1. The present study aimed at elucidating the effect of nitric oxide (NO) on blood-brain barrier (BBB) function with mouse brain capillary endothelial (MBEC4) cells.
2. Histamine (20–100 μM) evoked NO production (1.6–7 μM) in MBEC4 cells in a dose-dependent manner.
3. The permeability coefficient of sodium fluorescein for MBEC4 cells and the cellular accumulation of rhodamine 123 in MBEC4 cells were increased dose-dependently by the addition of NO solutions (14 and 28 μM) every 10 min during a 30-min period.
4. The present study demonstrated that NO increased the permeability and inhibited the P-glycoprotein efflux pump of brain capillary endothelial cells, suggesting that NO plays an inhibitory role in the dynamic regulation of the BBB function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The blood-brain barrier (BBB) contributes to brain homeostasis and fulfills a protective function by regulating the access of solutes and toxic substances to the central nervous system. The BBB is formed by brain capillary endothelial cells, which are closely sealed by tight junctions (Pardridge, 1999). The tight junctions in the BBB prevent significant passive movement of small hydrophilic molecules from the blood to the brain, but specialized transport systems mediate the entry of essential substances such as glucose, amino acids, choline, monocarboxylic acids, amines, thyroid hormones, purine bases, and nucleosides (Tsuji and Tamai, 1999; Kusuhara and Sugiyama, 2001). The efflux transporter, P-glycoprotein (P-gp) is a key element of the molecular machinery that confers special permeability properties on the BBB. P-gp, which was initially recognized for its ability to excrete anticancer drugs from multidrug-resistant cancer cells, is strongly expressed in brain capillaries. Its expression in the BBB limits the accumulation of many hydrophobic molecules and potentially toxic substances in the brain.
Nitric oxide (NO) is a transient product of inflammatory processes, generated from l-arginine by the enzyme NO synthase (NOS). NO appears to be involved in numerous vital cellular functions including neurotransmission, blood-pressure control, and the regulation of vascular tone. The basal production of NO appears to be required for biological regulation, and yet an excess of this same molecule can be cytotoxic to organism. But the molecular mechanisms mediating NO-induced tissue injury and breakdown of the BBB are not completely understood.
In the present study, to clarify the role of NO in the dynamic regulation of the BBB, we examined effect of NO on the function of tight junctions and P-gp in mouse brain capillary endothelial (MBEC4) cells. MBEC4 cells show the highly specialized characteristics of brain microvascular endothelial cells including P-gp expression (Tatsuta et al., 1992, 1994).
MATERIALS AND METHODS
A saturated NO solution (typically containing approximately 1.4 mM NO) was prepared according to Ikesue et al. (2000). Deionized water (2 mL) was bubbled with argon for 20 min to remove oxygen. Then, the solution was bubbled with pure NO gas for 20 min and kept in a glass flask with a rubber septum under a NO atmosphere prior to use.
MBEC4 cells, which were isolated from BALB/c mouse brain cortices and immortalized by SV40-transformation (Tatsuta et al., 1992), were cultured in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO BRL, Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum, 100 units/mL of penicillin, and 100 μg/mL of streptomycin. They were grown in 2.5-cm2 dish, 12-well Transwells (Costar, MA) and 24-well plates in a humidified atmosphere of 5% CO2/95% air at 37°C.
Direct and continuous electrochemical measurement of NO was performed with a three-electrode potentiostatic EMS-100 system (BIO-LOGIC, Grenoble, France), as previously described (Ikesue et al., 2000; Trevin et al., 1998). In brief, confluent MBEC4 cells in a 2.5-cm2 dish were washed three times with Krebs–Ringer solution (143.0 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.0 mM NaH2PO4, and 11.0 mM D-glucose, pH 7.4). The dish was placed on the stage of an inverted microscope (ECLIPSE TE300, Nikon, Tokyo, Japan) mounted with an NO monitoring system. The NO-biosensor (ASTEC, Fukuoka, Japan) was positioned about 10 μm above the cell surface. Ten minutes after treatment with 1-mM l-arginine (Sigma, St. Louis, MO), histamine (Wako, Osaka, Japan) in a volume of 10 μL was added to the cells in 1 mL of Krebs–Ringer solution with a transient mixing step to give the final concentration indicated. The level of production of NO in MBEC4 cells was monitored for a 15-min period after the addition of histamine.
