Abstract
In this study, the preparation methods, types, and developments of cellulose aerogels are comprehensively reviewed and evaluated. Aerogels can be prepared from synthetic or natural organic or inorganic materials. However, cellulose-based aerogels have gained tremendous attention in the past few years because of their biodegradability, abundance, and nontoxicity. In this study, the extraction of cellulose from various sources using different methods is discussed. Moreover, preparation of nanocellulose materials using different processes is reviewed. The different types of solvents used to dissolve cellulose and the various drying methods employed in the preparation of cellulosic aerogels are reviewed critically with a focus on green methods. The recent developments in hybrid aerogels for various applications are also discussed. In addition, the areas requiring further investigation are highlighted, such as the effect of the cellulose source on aerogel properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
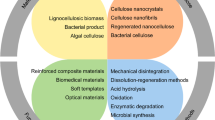
Aerogels are defined as ultralight materials (density below 0.05 g/cm3) with a high specific area (100–1000 m2/g) and high porosity (above 90%) (Rahmanian et al. 2021). Aerogels are initially produced by the sol-gel process. It involves the preparation of a gel at a low temperature. However, aerogels are prepared using drying techniques other than the conventional evaporation drying of the gel, such as supercritical drying or freeze-drying. This enables the material to retain its porous characteristic during the wet gel phase and maintain its unique properties such as the high porosity, high specific surface area, low density, low thermal conductivity, low dielectric constant, and special three-dimensional network structure (Pierre and Pajonk 2002; Chen et al. 2016; Wu et al. 2018; Wan et al. 2019; Garemark et al. 2020). The different types of aerogels are denoted by various technical terms based on the drying method. For example, aerogel is used to represent the material prepared by supercritical drying, whereas those prepared by freeze-drying are denoted as cryogels. In contrast, the oven-dried materials are named xerogels (Rahmanian et al. 2021). Another terminology used to distinguish aerogels from foams is based on the pore size and porosity. The materials with a pore size larger than 50 nm and porosity of at most 50% are generally described as foams. Meanwhile, materials with a porosity of at least 90% and pore size of 2–50 nm are called aerogels or mesoporous solid material (Lavoine and Bergström 2017). The potential applications of aerogels include thermal insulation, oil–water separation, CO2 capture, catalysis, and medicine (Zhao et al. 2018). The other applications include biomedical scaffolds, thermal devices for energy storage and generation, superabsorbents, supercapacitors, and aerospace-related applications (Chen et al. 2016; Lavoine and Bergström 2017; Wan et al. 2019). Kistler developed the first aerogel in 1931 by drying silica gel with supercritical CO2 (Kistler 1931). Since then, aerogels have mainly been applied for thermal insulation owing to the exceptionally low thermal conductivity obtained by silica aerogels (0.012–0.014 W/(m K) compared with that for air (0.025 W/(m K) (Budtova 2019). For example, the NASA Kennedy Space Center worked on developing silica aerogels for space launch applications owing to their high thermal insulation property (which is required for exceptionally cold environments in space) (Fesmire 2006). The key drawbacks of silica-based aerogels are their inferior mechanical properties and brittleness. These have motivated researchers to examine other options while maintaining the desired properties of aerogels (Novak et al. 1994). The second generation of aerogels was developed in the 1970 and 1980 s. These are composed of metal oxides such as titanium, zirconium, and aluminum, and synthetic polymers such as resorcinol–formaldehyde, polyurethane, polyimide, and the hybrids of all the previously mentioned materials with silica (Budtova 2019). Several polymer aerogels achieved better mechanical properties and exhibited superior electrochemical characteristics compared with silica aerogels, when pyrolyzed (Budtova 2019). Nonetheless, the key problem with the aforementioned class of aerogels is that these are derived from nonrenewable sources and generate large amounts of waste. This can be a massive challenge owing to the tedious process of aerogel fabrication (Gupta et al. 2018). A third class of aerogels termed bio-aerogels emerged in the 2000s. These are primarily made of polysaccharides and are based on biomass such as starch, chitosan, pectin, cellulose, and cellulose derivatives (Chen et al. 2016; Budtova 2019). Their production is similar to that of conventional aerogels. It starts with polymer dissolution and progresses to solution gelation (which can be omitted in certain cases). This is followed by solvent exchange and drying using supercritical carbon dioxide, freeze drying, or conventional oven drying. Bio-aerogels do not break straightforwardly under compression, with plastic deformation attaining 80% of the strain before pore-wall collapse. This is in contrast to silica aerogels, which are significantly brittle (Sescousse et al. 2011; Rudaz et al. 2014; Pircher et al. 2016). Bio = aerogels have a relatively large specific surface area and low density. Although the second parameter appears to be influenced substantially by the polysaccharide type, the causes and mechanism are unclear (Budtova 2019). The major advantage of bio-based aerogels is that these are sustainable and renewable and are not environmental hazards. Cellulosic aerogels are among the most prominent types of organic bio = aerogels that have been growing rapidly in recent years (Lavoine and Bergström 2017; Gupta et al. 2018; Rahmanian et al. 2021) Other types of bio-aerogels that are currently being developed and do not originate from cellulose include protein- and chitin-based aerogels (Long et al. 2018; Liu et al. 2021c). The preparation procedure for chitin and protein-based aerogels has been reviewed recently (Seantier et al. 2016; Illera et al. 2018). However, chitin-based aerogels including chitosan and nanochitin involve the problem of the low availability of the chitin raw material and an expensive and time-consuming extraction process. In addition, chitin-based aerogels are brittle and fragile, and display a low water resistance and limited solubility (Jackcina Stobel Christy et al. 2020). Compared with other types of bio-aerogels, cellulose-derived aerogels are characterized by the high readiness in the availability of cellulose. Cellulose aerogels are a novel category of sustainable and eco-friendly materials that provide several advantages over conventional inorganic and synthetic polymer-based aerogels. These display exceptional porosities (ranging from 84.0 to 99.9%), significantly large specific surface areas (10–975 m2/g), remarkably low densities (ranging from 0.0005 to 0.35 g/cm3), and biocompatibility. In addition, cellulose aerogels exhibit superior compressive strength (ranging from 5.2 to 16.67 MPa) and biodegradability compared with their counterparts. (Long et al. 2018). Cellulose aerogels are eco-friendly functional materials with a substantial potential for many disciplines including catalysis, air purification, wastewater treatment, thermal insulation, and medicine. Because of their remarkable biocompatibility, degradability, and nontoxicity, cellulose-based aerogels are exceptionally important for drug delivery (Liu et al. 2021c). Cellulose aerogels are studied extensively for thermal insulation, because of their reported low thermal conductivity that reaches down to 0.038 W/m K, and low diffusivity of 0.341 mm2/s. moreover, Cellulose aerogels are thoroughly studied for carbon capture, due to their high sorption rates that ranges between 3.8 and 28 mmol/g. Furthermore, cellulose aerogels are explored for electric devices and energy storages, where some types of cellulose aerogels achieved specific capacitance up to 916 F/g (Zaman et al. 2020a). There are several advantages of using cellulose and its derivatives as precursors for developing cellulose aerogels. First, cellulose is extracted from various sources and is the most prevalent renewable biopolymer. Second, few crosslinking agents are required to manufacture aerogels because cellulose chains contain many hydroxyl groups. Cellulose hydroxyl groups can be used to physically cross-link the hydrogen bonds between molecules to generate a stable 3D network structure (Long et al. 2018). This enables the creation of cellulose aerogels. Finally, a chemical alteration or physical blending of cellulose facilitates the enhancement of the structural properties of cellulose aerogels (Liu et al. 2021c). Cellulose aerogels are prepared using three methods, as illustrated in Fig. 1. In the first process (labeled (a), native cellulose nanomaterial (cellulose I) is suspended and dispersed in water rather than being dissolved. Heavy mechanical stirring combined with ultrasonication is applied to achieve a homogeneous dispersion of the cellulose particles. For example, this process was applied by Gupta et al. (2018) and Seantier et al. (Seantier et al. 2016). The second method is widely used. It involves the dissolution of cellulose material in a suitable solvent followed by solvent regeneration. In this process, cellulose I is converted into regenerated cellulose II, which possess higher mesopores and surface area due to this solubilization-regeneration process (Zhang et al. 2020a). Several solvents are used. These are discussed in detail subsequently. In the third process (c), the cellulose derivatives are combined by crosslinking to produce a cellulose derivative gel. For example, polyfunctional carboxylic acid was used to crosslink hydroxy propyl methyl cellulose, to reduce the water sensitivity for packaging materials that are based on cellulose derivates (Coma et al. 2003). The crosslinking was successful in reducing the water sensitivity and increasing the water vapor barrier. Moreover, another study used copper to crosslink carboxymethyl cellulose, to produce hydrogels for controlled release of fertilizers (Basta et al. 2021). The copper-crosslinked hydrogel achieved much higher swelling compared with other gels produced from biopolymers (trimethyl chitosan). Furthermore, cobalt chloride was also used to crosslink carboxymethyl cellulose (Abdel-Hadi et al. 1994). The final step for all the methods is drying using different drying techniques.
