Summary
1. Aims: Brain vascular endothelial cells secret Adrenomedullin (AM) has multifunctional biological properties. AM affects cerebral blood flow and blood–brain barrier (BBB) function. We studied the role of AM on the permeability and tight junction proteins of brain microvascular endothelial cells (BMEC).
2. Methods: BMEC were isolated from rats and a BBB in vitro model was generated. The barrier functions were studied by measuring the transendothelial electrical resistance (TEER) and the permeability of sodium fluorescein and Evans’ blue albumin. The expressions of tight junction proteins were analyzed using immunocytochemistry and immunoblotting.
3. Results: AM increased TEER of BMEC monolayer dose-dependently. Immunocytochemistry revealed that AM enhanced the claudin-5 expression at a cell–cell contact site in a dose-dependent manner. Immunoblotting also showed an overexpression of claudin-5 in AM exposure.
4.Conclusions: AM therefore inhibits the paracellular transport in a BBB in vitro model through claudin-5 overexpression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Adrenomedullin (AM), a potent vasodilator and hypotensive peptide, was originally isolated from pheochromocytomas. The AM gene is thus expressed in and the peptide is released by various cells and it is thus considered to play a pathological role in cerebrovascular and cardiovascular diseases (Tsukita and Furuse, 1999; Fujioka et al., 2000; Terata et al., 2000; Kastin et al., 2001; Wijdicks et al., 2001; Kis et al., 2001a,b, 2002, 2003a; Serrano et al., 2002; Kato et al., 2003; Tahan et al., 2003; Fernandez-Sauze et al., 2004; Hayashi et al., 2004; Nagoshi et al., 2004; Suzuki et al., 2004). AM is also secreted from cultured rat brain microvascular endothelial cells (BMEC) and its production is stimulated by astrocyte-derived factors (Kis et al., 2002).
AM dilates cerebral vessels and increases cerebral blood flow (Fujioka et al., 2000; Kis et al., 2001a). The dilation of vessels interacts with blood–brain barrier (BBB) tight–junction properties. The opening of tight junctions, which reflects the paracellular pathway, or transendothelial pathway including several endocytosis, transendocytosis and exocytosis, contribute to the passage of molecules through the BBB (Deli et al., 2000). The AM administration dose-dependently increases intracellular cyclic AMP (cAMP) level which increases TEER and P-glycoprotein activity and reduces paracellular permeability and the rate of fluid-phase endocytosis without any change in albumin permeability (Kis et al., 2001b).
Trans-membrane tight-junction proteins consist of three integral protein families i.e., claudins, occludin, and junctional adhesion molecules. In these, the claudins have been proven to be one of the essential proteins for tight junction strands and the composition of the claudin species directly determines the barrier function (Furuse et al., 1998, 1999; Tsukita and Furuse, 1999; Morita et al., 1999a,b; Wolburg and Lippoldt, 2002; Ishizaki et al., 2003).
The expression of claudin proteins after exposure to several agents has been studied in seven articles and substantial variations have been shown depending on the species, cell types, and experimental models used (Yi et al., 2000; Coyne et al., 2002; Han et al., 2003; Andras et al., 2005; Chen et al., 2005; Florin et al., 2005; Kuribayashi et al., 2005).
In claudins, only claudin-1 and claudin-5 have been detected in BMEC and the interaction with claudins and AM has also been studied (Morita et al., 1999a; Liebner et al., 2000; Lippoldt et al., 2000; Ishizaki et al., 2003; Kis et al., 2003b). According to these studies, AM has cAMP like functions and cAMP strengthens claudin-5 expression, but deteriorates claudin-1 expression (Kis et al., 2001b; Ishizaki et al., 2003). Between two claudins, claudin-5 was the microvascular endothelium specific protein (Kuribayashi et al., 2005). Only two articles have previously studied about the changes in claudin-5 expression (Andras et al., 2005; Kuribayashi et al., 2005).