MBEC4 cells (42,000 cells/cm2) were cultured on the collagen-coated polycarbonate membrane of the Transwell insert (3.0-μm pore size, 12-well type). After 3 days, they were washed three times with serum-free medium. Cells were exposed to 5 or 10 μL of NO solution (final concentration, 14 and 28 μM, respectively) injected into the inside of the insert (luminal side) every 10 min during a 30-min period. To initiate the transport experiments, the medium was removed, and cells were washed three times with Krebs–Ringer buffer (118 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1.2 mM MgCl2, 1.0 mM NaH2PO4, 25 mM NaHCO3, and 11 mM D-glucose, pH 7.4). Krebs–Ringer buffer (1.5 mL) was added to the outside of the insert (abluminal side). Krebs–Ringer buffer (0.5 mL) containing 100 μg/mL of sodium fluorescein (Na-F; Sigma) was loaded on the luminal side of the insert. Samples (0.5 mL) were removed from the abluminal chamber at 10, 20, 30, and 60 min and were immediately replaced with fresh Krebs–Ringer buffer. Aliquots (5 μL) from the abluminal chamber samples were mixed with 200 μL of Krebs–Ringer buffer, and then the concentration of Na-F was determined using a multiwell fluorometer (E x(λ) 485 nm; E m(λ) 530 nm; CytoFluor Series 4000, PerSeptive Biosystems, Framingham, MA). The permeability coefficient and clearance were calculated according to the method described by Dehouck et al. (1992). Clearance was expressed as microliters of tracer diffusing from the luminal to abluminal chamber and was calculated from the initial concentration of tracer in the luminal chamber and final concentration in the abluminal chamber: Clearance (μL) = [C]A × V A/[C]L where [C]L is the initial luminal tracer concentration, [C]A is the abluminal tracer concentration, and V A is the volume of the abluminal chamber. During a 60-min period of the experiment, the clearance volume increased linearly with time. The average volume cleared was plotted versus time, and the slope was estimated by linear regression analysis. The slope of clearance curves for the MBEC4 monolayer was denoted by PSapp, where PS is the permeability-surface area product (in microliters per minute). The slope of the clearance curve with a control membrane was denoted by PSmembrane. The real PS value for the MBEC4 monolayer (PStrans) was calculated from 1/PSapp = 1/PSmembrane + 1/PStrans. The PStrans values were divided by the surface area of the Transwell inserts to generate the permeability coefficient (Ptrans, in centimeters per minute).
The functional activity of P-gp was determined by measuring the cellular accumulation of rhodamine 123 (Sigma) according to the method of Fontaine et al. (1996). MBEC4 cells (21,000 cells/cm2) were cultured on collagen-coated 24-well plates. Three days after seeding, they were washed three times with serum-free medium and then exposed to 14–28 μM of NO solution every 10 min during a 30-min period. The medium was removed, and the cells were washed three times with assay buffer (143 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1.2 mM MgCl2, 1.0 mM NaH2PO4, 10 mM HEPES, and 11 mM D-glucose, pH 7.4). The cells were incubated with 0.5 mL of assay buffer containing 5 μM of rhodamine 123 for 60 min. Then, the solution was removed, and the cells were washed three times with ice-cold phosphate-buffered saline and solubilized in 1 M NaOH (0.2 mL). The solution was neutralized with 1 M HCl (0.2 mL), and the rhodamine 123 content was determined using a multiwell fluorometer (E x(λ) 485 nm; E m(λ) 530 nm). The cellular protein was measured by the method of Bradford (Bradford, 1976).
The effect of the NO solution on cell viability was assessed using a WST-8 assay (Cell Counting Kit, DOJINDO, Kumamoto, Japan). A highly water-soluble formazan dye, reduced by mitochondrial dehydrogenase, was measured by determining the absorbance of each sample with a 450-nm test wavelength and a 700-nm reference wavelength.
The values are expressed as means ± SEM. Statistical analysis was performed using Student’s t-test. One-way analysis of variance (ANOVA) followed by the Dunnett test was applied to multiple comparisons. The differences between means were considered to be significant when P values were less than 0.05.
RESULTS
Figure 1A shows a representative current-time curve obtained with the NO biosensor in MBEC4 cells. When l-arginine (1 mM) was added to cells 10 min before the addition of histamine, the signal became stable within 1–2 min. The NO-biosensor signal increased rapidly to reach a peak within 1 min after the injection of histamine. Following this period, the signal decreased slowly to the baseline about 5–7 min post-injection. Histamine at concentrations of 20–100 μM dose-dependently increased NO production (1.55 ± 0.65–6.94 ± 1.43 μM) in MBEC4 cells (Fig. 1B).