The three methods of preparing cellulose aerogels, a through dispersion of nanocellulose particles in water, followed by drying, b by dissolving cellulose particles in a suitable solvent to form a solution/gel, followed by regeneration of solvent and drying, and c by cellulose derivatives combined with crosslinking to from gel, and finally drying. This figure was regenerated based on a figure from Liu et al. (2021c)
In this study, the overall process of cellulose aerogels preparation is reviewed, in addition to cellulose hybrid aerogels and their application. To begin with, cellulose extraction from biomass sources using different techniques and the specifications of the obtained cellulose forms are discussed and summarized. Subsequently, the cellulose aerogel processes are discussed in detail, with the different solvents used to dissolve cellulose particles, solvent exchange and regeneration steps, and drying steps using different drying methods. The specifications of the cellulose aerogels produced using these methods at each step are compared. Finally, the cellulose aerogel hybrids and composites and their applications are reviewed.
Cellulose resources and extraction methods
Cellulose extraction from biomass
Cellulose is the most abundant biopolymer on our planet. It has desirable features such as biodegradability, sustainability, and biocompatibility. Its estimated annual production is 1.5 × 1012 tons (Illera et al. 2018; Sen et al. 2022). Biomass is generally referred to as a lignocellulosic material because it consists of three main biopolymers: cellulose, hemicellulose, and lignin (Raza and Abu-Jdayil 2022). Cellulose is the backbone and main component of biomass (Chen et al. 2016). It can be extracted from various biomasses such as cotton stalk, okra stalk, bean stalk, pepper stalk, wheat straw, date palm leaves, rice straw, potato tubers, bagasse, and cannabis (Long et al. 2018; Raza and Abu-Jdayil 2022). Cellulose can be separated from the biomass through chemical processes that remove hemicellulose, lignin, and other impurities (Raza and Abu-Jdayil 2022). The process of lignin removal is termed delignification. A common chemical treatment for delignification is alkali treatment. Here, wood fibers are treated with solutions of different concentrations of sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2) (Singh et al. 2014). This is followed by bleaching, known as wood pulping, to remove lignin and hemicellulose (Raza and Abu-Jdayil 2022). However, bleaching can be begun before delignification. This renders the two steps interchangeable. For example, in the studies by Alothman et al. (2021) and Raza et al. (2022), bleaching was performed before delignification.
Nanocellulose preparation methods
Different forms of cellulose can be obtained by chemical treatment. The familiar cellulose fibers have a diameter of 20–50 mm and length of 1–4 mm. These consist of smaller forms called cellulose macrofibrils, with diameters of 15–60 nm. A further analysis of the molecular structure would indicate that the cellulose macrofibrils consist of smaller units called cellulose nanofibrils (CNFs). These have a diameter of 3–4 nm and length of 1–2 mm. the highly crystalline and rigid nanoparticles remaining after processing are CNCs (Lavoine and Bergström 2017).
After separating the cellulose fibers, the final step to obtain nanocellulose is acid hydrolysis. Here, acidic chemicals such as sulfuric acid, hydrochloric acid, or acetic acid are used to obtain CNFs or CNCs (Yu et al. 2013; Sen et al. 2022; Basta and Lotfy 2023). Moreover, Ammonium Persulphate is another chemical that is used to obtain nanocellulose material (Filipova et al. 2018). Another technique used after bleaching (rather than acid hydrolysis to convert cellulose into CNFs/CNCs) is 2,2,6,6-tetramethylpiperidine-1-oxylradical (TEMPO)-mediated oxidation followed by mild disintegration in water (Isogai et al. 2011). Several approaches can be employed to oxidize cellulose using TEMPO. One involves dissolving catalytic amounts of TEMPO and sodium bromide (NaBr) in water at a pH of 10–11, and adding sodium hypochlorite (NaClO) as the main oxidant (Isogai et al. 2011). When cellulose is oxidized with TEMPO, the primary hydroxyls on cellulose are converted to carboxylates. This produces repulsive forces between the fibrils, thereby facilitating fibrillation (Liu et al. 2018). Oxidized cellulose maintains its fibrous morphology and crystalline structure (Isogai and Zhou 2019). An alternative to TEMPO/NaBr/NaClO is the application of a TEMPO/NaClO/NaClO2 mixture in water at pH 4.8–6.8 at elevated temperatures for a period of one–three days (Isogai and Zhou 2019).
Nonetheless, owing to the hazardous characteristic of acids, environmental awareness, difficulty in controlling reactions involving TEMPO, and relatively low carboxyl content of CNCs (Yang et al. 2019), ionic liquids have emerged as green and effective solvents for nanocellulose extraction. Ionic liquids (ILs) are types of salts that have melting points below 100 °C. These consist of bulky organic cations and organic or inorganic anions that may also be bulky (Glińska et al. 2021). Several studies have investigated various ionic liquids for cellulose extraction from biomass, as alternatives to volatile and hazardous organic solvents and acids for cellulose extraction from biomass. In a study by Glinska et al., five ionic liquids, three tetra-alkyl-phosphonium-based and two imidazolium-based, were used to dissolve and obtain cellulose from corn stover (Glińska et al. 2021). Imidazolium ILs form microemulsions that can be separated conveniently. This makes them good candidates for recovery. These are also highly effective in removing ash from stover. Phosphonium ILs are more suitable for recovering cellulose. However, these cannot remove ash from corn stover. Two reviews have been published in recent years on the recent progress in nanocellulose extraction and cellulose processing using ionic liquids, including the synthesis of ionic liquids and the properties of the obtained cellulose (Hermanutz et al. 2019; Shamsuri et al. 2022).
Several studies have examined different techniques and chemical treatments for nanocellulose production. Table 1 summarizes these techniques and the reported outcomes including the particle size and yield. The use of hydrochloric acid (HCl) for acid hydrolysis provided higher yields (entry 8) than that of other acids such as sulfuric acid (entries 2, 5, 6 and 7) and acetic acid with nitric acid (HNO3) (entry 1). The compared entries used the same delignification reagent (i.e., NaOH). However, the biomass material and bleaching reagent were not necessarily the same. Nevertheless, this table is not exhaustive and is inconclusive because numerous studies have examined the extraction of nanocellulose, which is not addressed here. This table lists the commonly used state-of-the-art techniques and their results.