Owing to the importance of claudin-5 and AM in maintaining the BBB integrity, the present study focused on how AM influences the claudin-5 expression using a BBB in vitro model culturing rat BMEC. This is the first study to elucidate the relationship between AM and claudin-5 with trans-endothelial electrical resistance (TEER), a permeability study, immunocytochemistry, and immunoblotting.
MATERIALS AND METHODS
Cell Cultures
Primary BMEC were isolated as described previously from 3-week-old male Wistar rats (Japan SLC, Hamamatsu, Japan) with the permission of the Ethics Committee of Nagasaki University, Nagasaki, Japan (Hayashi et al., 2004). Briefly, meninges were carefully removed from the forebrain and gray matter was minced, and then it was digested with collagenase 2 (Worthington) in Dulbecco's modified Eagles medium (DMEM, Sigma, St. Luis, MO) in a shaker for 1.5 h at 37°C. The cell pellet was separated by centrifugation then the microvessels obtained in the pellet were further digested with collagenase-dispase (Roche) in DMEM for 1.5 h at 37°C. The endothelial cell clusters were separated on Percoll gradient and washed twice. Primary BMEC were seeded onto collagen type IV and fibronectin coated 35-mm plastic dishes. The cultures were maintained in the endothelial culture medium consisted of DMEM nutrient mixture F-12 HAM (DMEM/F12; Sigma) supplemented with bovine plasma derived serum (First Link, Brierly Hill, UK). The confluent BMEC were seeded on inside of the inserts coated with collagen type IV and fibronectine, and then placed into 12-well plates (Transwell™ inserts, diameter 12 mm, 0.45 μm pore size; Corning, Midland, MI).
AM Treatments
AM (10−6, 10−7, and 10−8 M) with cAMP-specific phosphodiesterase IV inhibitor 4-(3-butoxy-4-methoxy-benzyl) imidazolidin-2-one (RO, 20-1724; 17.5 μM, Calbiochem, San Diego, CA) was added. The concentrations of AM and RO have been described in a previous report (Kis et al., 2001b).
Transendothelial Electrical Resistance (TEER)
The electrical resistance across the membrane was measured using an EVOM resistance meter (World Precision Instruments, Sarasota, FL). The extracellular matrix-treated Transwell inserts were placed in a 12-well plate containing culture medium and then were used to measure background resistance. The resistance measurements of these blank filters were then subtracted from those of filters with cells. The values are shown as Ω cm2 based on culture inserts.
Transendothelial Permeability
Inserts were transferred to 12-well plates containing Ringer Hepes solution. In apical chambers, the culture medium was replaced by Ringer Hepes containing 10 μg/mL sodium fluorescein (mw: 376 Da) and 165 μg/mL Evans’ blue bound to 0.1% BSA (mw: 67 kDa). The absorbency of Evans’ blue was measured at 620 nm (Labsystems Multiscan plate reader), while the emission of sodium fluorescein at 525 nm (Shimadzu RF-5000 fluorometer; excition: 440 nm). Transport was expressed as μl tracer diffusing from luminal to abluminal compartments, and endothelial permeability coefficient (P e; in cm/min) was calculated and compared to control group. The control flux across cell-free, collagen-coated inserts was also measured and it was regarded as 100%. The transport was expressed as percentage of the tracer accumulation of the control diffusing from the luminal to abluminal compartments (Dehouck et al., 1992; Dohgu et al., 2004).
Claudin-5 Immunocytochemistry
The cells were grown on the coverslips according to the method mentioned above. On the fourth day of culture, AM (10−6, 10−7, and 10−8 M) with RO was added to each slip. Next, the cells were fixed in 1% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min after 6 h of administration. Nonspecific reactions were blocked by normal horse serum and then cells were incubated with primary antibody (claudin-5; Zymed, San Francisco, CA) for 1 h at 37°C. The cells were rinsed with PBS and incubated for 1 h at room temperature with appropriate secondary antibodies labeled with Alexa Fluor 488 (green) (Molecular Probes, Eugene, OR). All samples were examined using a laser-scanning confocal microscope (MRC 1024; Bio-Rad, Hercules, CA) with excitation at 488 nm and a detection range from 500 to 535 nm.