Histamine-induced NO production in MBEC cells. (A) A representative differential pulse amperogram obtained using a NO biosensor shows the level of NO production evoked by histamine (100 μM). (B) Concentration-response relationship of histamine-induced NO production using direct electrochemical monitoring in MBEC cells. Values are means ± SEM (n = 3). * p<0.05, significant difference from 20 μM histamine.
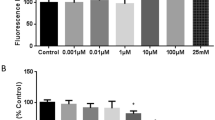
Changes in the permeability coefficient of Na-F (A) and the cellular accmulation of rhodamine 123 (B) in MBEC4 cell monolayers after addition of NO solutions every 10 min during a 30-min period. Data are expressed as a percentage of the corresponding control value (A: 1.47 ± 0.21 × 10−4 cm/min, B: 0.95 ± 0.07 nmol/mg protein). Values are shown as means ± SEM (n = 3–18). ** P < 0.01, significant difference from control.
To evaluate effect of NO on the BBB function, the concentration and the exposure time of NO were determined on the basis of NO production evoked by histamine. When the NO solution at the final concentrations of 14 and 28 μM was added every 10 min during a 30-min period, the permeability coefficients of Na-F for MBEC4 cells increased dose-dependently to 113.6 ± 12.9 and 123.7 ± 2.8%, respectively (Fig. 2A). The exposure to NO solution had no effect on cell viability assessed with the WST-8 assay (14 μM: 93.7 ± 3.3%, 28 μM: 101.3 ± 3.2% of control).
As shown in Fig. 2B, the accumulation of rhodamine 123 in MBEC4 cells increased dose-dependently to 108.3 ± 4.8 and 164.5 ± 14.6% of the control value after a 30-min exposure to the NO solution at concentrations of 14 and 28 μM, respectively.
DISCUSSION
As shown in Fig. 1, direct and continuous electrochemical monitoring revealed that histamine stimulated MBEC4 cells to release NO over a short period (about 5 min). This phenomenon appears to be due to an activation of the constitutive endothelial form of NO synthase (eNOS) through a Ca2+/calmodulin-dependent pathway in response to H1 receptor-mediated increases in intracellular Ca2+ (Daum et al., 1983; Kishi et al., 1996). Various endogenous substances including histamine stimulate NO production in the microvascular endothelium and/or glial and neuronal cells in the brain (Mayhan, 1996), probably contributing to dynamic regulation of the BBB function.
The permeability of MBEC4 cells to Na-F was apparently increased by a brief exposure to NO solutions at 2- to 4-fold the concentrations induced by histamine (Fig. 2A). The NO solutions employed here are more prominent than convenient NO donors such as sodium nitroprusside with respect to biological properties including distribution and degradation. The cell viability was not influenced by NO at the concentrations employed here (14–28 μM), suggesting that the increased paracellular permeability of MBEC4 cells was not due to the cytotoxicity of NO. Therefore, NO is highly likely to lower the functions of the tight junctions at the BBB, supporting early reports (Hurst and Fritz, 1996; Mayhan, 2000; Shukula et al., 1996). The mechanisms by which NO donors increased vascular endothelial permeability involved an increase in the level of cyclic guanosine monophosphate (cGMP) (Gimeno et al., 1998) or the formation of peroxynitrite (Menconi et al., 1998). These substances conceivably influence the intrinsic tight junction proteins and the associated actin cytoskeleton through a direct or second signaling pathway. Further studies are required to clarify this mechanism.
In the present study, the most important finding was that the accumulation of rhodamine 123, a substrate of P-gp (Fontaine et al., 1996), in MBEC4 cells was increased by treatment with NO solutions (Fig. 2B). P-gp is an energy-dependent efflux pump mediating the transportation of substances from the basement membrane to the luminal surface. NO induced a depletion of endothelial ATP by inhibiting glyceraldehyde-3-phosphate dehydrogenase (glycolytic enzyme) activity (Hurst et al., 2001). This inhibition of energy metabolism is probably associated with the NO-triggered inhibition of P-gp function.
In the present study, NO induced hyperpermeability in MBEC4 cells and inhibition of the P-gp efflux pump. These findings suggest that NO in the brain plays an inhibitory role in the dynamic regulation of the BBB function.
REFERENCES
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254.
Daum, P. R., Downes, C. P., and Young, J. M. (1983). Histamine-induced inositol phosholipid break-down mirrors H1-receptor density in brain. Eur. J. Pharmacol. 87:497–498.