Cellulose-based aerogels: preparation methods
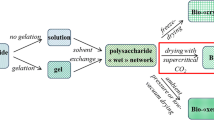
Cellulose aerogels are remarkable materials with unique features and characteristics such as ultralow density, ultralow thermal conductivity, high porosity, and high surface area. These are prepared by dissolving cellulose in a solution. The next step is the gelation or sol-gel process. It can be omitted when the solution (no gelation) route is used, as shown in Fig. 2. Unlike synthetic or inorganic aerogels, the starting material in cellulosic aerogels is a solution of “ready” polymer rather than a colloidal suspension or solution of monomers. The prepared polymeric chains are combined to form a three-dimensional, linked network that resembles a sponge and is more commonly referred to as a gel. Herein, the clusters are filled with different materials (generally a liquid) (Zaman et al. 2020b). Subsequently, the material undergoes solvent exchange to induce non-solvent phase separation and produce cellulose II hydrogel. Coagulation is typically performed with alcohol or water (Zhang et al. 2017). Finally, the solvent is replaced with air in the drying step to maintain the porous structure and produce aerogels (Budtova 2019; Dahlem et al. 2019). Figure 2 shows the process of preparing the polysaccharide (including cellulose) aerogels. Moreover, samples of different types of aerogels produced using different drying methods are presented. The potential of cellulose aerogels has been studied for several applications including thermal insulation (Gupta et al. 2018), food packaging (de Oliveira et al. 2019), energy storage devices (Liu et al. 2021a), oil absorption (Feng et al. 2015), water purification, dye removal, heavy metal removal (Ganesamoorthy et al. 2021), and biomedical applications (Abdul Khalil et al. 2020).
Nonetheless, nanocellulose aerogels can be prepared without dissolving cellulose in a solvent. Rather, these can be dispersed in water to form a cellulose suspension. In this method, naturally occurring cellulose I nanomaterials (CNCs and CNFs) are used to prepare cellulose suspensions in water (Liao et al. 2019). This method is combined with ultrasonication and mechanical stirring to achieve a homogeneous dispersion. This is followed by drying. It occasionally includes a solvent exchange before the drying step (Long et al. 2018). The third method to prepare cellulose aerogels is using cellulose derivatives. In this method, surface modification is performed to functionalize cellulose and alter its physical and chemical properties. The solubility of cellulose derivatives in water or specific organic solvents facilitates their gelation. This makes these highly suitable for this purpose. Cellulose acetate (CA) can dissolve in acetone, dichloromethane, dimethylacetamide, and dimethylformamide. Carboxymethyl cellulose (CMC) and hydroxypropyl methyl cellulose (HPMC) dissolve straightforwardly in water. Triacetyl cellulose (TAC) is soluble in a mixture of dioxane and isopropanol (Liu et al. 2021c). Because of its self-association and remarkable solubility in water and certain organic solvents, CMC produced by carboxymethylation is one of the most frequently employed cellulose derivatives to produce foams and aerogels. In addition, the functional groups on CMC can facilitate the sol-gel process and can be crosslinked by a few crosslinkers. This enables the creation of foams and aerogels with a high mechanical strength (Jiang and Hsieh 2014a, b, 2017).
Moreover, it is important to consider the effects of cellulose on the aerogel properties. Rostamitabar et al. prepared cellulose aerogels using high- and low-molecular-weight microcrystalline cellulose after dissolution in a calcium thiocyanate tetrahydrate solution. It was observed that cellulose aerogels obtained from cellulose with a higher molecular weight and lower crystallinity had a higher surface area, denser structure, and finer nanofibrils with better thermal stability than aerogels obtained from cellulose with a lower molecular weight and higher crystallinity. For example, aerogels with high molecular weights have a surface area of approximately 197 m2/g and porosity of 71%, whereas aerogels with low molecular weights have 85 m2/g and 77%, respectively (Rostamitabar et al. 2021). This indicates that the source of cellulose used to prepare cellulose aerogels can significantly affect their properties. Different sources of cellulose can produce cellulose with various structures and chemical properties even when the same extraction and preparation methods are used (Yu et al. 2021). The selection of the cellulose source can also affect the production process and overall cost of aerogels because different biomasses have different proportions of cellulose (Chai et al. 2022). Therefore, the effect of the cellulose source on aerogels needs to be investigated further because there are few studies on this matter.
Cellulose aerogels production process, with sample shapes of the cellulose precursor, and the final aerogels after the three drying techniques (Budtova 2019)
Cellulose treatment
Nanocellulose suspensions
A method to obtain cellulose hydrogels that are subsequently freeze-dried or supercritically dried is to generate a suspension of nanocellulose in water or other organic solvents and then, crosslink it physically or chemically. If CNFs are suspended in water, these can create a 3D structure through the combination of hydrogen bonds and entanglement of long fibrils. These nanofibrils function as the core framework of the network and improve the strength and modulus of the shaped gel. Consequently, the gel retains its shape during the drying process. This enables the resulting aerogel to maintain its structural integrity even at a low concentration of solid nanocellulose (Chen et al. 2021). Several studies have investigated different crosslinking techniques, both chemical and physical, during the gelation step after preparing nanocellulose suspensions in water. Several chemicals are used to crosslink the suspension and form gel. These include methylene diphenyl diisocyanate (MDI) (Jiang and Hsieh 2017), citric acid (CiA) (Berglund et al. 2018), nitric acid (NA) (de Morais Zanata et al. 2018), poly(methyl vinyl ether-co-maleic acid) (PMVEMA) and poly(ethylene glycol) (PEG) (Liang et al. 2020), polyethylene imine (PEI) with glutaraldehyde (Hong et al. 2021), polyvinyl alcohol (PVA) (Zheng et al. 2014), poly (acrylic acid) (PAA) (Zhou and Hsieh 2020), styrene acrylic emulsion (SAE) (Gong et al. 2021), polyamideamine-epichlorohydrin (PAE) (Dilamian and Noroozi 2021), methyltrimethoxysilane (MTMS) (Jiang et al. 2020), polyethylene imine (PEI) with 1,4-butanediol diglycidyl ether (Chatterjee et al. 2020), hexamethylene diisocyanate (HDI), hexamethylene diisocyanate biuret (HB) with hexamethylene diisocyanate trimer (HT) (Zhu et al. 2019), and adipic acid dihydrazide (ADH) (Ma et al. 2017). Aerogels obtained from the abovementioned crosslinkers display superior properties such as high porosity, low density, high absorbing capability, and low thermal conductivity. The recently developed aerogels obtained through dispersion in water and crosslinking using different chemicals are summarized in Table 2.
–*Not mentioned
Cellulose dissolution
This is another method of preparing aerogels by dissolving cellulose in a solvent. To obtain the gel shape, cellulose should first be dissolved in a solvent to produce a gel. This is the starting point of aerogel production. However, cellulose does not display thermoplastic polymeric properties. That is, it is not a melting polymer and degrades before melting (Zaman et al. 2020b). It has a well-defined structure with high crystallinity and high inter-and intra-molecular hydrogen bonds formed through hydroxyl groups. These characteristics prevent organic and inorganic solvents from penetrating and dissolving the cellulose chains (Zaman et al. 2020b). Another factor that hinders cellulose from dissolving is its long chains. These cause a substantial reduction in entropy and prevent dissolution (Navard et al. 2013). For cellulose disintegration, the solvent should be capable of diffusing into cellulosic chains and separating their crystalline and amorphous regions. It should directly attack the strong intramolecular bonds (hydrogen bonds and hydrophobicity) inside the crystalline regions and separate the amorphous regions from one another (Zaman et al. 2020b). Therefore, to release or untangle the cellulosic chains, an active solvent that dissolves cellulose requires a high diffusivity and disruption of the crystalline network. Figure 3 illustrates the cellulose dissolution process.