Immunoblotting
For the Western blot assay, BMEC were cultured in 6 well culture dishes and then were treated with AM (10−6, 10−7, and 10−8 M) with RO for 6 h. Next, the cells were harvested by scraping in NP40 lysis buffer supplemented with proteinase inhibitors (1 μg/mL aprotinin, 50 μg/mL phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin). The lysates were centrifuged at 12000×g for 5 min at 4°C. The supernatants were collected and protein concentrations were determined with DC Protein Assay (Bio-Rad, Hercules, CA). The samples were mixed with 5×Laemmli sample buffer and heated at 95°C for 5 min. An equal amount of protein for each sample was separated by 10% SDS–PAGE and then was transferred onto a polyvinylidine difluoride sheet (Polyscreen PVDF; Perkin Elmer Life Sciences, Boston, MA). The membranes were then incubated in a blocking buffer (Tris buffered saline, 0.1% Tween 20, 5% Problock powder, Sigma, St. Louis, MO) for 1 h at 37°C. The blots were subsequently incubated with anit-claudin (1:500; Zymed, San Francisco, CA) antibodies 1 h at room temperature. After washing with Tris buffered saline with 0.1% Tween 20 three times, the blots were incubated with secondary antibody, conjugated with horseradish peroxidase for 1 h at room temperature. For visualization, the immunoblots were analyzed using an ECL Western blot detection kit (Amersham Biosciences, Piscataway, NJ). To quantify the relative levels of claudin-5, the intensity of the specific bands was estimated by the Scion image analysis software package (Scion Corporation, Frederick, MD).
Statistical Analysis
The statistical analysis was performed using the ystat2004.xls software package (IGAKU TOSHO SHUPPAN CO., LTD , Tokyo, Japan). All data are presented as the means ± standard error. Differences between the two groups were assessed by an unpaired t-test, and among three or more groups by a one-way analysis of variance followed by Scheffe's or Dunnett T3 multiple comparison tests. A p value of less than 0.05 was considered to indicate statistical significance.
RESULTS
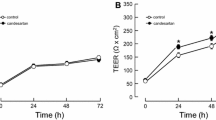
AM administration elevated TEER in a dose-dependent manner (Fig. 1). At 3 h, % TEER was 119.4±25.2% in AM 10−8 M, 127.7±10.3% in 10−7 M and 148.2±17.9% in 10−6 M. TEER changes in the first 0.5 h of AM administration showed a similar tendency.
Adrenomedullin increased the transendothelial electrical resistance (TEER) dose-dependently in BMEC monolayer. Confluent BMEC cultures were exposed to AM at the doses of 10−6, 10−7, and 10−8 M for up to 6 h. The TEER value was expressed in the percent of the control value (100%=158.8±10.4 Ωcm2, mean±SEM, n=4,* p < 0.05 vs. control).
The permeability of sodium fluorescein, which has of low molecular weight, is regarded as a marker for paracellular permeation. The accumulation of sodium fluorescein diffusing across the endothelial monolayer was 96.7±4.5% in AM 10−8 M, 74.7±4.1% in 10−7 M and 64.4±3.2% in 10−6 M. On the other hand, the permeability of Evans’ blue, which is bound to albumin, which is regarded as a transcellular transport for large molecules, was not affected by the AM administration (Fig. 2(B)).
