glycoprotein expression in brain capillary endothelial cells after focal ischemia in rat. Acta Neurochir Suppl 60:257–260
Serkova NJ, Christians U, Benet LZ (2004) Biochemical mecahisms of cyclosporine neurotoxicity. Mol Interv 4:97–107
Shuto H, Kataoka Y, Kanaya A, Matsunaga K, Sueyasu M, Oishi R (1998) Enhancement of serotonergic neural activity contributes to cyclosporine-induced tremors in mice. Eur J Pharmacol 341:33–37
Shuto H, Kataoka Y, Fujisaki K, Nakao T, Sueyasu M, Miura I, Watanabe Y, Fujiwara M, Oishi R (1999) Inhibition of GABA system involved in cyclosporine-induced convulsions. Life Sci 65:879–887
Takata F, Dohgu S, Yamauchi A, Nakagawa S, Naito M, Tsuruo T, Shuto H, Kataoka Y (2007) Inhibition of transforming growth factor-β production in brain pericytes contributes to cyclosporin A-induced dysfunction of the blood-brain barrier. Cell Mol Neurobiol 27:317–328
Tatsuta T, Naito M, Oh-hara T, Sugawara I, Tsuruo T (1992) Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem 267:20383–20391
Thompson CB, June CH, Sullivan KM, Thomas ED (1984) Association between cyclosporine neurotoxicity and hypomagnesia. Lancet 2:1116–1120
Tsuji A, Tamai I, Sakata A, Tenda Y, Terasaki T (1993) Restricted transport of cyclosporin A across the blood-brain barrier by a multidrug transporter P-glycoprotein. Biochem Pharmacol 46:1096–1099
Utepbergenov DI, Mertsch K, Sporbert A, Tenz K, Paul M, Haseloff RF, Blasig IE (1998) Nitric oxide protects blood-brain barrier in vitro from hypxia/reoxygenation-mediated injury. FEBS Lett 424:197–201
Wachtel M, Frei K, Ehler E, Bauer C, Gassmann M, Gloor SM (2002) Extracellular signal-regulated protein kinase activation during reoxygenation is required to restore ischaemia-induced endothelial barrier failure. Biochem J 367:873–879
Wardlaw JM, Sandercock PA, Dennis MS, Starr J (2003) Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 34:806–812
Wartenberg M, Ling FC, Schallenberg M, Baumer AT, Petrat K, Hescheler J, Sauer H (2001) Down-regulation of intrinsic P-glycoprotein expression in multicellular prostate tumor spheroids by reactive oxygen species. J Biol Chem 276:17420–17428
Yamauchi A, Dohgu S, Nishioku T, Shuto H, Naito M, Tsuruo T, Sawada Y, Kataoka Y (2007) An inhibitory role of nitric oxide in the dynamic regulation of the blood-brain barrier function. Cell Mol Neurobiol 27:263–270
Yamauchi A, Shuto H, Dohgu S, Nakano Y, Egawa T, Kataoka Y (2005) Cyclosporin A aggravates electroshock-induced convulsions in mice with a transient middle cerebral artery occlusion. Cell Mol Neurobiol 25:923–928
Acknowledgments
This work was supported, in part, by Grants-in-Aid for Scientific Research [(B) 17390159], Grants-in-Aid for Young Scientists [(Start-up) 18890227], Grants-in-Aid for Young Scientists [(B) 19790199] from JSPS, Japan and the Ministry of Health, Labor and Welfare of Japan (H19-nanchi-ippan-006). The authors thank Dr. Mária A. Deli (Institute of Biophysics, Biological Research Centre of the Hungarian Academy of Sciences) and Dr. Masami Niwa (Nagasaki University School of Medicine) for pertinent comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dohgu, S., Nishioku, T., Sumi, N. et al. Adverse Effect of Cyclosporin A on Barrier Functions of Cerebral Microvascular Endothelial Cells After Hypoxia-reoxygenation Damage In Vitro. Cell Mol Neurobiol 27, 889–899 (2007). https://doi.org/10.1007/s10571-007-9209-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9209-2