The main process through which cellulose dissolves occurs at the cellulose–solvent contact. a Cellulosic solid phase in contact with solvent, b solid phase swelling, c point of disentanglement, d cellulosic chains moving from swollen phase to solvent, and e solubilization advanced inside the solid material. Extracted from reference (Navard et al. 2013) and edited
The complex structure and characteristic of cellulose prevents it from dissolving in common solvents. Therefore, complex solvents are used (Zhang et al. 2017). Different types of solvents were studied for dissolving cellulose. These can generally be divided into two main groups: non-derivatizing and derivatizing. The first type does not form bonds with cellulose, whereas the other produces cellulose derivatives before dissolving these. Another classification of cellulose solvents is into aqueous and non-aqueous solvents (Zaman et al. 2020b). A limited number of solvents are used to dissolve cellulose. These include a common solvent for cellulose used in cellulosic aerogel preparation, namely, N-methylmorpholine-N-oxide (NMMO) (Long et al. 2018). Other complex solvents such as N,N-dimethylacetamide/lithium chloride (DMAc/LiCl), N,N-dimethylformamide/dinitrogen tetroxide (DMF/N2O4), and dimethyl sulfoxide/tetrabutylammonium fluoride (DMSO/TBAF) have also been used to dissolve cellulose (Zhang et al. 2017). Molten salt hydrates such as zinc chloride (ZnCl2·6H2O) and two forms of calcium thiocyanate (Ca(SCN)2·6H2O and Ca(SCN)2-8H2O/LiCl) have been used for this purpose (Budtova 2019). LiClO4·3H2O and LiSCN·2H2O are also considered to be good solvents for cellulose (Xu et al. 2010). Moreover, Lithium Bromide Trihydrate (LBTH) successfully dissolved cellulose in the work of Liao et al. (Liao et al. 2019). LBTH, which has a chemical formula of LiBr·6H2O, was used to create aerogels from a whole biomass of Douglas fir wood including cellulose, hemicellulose, and lignin. The dissolution of the biomass required an elevated temperature of 135 or 145 °C for the solvent. Two approaches were used to produce aerogels containing lignin, and aerogels excluding lignin. Unlike cellulose and hemicellulose, the lignin did not dissolve in LBTH solvent. In the first approach, the solution was homogenized by stirring, then cooled down to room temperature to form hydrogel, followed by regeneration using water and alcohol, and finally freeze dried to obtain lignin-including aerogels. On the other hand, to produce lignin-excluding aerogels, the solution prepared after dissolving the biomass in LBTH was filtered to remove lignin and undissolved particles, then it was followed by the same procedure for producing lignin-including aerogels. It is important to highlight that no gelation step was required in this process, since the hydrogel was formed simply by cooling down the solution to room temperature after dissolving the biomass. Nonetheless, these solvents have key disadvantages such as the toxicity, high cost, harsh processing conditions, and difficulty in solvent regeneration (Xu et al. 2010).
Therefore, other types of solvents such as ionic liquids and alkali solutions have been synthesized and studied. NaOH and LiOH solutions of different concentrations are used to dissolve the cellulose (Cai et al. 2008). For example, cellulose crystals are dissolved in an NaOH solution with a concentration corresponding to 7.6 g of NaOH in 100 g of cellulose/water/NaOH mixture (Gavillon and Budtova 2008). However, the dissolution occurs only at low temperatures (− 10–5 °C). In the cited study, the mixing was performed at − 6 °C. Moreover, cellulose dissolves in aqueous NaOH/urea and LiOH/urea systems (Cai et al. 2008). The cellulose gel was prepared by dissolving cellulose in an NaOH/thiourea/urea solution containing 9 wt% NaOH, 6.5 wt% thiourea, and 6 wt% urea (Yang et al. 2017).
Ionic liquids are a unique class of green solvents with several advantages such as chemical and thermal stability, non-flammability, and non-detectable vapor pressures (Xu et al. 2010). Therefore, several studies have investigated the capability of ionic liquids to dissolve cellulose (Zaman et al. 2020b). The first study to examine ionic liquids for dissolving cellulose was conducted in 2002 by Rogers et al. They synthesized 1-N-butyl-3-methylimidazolium chloride [C4mim]Cl or [Bmim]Cl. It was observed to be highly efficient in the dissolution of cellulose (Swatloski et al. 2002). However, they observed that unlike Cl, [BF4]– and [PF6]– anions produce inferior solvents. The research on the ionic liquid field continued to examine the possibilities in dissolving cellulose. Zhang et al. synthesized 1-N-allyl-3-methylimidazolium chloride [Amim]Cl in 2005. It appeared to be a remarkable solvent for cellulose without pretreatment (Zhang et al. 2005). Subsequently, 1-Ethyl-3-methylimidazolium acetate ([C2mim][OAc]) or [Emim]Ac was reported to be the best solvent for cellulose among ionic liquids (Bowron et al. 2010). Moreover, 1-N-butyl-3-methylimidazolium cation [C4mim]+ or [Bmim]+ was coupled with Brønsted basic anions [CH3COO]–, [HSCH2COO]–, [HCOO]–,[(C6H5]COO]–, [H2NCH2COO]–, [HOCH2COO]–, [CH3CHOHCOO]–, and [N(CN)2]– to synthesize ionic liquids that dissolves cellulose (Xu et al. 2010). It was determined that the solubility of cellulose in the prepared ionic liquids is a function of the temperature. The solubility increases with an increase in the temperature. Moreover, when 1 wt% lithium salt (LiCl, LiBr, LiAc, LiNO3, or LiClO4) was added to [C4mim][CH3COO], the solubility of cellulose increased. The cellulose produced showed a high thermal stability (Xu et al. 2010). Different solvents produced aerogels with different properties (Long et al. 2018). However, in terms of solubility, the use of DMSO combined with trihydrate ionic liquids (namely, tetra-n-butylammonium fluoride (TBAF) and tetrabutylammonium hydroxide (TBAH) afforded the highest solubilities of 100 and 95%, respectively (Östlund et al. 2009; Cao et al. 2018). The selection of a solvent is a critical aspect in the process of aerogel production.
Imidazolium-based ionic liquids (ILs) are currently considered highly potential solvents for cellulose and have been the subject of extensive research. These ILs display a range of desirable characteristics such as nonvolatility, a high solvency power for cellulose, and exceptional thermal stability. These are superior to those of other cellulose solvents and cellulose. [Bmim]Cl and [Emim]Ac are widely considered at present for direct dissolution of cellulose. It has the capacity to dissolve cellulose up to approximately 15–20% (Sescousse et al. 2011). Cellulose aerogels were prepared using the two mentioned ILs. These yielded highly similar results in dissolving cellulose, providing aerogels with a surface area of approximately 150–200 m2/g and density of 0.06–0.20 g/cm3. Cellulose was dissolved in a mixture of DMSO and [Emim]Ac (60/40 wt. /wt%) with a (cellulose) concentration of 3–11 wt% to prepare aerogels (Buchtová and Budtova 2016a). The characteristics of the produced aerogels depended on the drying method, as illustrated subsequently. Bamboo dissolved pulp and cotton were dissolved in [Amim]Cl to obtain aerogels with a density of 0.034 g/cm3 and porosity of 98.5%, as reported earlier (Zhang et al. 2016). A survey of the capability of different ionic liquids to dissolve cellulose was conducted to determine the conditions and maximum weight% of possible dissolved cellulose (Pinkert et al. 2009). It was determined that 1,3-dimethylimidazolium, [Mmim]MeSO4 and [Bmim]CF3SO3 achieved the highest capacities with over 50 wt% of dissolved cellulose within 24 h of mixing under 100 °C. [Bmim]Cl achieved a maximum concentration of 25 wt% under microwave heating and approximately 20 wt% under 100 °C for 2 h. [Emim]Ac and [Amim]Cl achieved the maximum adsorption capacities at 30 wt% under 90 °C with 24 h of mixing.