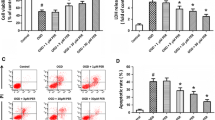
An immunohistochemical study revealed that claudin-5 expression changed from a zigzag to a linear shape at the cell–cell junctions in a dose-dependent manner (Fig. 3(A)). A Western blot analysis also showed an overexpression of claudin-5 dose-dependently (Fig. 3(B)). The relative expression ratio of AM 10−8, 10−7, and 10−6 M versus control was 1.39, 1.89, and 2.20, respectively (Fig. 3(C)), which closely correlated with the immunocytochemsitry findings.
Dose-dependent effects of AM on claudin-5 protein expression in BMEC. Confluent BMEC cultures were exposed to the indicated doses of AM for 6 h. A: Claudin-5 protein expression was analyzed by immunocytochemistry using anti-claudin-5 antibody. The photographs shown are representative images of at least five different fields observed in three independent experiments. Original magnification, ×400. Single white bar indicates 10 μm. B: Cells were analysed and subjected to immunoblot analysis using anti-claudin-5 antibody. C: The bar graph reflects the combined densitometry data from four independent experiments (mean±SEM, n=4, * p < 0.05 vs. control, § § p < 0.05 vs. AM 10−8 M).
DISCUSSION
Claudins are located in the superficial part of the tight junction and form a primary seal and the expression of claudins in the BBB seems to vary from species to species and it has not been clarified until now. In contrast, occludins induce short strands and connect claudins with ZO-1 or ZO-2, the claudin-induced strands are relatively long and branched (Furuse et al., 1998; Tsukita and Furuse, 1999; Huber et al., 2001). Among the known claudins, claudin-1 and claudin-5 have been detected in cerebral endothelial cells (Morita et al., 1999a,b; Liebner et al., 2000; Lippoldt et al., 2000). As illustrated in Fig. 3, claudin-5 expression was induced dose-dependently by AM administration with a pattern change from zipper-like to linear and the location was also changed from the cytoplasm to cellular membrane.
AM is an autocrine mediator which maintains the intraendothelial cyclic cAMP levels, and it is also known to be a critical regulator of BBB (Kis et al., 2003a). cAMP induces the phosphorylation of claudin-5 immunoprecipitates and the expression of clauidn-5 via PKA-dependent and—independent pathways (Ishizaki et al., 2003). cAMP also activates the gene expression of claudin-5 via protein kinase A-(PKA) independent pathway and the activation of PKA increased the claudin-5 signals along cell–cell adhesive sites (Ishizaki et al., 2003).
AM/cAMP/PKA cascade promotes cell proliferation, migration, and regeneration in vivo and in vitro with endothelial Akt activation (Miyashita et al., 2003a,b). Though the experimental conditions differ from study to study, the type of cultured cells and the serum concentration may influence the AM effect. As a result, this theory remains controversial (Miyashita et al., 2003a,b). On the other hand, the abluminal administration of AM caused significant cell migration into the abluminal chamber, therefore our biluminal application might prevent cell migrations and cause appropriate cell proliferation, thus resulting in a high TEER voltage in primary rat BMECs (Miyashita et al., 2003b). This cell proliferation may increase the physical monolayer structural strength of the insert membrane, however further study is called for to elucidate this report.
Not only claudin-5 activation, but also occludins, ZO-1 or -2, and F-actin or nonmuscle myosin may also support tight junction strength. Similar to cAMP, AM may strengthen the expression of these proteins (Kis et al., 2003a,b). Including translocation, phophorylation, and stress fiber rearrangement, many factors participate in an improvement of the BBB tight–junction properties.
In an AM study using cerebral endothelial cells, the administration of AM is performed either chronically or tentatively (Kis et al., 2003a,b). Similar to cAMP, AM has a short half life (about 20 min) (Beltowski and Jamroz, 2004). Therefore, the chronic effect of AM was thought to be related to a cAMP-independent mechanism (Kis et al., 2003b). We observed a rapid increase of TEER for first 0.5 h after administration and the increase was dose-dependent. This effect lasted about 24–48 h (data not shown) without any replacement of the medium. Therefore, both cAMP-dependent/-independent mechanisms involving the PKA-dependent/-independent pathway may be the basis for the complex intracellular mechanism of AM. On the other hand, this acute phase administration may help hypoxia/reperfusion injury of the brain or heart caused by stroke and acute myocardial infarction, respectively. Further, experimental and clinical studies are thus called for to evaluate the downstream molecules of the cAMP/PKA cascade.