Dehouck, M.-P., Jolliet-Riant, P., Brée, F., Fruchart J.-C., Cecchelli, R., and Tillement, J.-P. (1992). Drug transfer across the blood-brain barrier: Correlation between in vitro and in vivo models. J. Neurochem. 58:1790–1797.
Fontaine, M., Elmquist, W. F., and Miller, D. W. (1996). Use of rhodamine 123 to examine the functional activity of P-glycoprotein in primary cultured brain microvessel endothelial cell monolayers. Life Sci. 59:1521–1531.
Gimeno, G., Carpentier, P. H., Desquand-Billiald, S., Hanf, R., and Finet, M. (1998). l-arginine and NG-nitro-l-arginine methyl ester cause macromolecular extravasation in the microcirculation of awake hamsters. Eur. J. Pharmacol. 346:275–282.
Hurst, R. D., and Fritz, I. B. (1996). Properties of an immortalized vascular endothelial/glioma cell co-culture model of blood-brain barrier. J. Cell. Physiol. 167:81–88.
Hurst, R. D., Azam, S., Hurst, A., and Clark, J. B. (2001). Nitric-oxide-induced inhibition of glyceraldehyde-3-phosphate dehydrogenase may mediate reduced endothelial cell monolayer integrity in an in vitro model blood-brain barrier. Brain Res. 894:181–188.
Ikesue, H., Kataoka, Y., Kawachi, R., Dohgu, S., Shuto, H., and Oishi, R. (2000). Cyclosporine enhances α1-adrenoceptor-mediated nitric oxide production in C6 glioma cells. Eur. J. Pharmacol. 407:221–226.
Kishi, F., Nakaya, Y., Takahashi, A., Miyoshi, H., Nomura, M., and Saito, K. (1996). Intracellular and extracellular Ca2+ regulate histamine-induced release of nitric oxide in vascular endothelial cells as shown with sensitive and selective nitric oxide electrodes. Pharmacol. Res. 33:123–126.
Kusuhara, H., and Sugiyama, Y. (2001). Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 1). Drug. Discov. Today 6:150–156.
Mayhan, W. G. (1996). Role of nitric oxide in histamine-induced increases in permeability of the blood-brain barrier. Brain Res. 743:70–76.
Mayhan, W. G. (2000). Nitric oxide donor-induced increase in permeability of the blood-brain barrier. Brain Res. 866:101–108.
Menconi, M. J., Unno, N., Smith, M., Aguirre, D. E., and Fink, M. P. (1998). Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: Role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochim. Biophys. Acta 1425:189–203.
Pardridge, W. M. (1999). Blood-brain barrier biology and methodology. J. Neurovirol. 5:556–569.
Shukula, A., Dikshit, M., and Srimal, R. C. (1996). Nitric oxide-dependent blood-brain barrier permeability alteration in the rat brain. Experientia 52:136–140.
Tatsuta, T., Naito, M., Oh-hara, T., Sugawara, I., and Tsuruo, T. (1992). Functional involvement of P-glycoprotein in blood-brain barrier. J. Biol. Chem. 267:20383–20391.
Tatsuta, T., Naito, M., Mikami, K., and Tsuruo, T. (1994). Enhanced expression by the brain matrix of P-glycoprotein in brain capillary endothelial cells. Cell Growth Differ. 5:1145–1152.
Trevin, S., Kataoka, Y., Kawachi, R., Shuto, H., Kumakura, K., and Oishi, R. (1998). Direct and continuous electrochemical measurement of noradrenaline-induced nitric oxide production in C6 glioma cells. Cell. Mol. Neurobiol. 18:453–458.
Tsuji, A., and Tamai, I. (1999). Carrier-mediated or specialized transport of drugs across the blood-brain barrier. Adv. Drug. Deliv. Rev. 36:277–290.
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Scientific Research ((B)(2) 14370789) and ((C)(2) 15590475) from JSPS, Japan, by a Grant-in-Aid for Exploratory Research (16659138) from MEXT, Japan, and by funds (No.: 031001) from the Central Research Institute of Fukuoka University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamauchi, A., Dohgu, S., Nishioku, T. et al. An Inhibitory Role of Nitric Oxide in the Dynamic Regulation of the Blood-Brain Barrier Function. Cell Mol Neurobiol 27, 263–270 (2007). https://doi.org/10.1007/s10571-007-9139-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9139-z