Nonetheless, in the work of Le et al. (2012), the effect of adding water and diluting the ionic liquid on the dissolution of cellulose was studied. The dissolution of cellulose decreases when the water content exceeds 15 wt% in the solvent mixture of water and [Emim]Ac. Therefore, aqueous solutions of ionic liquids cannot be used as diluted solvents to dissolve cellulose. This can be a challenge for this purpose because of their relatively high cost. However, this challenge can be resolved using other materials as co-solvents to ionic liquids to dissolve cellulose. The incorporation of co-solvents with polar and aprotic properties, such as DMAc, DMSO, and DMF, into ILs can increase their capability to dissolve substances by reducing the viscosity of the solvent. This would, in turn, improve mass transportation. This effect was achieved without significantly altering the specific interactions between cations and anions or between the ILs and cellulose (Zhang et al. 2017). DMSO was used as a co-solvent with 1-ethyl-3-methylimidazolium diethyl phosphate [Emim]DEP to dissolve microcrystalline cellulose into high stiffness cellulose fibers. It was observed that the optimal ratio was 7:3 [Emim]DEP/DMSO (Zhu et al. 2018). Mussana et al. prepared aerogels from lignocellulose extracted from cotton stalks using a mixture of 50:50 [Amim]Cl and DMSO (Mussana et al. 2018). Cellulose was dissolved in 5, 7, 9, or 11 wt%. The maximum surface area was of the 11 wt% system with 102 m2/g. Meanwhile, the highest porosity achieved was 96.8%.
DMSO is an affordable, nontoxic, polar aprotic solvent that can be mixed with a variety of solvents including ionic liquids. As a co-solvent, it can expedite the dissolution of cellulose solutions in ILs by reducing the dissolution time, temperature, and viscosity without causing precipitation. By adding inexpensive DMSO to the mixture, the overall cost of the cellulose solvent can be reduced by decreasing the amount of ionic liquid required for dissolution (Zhu et al. 2018). An outstanding study on the effect of water and DMSO and co-solvents beside [Emim]Ac for dissolving cellulose, in which phase diagrams for cellulose-water-[Emim]Ac and cellulose-DMSO-[Emim]Ac were constructed (Le et al. 2014). The effect of water on the solubility of cellulose in [Emim]Ac is significantly higher than that in DMSO. [Emim]Ac and water are involved in hydrogen bonding. Therefore, these compete with cellulose for use as ionic liquid (Le et al. 2014). However, as illustrated in Fig. 4a, when pure [Emim]Ac (i.e., at a DMSO wt% of zero) was used, the maximum amount of cellulose to be dissolved was 25–27 wt%. Moreover, a correlation was established for the [Emim]Ac/DMSO mixture. Herein, it was verified that a minimum of 2.5–3 moles of ionic liquids is required to dissolve 1 anhydroglucose unit (AGU). Even at high DMSO contents of up to 90 wt%, dissolving approximately 5 wt% of cellulose is feasible. In contrast, the maximum feasible ratio of water to dissolved cellulose in the IL/water mixture was approximately 15 wt% (as illustrated in Fig. 4b). Furthermore, even at a low IL/water ratio, the cellulose weight% that the mixture could dissolve was significantly low compared with that for the IL/DMSO composition. The aerogels prepared through direct dissolution in different solvents and their physical characteristics are summarized in Table 3.
Nevertheless, there are few studies on the effects of using other polar solvents such as DMF and DMA as co-solvents with ionic liquids for aerogel preparation. The preparation of aerogel monoliths using [Bmim]Cl and DMF as co-solvent and was studied by Lin and Jana (2021). The aerogels exhibited superior properties, with porosities between 96.4 and 99%, densities between 0.0156 and 0.0548 g/cm3, and specific surface areas between 336 and 358 m2/g. However, the study was performed on a narrow range of cellulose weight percentages (between 1 and 4 wt%).
The phase diagram illustrates the cellulose concentration in EmimAc–DMSO and EmimAc-water. The solid line represents the highest achievable concentration of cellulose that can be dissolved in each solvent: a EmimAc–DMSO and b EmimAc–water. The solid line functions as a visual reference for the approximate maximum limit of cellulose solubility in the respective solvents (Le et al. 2014)
Cellulose gelation
After the treatment of cellulose through suspension (3.1.1) or dissolution (3.1.2), gelation is performed. However, it is important to note that in the case of aerogels derived from polysaccharides, a separate gelation process is not required, unlike in other organic or inorganic aerogels. Thus, it is feasible to produce aerogels when the substance is in a solution or gel prior to the solvent exchange, as illustrated in Fig. 2. A coagulated polysaccharide “wet” network (with non-solvent in the pores) is generated in both situations. However, the mechanisms by which the structure is generated vary. Non-solvent-induced phase separation occurs when a material is in solution prior to the solvent exchange. Another characteristic of polysaccharides is that although macromolecules experience certain volume contraction during the exchange of a solvent for another (although these are not gelled), these do not collapse completely. A 3D network is formed above the concentration of the polymer overlap, producing stable polysaccharide networks and chain stiffness (Budtova 2019). In contrast, the aerogel network structure was formed in the gel state prior to the solvent exchange. This is evident in cases such as alginate or pectin (which are cross-linked with polyvalent metal ions) or aged cellulose treated with a mixture of 7–9% NaOH and water (Budtova 2019). Unlike most polysaccharide-based aerogels, till date, cellulose aerogels have been produced by a non-solvent-induced phase separation method without the need for physical or chemical gelation. The gelation process for cellulose solutions is more challenging than that for other polysaccharides such as alginate, pectin, or carrageenan (which can undergo gelation by altering the pH of the solution or introducing metal ions). Similarly, aqueous starch pastes form gels via retrogradation (Zaman et al. 2020b). Except for cellulose-(7–9)%NaOH/water, cellulose solutions are not gelled straightforwardly. Thus, the cellulose aerogel precursor is typically stable during the solvent–non-solvent exchange. Monoliths are created either by gelled solutions (in this case, cellulose/8%NaOH/water), alkali/water (NaOH or LiOH added with urea and/or ZnO), LBTH solution, or a direct solvent–non-solvent exchange when the used cellulose solvents are ionic liquids. Unlike NaOH/water-based solvents, ILs enable the dissolution of cellulose at a wide range of concentrations and molecular weights. At normal temperatures, certain ionic liquids such as 1-butyl-3-methylimidazolium chloride [Bmim][Cl] and their cellulose solutions are solids (Budtova 2019).
Regeneration
Regeneration is a crucial phase in the fabrication of cellulosic aerogels. In this process, the intramolecular and intermolecular hydrogen bonds between the cellulosic chains are reconstructed when the solvent used to dissolve cellulose is removed (desolvation) or replaced by a non-solvent such as water. One or more soluble species are net transferred from one liquid phase to another (generally water (polar) or an organic solvent (nonpolar) (Zaman et al. 2020b). It is worth mentioning that the regeneration step is required only when a solvent is used to dissolve cellulose (rather than suspending the cellulose particles). The duration of the solvent exchange process in cellulose is influenced by various factors such as the quantity of cellulose, bath temperature, and shape of the sample. This process is characterized by a slow and diffusion-controlled mechanism. Here, the time required for the cellulose solvent to diffuse out and non-solvent to diffuse in depends on the aforementioned factors. The diffusion coefficient increased with the bath temperature and decreased with the cellulose concentration. Because of the microphase separation and pores that are significantly larger than the diffusing solvent molecule, the solvent exchange process for cellulosic gels is typically faster than that for cellulose solutions (Sescousse and Budtova 2009). Nevertheless, a significant shrinkage of the cellulose bodies was observed during the regeneration and solvent exchange phases. The selection of suitable pretreatment conditions helps control this behavior in certain cases (e.g., a higher cellulose concentration results in a lower shrinkage). When cellulose and a solvent interact, the molecules react relatively rapidly with the external polymer layer before penetrating the surface. This results in the immediate formation of a gel-like structure (Gupta et al. 2013). Water, which interacts with dissolved cellulose to form molecular chains and precipitates, is a non-solvent or coagulant bath used to continue the gelation process. The “wet” network, generally referred to as “cellulose hydrogels”, is then created with water-filled pores (Lindman et al. 2017). Many solvent properties of systems containing ionic liquids are affected by the presence of water. The experiments on cellulose/IL/non-solvent ternary systems have not produced conclusive results. It is still unclear how water, IL, and cellulose interact mechanistically (Medronho and Lindman 2015). The solvents used for regeneration include water (Wang et al. 2016a) and alcohols such as ethanol (Nguyen et al. 2014) and methanol (Jin et al. 2004). Regeneration was performed by pouring the solution/gel of the dissolved cellulose into molds and then, immersing these inside the regeneration bath (as illustrated in Fig. 5).