Considering the facts mentioned above, we speculate that AM has cAMP-dependent/-independent cascades to claudin-5 expression. A tentative administration may trigger the cAMP-dependent cascades which also have downstream PKA-dependent/-independent pathways. Chronic AM administration directly affects the claudin-5 expression. However, these mechanisms can be influenced by the study protocols or culture conditions including the contamination or coculturing of other types of cells. The precise mechanism is thus currently under investigation.
AM may be involved in the astrocytic and/or pericytic regulation. In fact, astrocyte-derived factors could further increase the AM release from primary BMEC. Moreover, AM elevated TEER on BMEC, especially when cocultured with astrocytes (Kis et al., 2003b; Nagoshi et al., 2004). In addition to astrocytes, pericytes have been investigated to clarify tight-junction properties of the BBB (Balabanov and Dore-Duffy, 1998; Fernandez-Sauze et al., 2004; Hayashi et al., 2004).
Though we could find dose-dependent claudin-5 expression changes on immunoblotting after only 6 h of conditioning, these results may have been influenced by the cultured cell types and thus they may too be controversial. As a result, further investigation is needed.
In summary, AM introduces the expression of claudin-5 while also activating the BBB function, which may thus help to prevent the brain from developing pathological conditions.
REFERENCES
Andras, I. E., Pu, H., Tian, J., Deli, M. A., Nath, A., Hennig, B., and Toborek, M. (2005). Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J. Cereb. Blood Flow Metab. 25:1159–1170.
Balabanov, R., and Dore-Duffy, P. (1998). Role of the CNS microvascular pericyte in the blood brain barrier. J. Neurosci. Res. 53:637–644.
Beltowski, J., and Jamroz, A. (2004). Adrenomedullin-what do we know 10 years since its discovery? Pol. J. Pharmacol. 56:5–27.
Chen, S. P., Zhou, B., Willis, B. C., Sandoval, A. J., Liebler, J. M., Kim, K. J., Ann, D. K., Crandall E. D., and Borok, Z. (2005). Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J. Appl. Physiol. 98:322–328.
Coyne, C. B., Vanhook, M. K., Gambling, T. M., Carson, J. L., Boucher, R. C., and Johnson, L. G. (2002). Regulation of airway tight junctions by proinflammatory cytokines. Mol. Biol. Cell 13:3218–3234.
Dehouck, M. P., Jolliet-Riant, P., Bree, F., Fruchart, J. C., Cecchelli, R., and Tillement, J. P. (1992). Drug transfer across the blood–brain barrier: correlation between in vitro and in vivo models. J. Neurochem. 58:1790–1807.
Deli, M. A., Abraham, C. S., Takahata, H., Katamine, S., and Niwa, M. (2000). Pentosan polysulfate regulates scavenger receptor-mediated, but not fluid-phase, endocytosis in immortalized cerebral endothelial cells. Cell. Mol. Neurobiol. 20:731–745.
Dohgu, S., Yamauchi, A., Takata, F., Naito, M., Tsuruo, T., Higuchi, S., Sawada, Y., and Kataoka, Y. (2004). Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell. Mol. Neurobiol. 24:491–507.
Fernandez-Sauze, S., Delfino, C., Mabrouk, K., Dussert, C., Chinot, O., Martin, P. M., Grisoli, F., Ouafik, L., and Boudouresque, F. (2004). Effects of Adrenomedullin on endothelial cells in the multistep process of angiogenesis: Involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int. J. Cancer 108:797–804.
Florin, A., Maire, M., Bozec, A., Hellani, A., Chater, S., Bars, R., Chuzel, F., and Benahmed, M. (2005). Androgens and postmeiotic germ cells regulate claudin-11 expression in rat Sertoli cells. Endocrinology 146:1532–1540.
Fujioka, M., Nishio, K., Sakaki, T., Minamino, N., and Kitamura, K. (2000). Adrenomedullin in patients with cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 31:3079–3083.
Furuse, M., Fujita, K., Hiiragi, T., Fujimoto, K., and Tsukita, S. (1998). Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141:1539–1550.
Furuse, M., Sasaki, A., and Tsukita, S. (1999). Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 147:891–903.
Han, X., Fink, M. P., and Delude, R. L. (2003). Proinflammatory cytokines cause NO*-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock 19:229–237.
Hayashi, K., Nakao, S., Nakaoke, R., Nakagawa, S., Kitagawa, N., and Niwa, M. (2004). Effects of hypoxia on endothelial/pericytic co-culture model of the blood–brain barrier. Regul. Pept. 123:77–83.
Huber, J. D., Egleton, R. D., and Davis, T. P. (2001). Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci. 24:719–725.
Ishizaki, T., Chiba, H., Kojima, T., Fujibe, M., Soma, T., Miyajima, H., Nagasawa, K., Wada, I., and Sawada, N. (2003). Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood–brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp. Cell Res. 290:275–288.
Kastin, A. J., Akerstrom, V., Hackler, L., and Pan, W. (2001). Adrenomedullin and the blood–brain barrier. Horm. Metab. Res. 33:19–25.
Kato, K., Yin, H., Agata, J., Yoshida, H., Chao, L., and Chao, J. (2003). Adrenomedullin gene delivery attenuates myocardial infarction and apoptosis after ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 285:H1506–1514.
Kis, B., Abraham, C. S., Deli, M. A., Kobayashi, H., Wada, A., Niwa, M., Yamashita, H., and Ueta, Y. (2001a). Adrenomedullin in the cerebral circulation. Peptides 22:1825–1834 Review.
Kis, B., Deli, M. A., Kobayashi, H., Abraham, C. S., Yanagita, T., Kaiya, H., Isse, T., Nishi, R., Gotoh, S., Kangawa, K., Wada, A., Greenwood, J., Niwa, M., Yamashita, H., and Ueta, Y. (2001b). Adrenomedullin regulates blood–brain barrier functions in vitro. Neuroreport 12:4139–4142.
Kis, B., Kaiya, H., Nishi, R., Deli, M. A., Abraham, C. S., Yanagita, T., Isse, T., Gotoh, S., Kobayashi, H., Wada, A., Niwa, M., Kangawa, K., Greenwood, J., Yamashita, H., and Ueta, Y. (2002). Cerebral endothelial cells are a major source of adrenomedullin. J. Neuroendocrinol. 14:283–293.
Kis, B., Abraham, C. S., Deli, M. A., Kobayashi, H., Niwa, M., Yamashita, H., Busija, D. W., and Ueta, Y. (2003a). Adrenomedullin, an autocrine mediator of blood–brain barrier function. Hypertens. Res. 26 (Suppl.):S61–70 Review.
Kis, B., Snipes, J. A., Deli, M. A., Abraham, C. S., Yamashita, H., Ueta, Y., and Busija, D. W. (2003b). Chronic adrenomedullin treatment improves blood–brain barrier function but has no effects on expression of tight junction proteins. Acta Neurochir. Suppl. 86:565–568.
Kuribayashi, M., Wang, J., Fujiwara, O., Doi, Y., Nabae, K., Tamano, S., Ogiso, T., Asamoto, M., and Shirai, T. (2005). Lack of effects of 1439 MHz electromagnetic near field exposure on the blood–brain barrier in immature and young rats. Bioelectromagnetics 26:578–588.