Nonetheless, certain studies have investigated the interaction between the cellulose solvent and the antisolvent used as a coagulation bath. Hedlund et al. (2019) compared water and 2-propanol (2PrOH) as coagulants for cellulose dissolved in [Emim]Ac and DMSO. The [Emim]/DMSO ratios were 99:1 and 50:50, respectively. The purpose was to dissolve the cellulose and obtain cellulose nanofibrils through regeneration. It was concluded that 2PrOH decreased the crystallinity of cellulose compared with water. Moreover, the higher the cellulose and DMSO concentrations in the solvent mixture, the lower is the crystalline order. Figure 6 shows different aerogels obtained using different solvents and those regenerated using different antisolvents.
Aerogels obtained from cellulose dissolved in [Emim]Ac/DMSO, coagulated in a water and b ethanol (Rudaz 2013). Aerogels obtained by dissolving cellulose in c NaOH/water (Gavillon and Budtova 2008), d DMSO/DBU-TMG-DBN (Onwukamike et al. 2019), e NaOH-Water (Sescousse and Budtova 2009), and f water with HT as crosslinker (A-1, A-2, etc., denote different cellulose ratios) (Zhu et al. 2019)
Drying
Drying is the final and most crucial step in the process of fabricating aerogels. It is applied regardless of the starting route for preparing the aerogels (Long et al. 2018; Budtova 2019). The main requirement for generating cellulosic aerogels is to prevent network collapse (a collapsed pore structure) that is caused by the bending of the air–liquid interface during the removal of the solvent or drying of wet gels (Zaman et al. 2020b). To prevent this, two drying processes are used: freeze-drying (FD) and supercritical drying (Lavoine and Bergström 2017).
Freeze drying is also known as ice templating, freeze casting, lyophilization, or cryodesiccation. It is a remarkable technique that rejects the solvent from wet gels while retaining the unique properties of low density, high porosity, and high surface area. In this process, the method includes three steps that achieve solvent rejection without generating a liquid/vapor interface. This prevents the creation of a capillary pressure that collapses the porous structure (Lavoine and Bergström 2017). In the first step, the temperature is reduced below the critical point of the liquid solvent. This causes the solvent to solidify. Subsequently, vacuum is applied to reduce the pressure in the second step. The final step is to elevate the temperature at the low pressure attained in the previous step. This sublimes the solid solvent into a gas and rejects it from the wet gel to produce aerogels or cryogels (aerogels produced by freeze-drying) (Zaman et al. 2020b). This process is presented in Fig. 7. The freeze-drying steps are illustrated in blue. The freeze-drying process should be conducted with caution to prevent damage to the structure while ice crystals are formed during freezing. If no precautions are adopted to minimize the growth of ice crystals, cryogels would have large pores with sizes of up to hundreds of microns. This results in thick and nonporous walls, a significantly low density, and a small surface area (Budtova 2019). This occurs because ice crystals push the walls of the network, thereby damaging the structure (Zaman et al. 2020b). A method to decrease the growth of ice crystals within the network is by submerging the wet gels in a liquid nitrogen bath (i.e., unidirectional freezing) or placing these inside a freezer (Lavoine and Bergström 2017). This rapid freezing process results in an undisturbed intrinsic gel network. However, slow freezing can cause segregation of the solvent inside the dispersion. This, in turn, disrupts the structure and yields large pores (Lavoine and Bergström 2017; Dahlem et al. 2019). For example, the rapid freezing of CNF gels in liquid ethanol at a temperature of − 114 °C yielded aerogels with pore size of 1–60 nm owing to the small ice crystals. Meanwhile, a slower freezing in liquid benzonitrile at − 13 °C resulted in aerogels with larger pores (Lavoine and Bergström 2017). Another technique for preventing the growth of large crystals is the use of mixed solvents, mostly tert-butanol (TBA)/water (Budtova 2019). For example, a TBA/water solvent with different ratios was used to prepare polysaccharide (pectin, starch, and alginic acid) aerogels. The results showed that the highest density of aerogels was achieved at ratios near the eutectic point in the phase diagram of TBA/water, which was equivalent to 0.114 kg/m3. Moreover, the lowest density was for the pure water solution (0.044 kg/m3) (Borisova et al. 2015). Furthermore, cellulose microfibril aerogels were prepared using a TBA/water solvent. This resulted in a surface area of 100 m2/g (Fumagalli et al. 2013).
Supercritical drying is another approach used to obtain aerogels while maintaining the desired properties of the gel, without the collapse of the structure through the liquid–vapor interface owing to heating. The first step of this process is the replacement of the existing solvent with a supercritical fluid that is miscible with the existing solvent (Lavoine and Bergström 2017; Dahlem et al. 2019) CO2 is mostly used as the supercritical fluid because of factors such as the low cost, non-flammability, and conveniently accessible supercritical conditions (a temperature of 31.3 °C and pressure of 72.9 atm (7.38 MPa) (Budtova 2019). If water is used as a solvent for the suspension of cellulose, a solvent-exchange step with alcohol is required because water is immiscible with CO2 (Lavoine and Bergström 2017; Dahlem et al. 2019). Moreover, water in the supercritical state exhibits oxidizing properties (Budtova 2019). After the introduction of CO2 into the gel structure, the supercritical conditions are attained by increasing the pressure and temperature while the content of CO2 increases. When there is no liquid phase in the pores owing to the presence of a supercritical mixture, the pressure is reduced to attain a gaseous state that leaves the pores without the formation of a liquid/vapor interface and prevents the formation of a meniscus and capillary pressure that can damage the structure of the gel during solvent removal (Lavoine and Bergström 2017; Long et al. 2018) This process is shown in Fig. 7, with the supercritical drying route illustrated in yellow. The process conditions may be optimized according to the aerogel type. For example, a pressure of 10 MPa during supercritical drying with CO2 produces aerogels with a density of 0.0078 g/cm3 and specific area of 605 m2/g (Heath and Thielemans 2010). However, supercritical drying is not commonly used because of the expensive equipment (it requires a high-pressure vessel) and process complexity (Lavoine and Bergström 2017; Long et al. 2018; Dahlem et al. 2019).
Another study compared CO2 supercritical drying with freeze-drying of cellulose gels prepared from Whatman filter paper and alpha cellulose as raw cellulose materials. In general, the reduction in the size of cellulose aerogels dried using supercritical CO2 was less than that of freeze-dried aerogels (Lee et al. 2017).