Liebner, S., Fischmann, A., Rascher, G., Duffner, F., Grote, E. H., Kalbacher, H., and Wolburg, H. (2000). Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. (Berl.) 100:323–331.
Lippoldt, A., Liebner, S., Andbjer, B., Kalbacher, H., Wolburg, H., Haller, H., and Fuxe, K. (2000). Organization of choroid plexus epithelial and endothelial cell tight junctions and regulation of claudin-1, -2 and -5 expression by protein kinase, C. Neuroreport 11:1427–1431.
Miyashita, K., Itoh, H., Sawada, N., Fukunaga, Y., Sone, M., Yamahara, K., Yurugi-Kobayashi, T., Park, K., and Nakao, K. (2003a). Adrenomedullin provokes endothelial Akt activation and promotes vascular regeneration both in vitro and in vivo. FEBS Lett. 544:86–92.
Miyashita, K., Itoh, H., Sawada, N., Fukunaga, Y., Sone, M., Yamahara, K., Yurugi, T., and Nakao, K. (2003b). Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens. Res. 26(Suppl.):S93–98.
Morita, K., Furuse, M., Fujimoto, K., and Tsukita, S. (1999a). Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. U.S.A. 96:511–516.
Morita, K., Sasaki, H., Furuse, M., and Tsukita, S. (1999b). Endothelial claudin: claudin-5/ TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 147:185–194.
Nagoshi, Y., Kuwasako, K., Cao, Y. N., Imamura, T., Kitamura, K., and Eto, T. (2004). Tumor necrosis factor-α downregulates adrenomedullin receptors in human coronary artery smooth muscle cells. Peptides 25:1115–1121.
Serrano, J., Alonso, D., Encinas, J. M., Lopez, J. C., Fernandez, A. P., Castro-Blanco, S., Fernandez- Vizarra, P., Richart, A., Bentura, M. L., Santacana, M., Uttenthal, L. O., Cuttitta, F., Rodrigo, J., and Martinez, A. (2002). Adrenomedullin expression is up-regulated by ischemia-reperfusion in the cerebral cortex of the adult rat. Neuroscience 109:717–731.
Suzuki, Y., Horio, T., Nonogi, H., Hayashi, T., Kitamura, K., Eto, T., Kangawa, K., and Kawano, Y. (2004). Adrenomedullin as a sensitive marker for coronary and peripheral arterial complications in patients with atherosclerotic risks. Peptides 25:1321–1326.
Tahan, V., Avsar, E., Karaca, C., Uslu, E., Eren, F., Aydin, S., Uzun, H., Hamazaoglu, H. O., Besisik, F., Kalayci, C., Okten, A., and Tozun, N. (2003). Adrenomedullin in cirrhotic and non-cirrhotic portal hypertension. World J. Gastroenterol. 9:2325–2327.
Terata, K., Miura, H., Liu, Y., Loberiza, F., and Gutterman, D. D. (2000). Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K(+). channels. Am. J. Physiol. Heart Circ. Physiol. 279:H2620–2626.
Tsukita, S., and Furuse, M. (1999). Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 9:268–273 Review.
Wijdicks, E. F. M., Heublein, D. M., and Burnett Jr., J. C. (2001). Increase and uncoupling of adrenomedullin from the natriuretic peptide system in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 94:252–256.
Wolburg, H., and Lippoldt, A. (2002). Tight junctions of the blood–brain barrier: development, composition and regulation. Vasc. Pharmacol. 38:323–337 Review.
Yi, X., Wang, Y., and Yu, F. S. (2000). Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol. Vis. Sci. 41:4093–4100.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honda, M., Nakagawa, S., Hayashi, K. et al. Adrenomedullin Improves the Blood–Brain Barrier Function Through the Expression of Claudin-5. Cell Mol Neurobiol 26, 109–118 (2006). https://doi.org/10.1007/s10571-006-9028-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-006-9028-x