Phase diagram of water, with the process of removal through a freeze-drying and b supercritical drying (Zaman et al. 2020b)
Buchtova and Budtova performed a comparative study of the effects of different drying methods (Buchtová and Budtova 2016b). Cellulose gels were prepared by dissolving 3–11 wt% microcrystalline cellulose in DMSO/[Emim]Ac (60/40 wt/wt%). Subsequently, three drying techniques (freeze-drying, supercritical CO2 drying, and oven vacuum drying) were applied. Moreover, freeze-drying was performed with and without pre-unidirectional freezing using liquid nitrogen at − 196 °C. The results are summarized in Table 4.
Freeze-dried aerogel samples subjected to pre-UD freezing had the lowest volume shrinkage, lowest density, and highest porosity. The (ScCO2) sample had the highest surface area. Another study examined the effect of using T-butanol as a medium for freeze-drying in nanocellulose aerogel preparation (Wang et al. 2017). In this study, nanocellulose was suspended in water, then added to calcium chloride solution (0.25 mol/L) for gelation, followed by gradual replacement of calcium chloride solution with tert-butanol. Finally, the hydrogels were freeze-dried to obtain nanocellulose aerogels. The aerogels achieved a surface area of 164. 9666 m2/g, and a low shrinkage of 5.89%.
Cellulose aerogel hybrids, composites, and their applications
Cellulose hybrid aerogels are lightweight porous materials that combine cellulose with other substances. The resulting material is exceptionally light, which makes it a remarkable material for applications where weight reduction is important. Because of their flammability, fragility, and low thermal stability, cellulose hybrids and composite aerogels have recently been investigated extensively. The purpose was to study the effect of combining cellulose with other organic and inorganic materials to produce aerogels, as well as the functionality and tunability provided by incorporating other materials into cellulosic aerogels (Rahmanian et al. 2021). Cellulose can be combined with a variety of other substances such as silica, graphene, graphene oxide, chitosan, metal oxides, and MOFs to produce functional cellulose-hybrid aerogels (Rahmanian et al. 2021). Hybrid aerogels are designed for various applications. For example, cellulose–MOF aerogels have been prepared for antibacterial applications, gas separation and adsorption, energy storage, drug delivery, and catalysis (Liu et al. 2021b). Cellulose–MOF aerogels have been fabricated also for heavy metal removal from water (Lei et al. 2019). Moreover, cellulose–graphene aerogels have been produced for applications in absorption, supercapacitors, and pressure sensing (Rahmanian et al. 2021). Furthermore, cellulose–silica and cellulose–zeolite aerogels have been prepared as superinsulation materials (Shi et al. 2013; Bendahou et al. 2015; Demilecamps et al. 2015; Wong et al. 2015).
Cellulose–silica aerogels
Silica aerogels were the first aerogels fabricated through the gelation of silica in particular solvents, followed by drying through CO2 supercritical drying. Silica aerogels are considered superinsulators owing to their exceptionally low thermal conductivities. While cellulose aerogels display good thermal insulation properties, cellulose is a hydrophilic material that can absorb moisture from air. This property may affect the thermal insulation of cellulose aerogels. Hence, incorporating silica into cellulose aerogels can hinder water moisture and sustain the insulation properties. Several studies have been conducted on combining silica and cellulose aerogels. Shi et al. produced cellulose–silica aerogels using a solvent mixture of NaOH/thiourea/H2O. The solvent aqueous solution ratio was 9.5% NaOH to 4.5% thiourea. Cellulose obtained from cotton linter was dissolved at weight percentages of 2–5% to produce cellulose hydrogels. After dissolving the cellulose, a mixture of tetraethyl orthosilicate (TEOS), water, and ethanol was prepared at a stoichiometric ratio (TEOS/H2O/EtOH molar ratio) of 1.0/3.4/8.5. Subsequently, the cellulose hydrogel was immersed in the TEOS solution and freeze-dried to obtain the cellulose–SiO2 aerogel. The FTIR results showed an increase in the hydrophobicity of the material. Moreover, the aerogel achieved a thermal conductivity of 0.026 W/(m K) (Shi et al. 2013). Demilecamps et al. (2015) prepared cellulose–SiO2 by incorporating polyethoxydisiloxane (PEDS) into a cellulose alcogel using two techniques: molecular diffusion and forced flow induced by a pressure difference. Low thermal conductivity values of 0.026 and 0.028 W/(m∙K) were achieved with these methods, respectively. The silica cellulose aerogel composites were prepared by mixing NFC with PEDS prior to gelation. The incorporation of NFC increased the thermal conductivity by 11% compared with silica aerogels of an equal density. However, the tensile strength increased by 25–40%. Wong et al. tested the effects of maleic anhydride (MA) (Wong et al. 2015). The sol-gel process was applied to fabricate silica–cellulose aerogels that were dried using CO2 supercritical drying. Initially, silica was in the form of TEOS. It was then converted to SiO2 using ammonia. The prepared cellulose–silica aerogels had different weight percentages of silica ranging from 24 to 62 wt%. This achieved a porosity between 70 and 83%, density between 0.34 and 0.58 g/cm3, surface area of 400–652 m2/g, and maximum thermal conductivity of 0.045 W/(m K) (Cai et al. 2012). For pure cellulose and pure silica aerogels prepared in this work, the porosity was 92 and 93%, respectively; density was 0.14 and 0.19 g/cm3, respectively; and surface area was 356 and 767 m2/g, respectively. We can observe an evident deterioration in the properties of the composite aerogels compared with those of the pure aerogels. However, these still displayed remarkable properties for various applications. Demilecamps et al. (2014) used a method that they called “one-pot” to make cellulose–silica composite aerogels by combining cellulose and 8% NaOH in water with sodium silicate as the silica source, in addition to ZnO. The mixture was coagulated and dried using supercritical carbon dioxide. The cellulose concentration of the initial mixture was 4 wt%, with 8 wt% NaOH and 1 wt% ZnO. Subsequently, the cellulose solution was mixed with a sodium silicate mixture. The final cellulose concentration was 4 wt%, while the sodium silicate varied between 2 and 5 wt%. As mentioned earlier for dissolving cellulose in NaOH, the mixing occurred at − 6 °C. Coagulation was performed in three solvents: HCl, citric acid, and water. The resulting densities of the materials were between 0.10 and 0.25 g/cm3. The specific surface area ranged from 100 to 200 m2/g. The samples used in this study are shown in Fig. 8.
Photographs of a aerocellulose from 5% cellulose–8% NaOH–1% ZnO and b cellulose–silica composite aerogel from 5% cellulose–8% NaOH–1% ZnO–5% SiO2, coagulated in 0.3 M HCl (Demilecamps et al. 2014)
Cellulose–graphene aerogels
Graphene and its derivatives such as graphene oxide and reduced graphene oxide have been widely studied as hybrid cellulose aerogels. The use of graphene in aerogels can potentially enhance their resistance to water and improve their capability to conduct heat and produce electricity. When combined with cellulose, these complementary properties provide a highly effective combination for many practical applications. Cellulose–graphene aerogels are used in pressure sensors, absorbents, and supercapacitors (Rahmanian et al. 2021). Among the recent studies on cellulose–graphene aerogels is the work of Wu et al. (2022) for developing bacterial cellulose combined with graphene for light, thermal, and electric conversion. The thermal conductivity was increased by adding different weight percentages of graphene from 0.3 (pure cellulose aerogels) to 1.17 W/(m K) (0.5 wt% graphene). Moreover, the energy storage increased by 96% and had a maximum light–thermal–electric energy conversion output power of 1.80 mW and maximum light–thermal conversion efficiency of 90.3%. A schematic representation of the application is shown in Fig. 9. In another study, cellulose aerogels and films reinforced with carbon nanotubes were prepared using single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs) (Gnanaseelan et al. 2018). Nanocomposites made of cellulose and SWCNTs, whether in the form of a film or an aerogel, demonstrated a significantly better power factor and figure-of-merit than cellulose/MWCNT composites. The highest power factor achieved was 1.1 µW/(m K2) for films containing 10 wt% of SWCNTs, which also exhibited a Seebeck coefficient of 47.2 µV/K. This indicated that nanocomposites composed of cellulose and SWCNTs are highly effective for energy-related applications. Another study prepared aerogels consisting of carbon nanotubes (CNTs) and cellulose for their use as vapor sensors for the first time (Qi et al. 2015). This study examined the capability of aerogels to detect vapors by observing the variations in their electrical resistance when exposed to various volatile organic compounds (VOCs) including methanol, ethanol, and toluene. The study also analyzed how the type of vapor, amount of CNTs, and concentration of vapor affected the relative variation in electrical resistance.
Schematic diagram of light-thermal conversion (Wu et al. 2022)
Cellulose–polymer aerogels
Polymers are a class of materials that can be combined with cellulose to form hybrid aerogels. Two types of polymers are combined with cellulose to produce hybrid aerogels, which are inorganic polymers, such as polypyrrole (PPy) and polyvinyl alcohol (PVA), and organic polymers such as chitosan, alginic acid, pectin, and gelatin.
Inorganic polymer composite aerogel
Polymers such as polypyrrole (PPy) and polyvinyl alcohol (PVA) are integrated into cellulose solutions to obtain composite aerogels with modified properties for specific applications. For example, Zhuo et al. (2019) prepared PPy/cellulose carbon aerogels for supercapacitors. The hybrid material that was produced exhibited a large specific capacitance of 387.6 F/g when tested at 0.5 A/g in a 1 M H2SO4 solution. This indicated its capability to store electrical energy efficiently. In addition, the material exhibited an exceptional cycling stability, retaining 92.6% of its capacitance after being cycled 10,000 times. A schematic diagram for the application is shown in Fig. 10. Meanwhile Feng et al. (2020) prepared PPy/cellulose aerogels with controllable microwave absorption performance. The PPy/CA composite material shows the best performance in terms of its capability to absorb microwave energy when compressed at a ratio of 65%. At this ratio, the material has a minimum reflection loss (RL) of − 12.24 dB at a frequency of 8.53 GHz and thickness of 5 mm. Furthermore, the effective bandwidth, where RL is less than − 10 dB (dB), of the PPy/CA composite can cover the entire X-band frequency range (8.2–12.4 GHz) by varying the thickness of the material between 4 and 5 mm. Zhou et al. (Zhou et al. 2019) investigated the effects of gelatin and PVA on the mechanical properties of cellulose aerogels. The PVA/CNF/gelatin composite material had a modulus of 1.65 MPa. This was almost 8 and 91 times higher than that of the PVA/CNF aerogel and neat CNF aerogel, respectively. A microscopic analysis revealed that the aerogels had a three-dimensional network structure. In addition, the composite materials exhibited good thermal stability, low density, and low thermal conductivity.
A schematic diagram for Cellulose/PPy aerogels designed as supercapacitors (Zhuo et al. 2019)
Organic polymer composite aerogels
Due to characters, they share with cellulose such as abundance, sustainability and biodegradability, organic polymers such as chitosan and alginic acid, are being studied for preparation of cellulose-based aerogel composites for different applications. In the study of Zhang et al. (2020a), composite aerogels consisting of cellulose with chitosan, and cellulose with alginic acid were fabricated for the application of triboelectric nanogenerators (shown in Fig. 11), in addition to pure cellulose aerogel as control sample. Chitosan was first dissolved in 60% (w/w) lithium bromide trihydrate solution, and then cellulose was added with cellulose to chitosan mass ratios of 1:2, 1:4, and 1:8, while maintaining a total mass 1% to the solvent solution. Under 40 N stress, pure cellulose generated voltage output of 63 V. Increasing the chitosan content significantly increased the electrical voltage output compared to the control sample, with 269% increase for the 1:8 ratio, and 311% for the 1:2 ratio. Moreover, cellulose-alginic acid composite aerogels were fabricated by dissolving 0.75 wt% cellulose in 60% bromide trihydrate solution, followed by immersing the hydrogel by 0.5, 1, and 1.5% sodium alginic solution with sonication. The hydrogel was further immersed in 0.1 M HCl to precipitate alginic acid. Cellulose-alginic acid composite aerogels showed opposed behavior to the cellulose-chitosan, due to the alginic acid electron-withdrawing groups, unlike the electron-donating groups in chitosan. In another study, cellulose-chitosan aerogels were prepared via LiBr solution for removing formaldehyde from indoor air (Liao and Pan 2021). Composites were prepared with 1:4, 3:8, 1:2, 5:8, 3:4, and 1:1 chitosan to cellulose ratios, while maintaining the cellulose content 1% (w/w) to the LiBr solution. Aerogels with chitosan to cellulose ratio of 1:2 achieved the highest formaldehyde adsorption, with capacity up to 7.5 mmol/g. Pectin is another organic polymer that was used to prepare cellulose composite aerogels. In the study of Groult et al., cellulose in different mass ratios was dissolved NaOH/Urea solution, followed by immersion in pectin solution with different concentrations, for the application of drug delivery (Groult et al. 2022). Moreover, some of the samples were further immersed in CaCl2 solution (0.5 M) to test the effect of calcium crosslinking for pectin. The efficiency of the prepared composites varied based on the cellulose fraction, density of the composites and the calcium crosslinking. Gelatin is also another organic polymer that was used to fabricate cellulose-based aerogel composites for antibacterials application. The material exhibited great properties, with permeability range between 628 and 976 g/m2/day for water vapor, and fluid uptake capacity ranged between 250 and 390% (Chiaoprakobkij et al. 2022).
Fabrication process and characterization of the cellulose II network structured aerogel. a Flow chart showing fabrication steps of cellulose II hydrogel and aerogel. b Photograph of a well-shaped cellulose II aerogel (Zhang et al. 2020b)
Other materials
The other common materials incorporated into cellulose aerogels include MOFs. In the work of Wang et al. (2019), UiO-66/cellulose aerogels were prepared for adsorption. These showed a high effectiveness, with adsorption capability toward anionic methyl orange (71.7 mg/g) and cationic methylene blue (51.8 mg/g). In another study, Lei et al. (2019) combined two MOFs (UiO-66 and UiO-66-NH2) with cellulose to prepare aerogels for removing heavy metal ions from water. UiO-66-NH2–cellulose achieved adsorption of 89.40 mg/g for Pb2+. Garemark et al. (2020) prepared cellulose aerogels reinforced with nanoparticles (Ag) and metal oxide nanoparticles (TiO2). The aerogels exhibited a surface area up to 247 m2/g and porosity up to 95%. Cu‑BTC/nanocellulose aerogel composites were synthesized for adsorption of organic dyes and heavy metal ions (Shaheed et al. 2021). A remarkable adsorption capacity of 39 mg/g was achieved for Congo red. Three components’ aerogels from Microfibrillated Cellulose/Polypyrrole/Silver Nanoparticles were prepared. These MFC/PPy/Ag hybrid aerogels displayed improved antimicrobial and electrical conductivity characteristics compared with MFC and MFC/PPy hybrid aerogels because of the combination of PPy and Ag (Zhou et al. 2015). Cellulose–chitosan framework/polyaniline hybrid aerogels have been prepared for microwave absorption applications (Zhang et al. 2020c). It is noteworthy that the aerogel can achieve a wide frequency range of 6.04 GHz and high level of absorption intensity (− 54.76 dB). The hybrid aerogels and their physio-thermal properties are summarized in Table 5.
Cellulose–silica aerogels appear to be the best thermal insulation materials because of their ultralow thermal conductivity. Meanwhile, cellulose–PPy aerogels have the lowest density, which makes these suitable for applications that require ultralightweight materials.
Conclusion
Cellulose aerogels have emerged as a potential class of materials with exceptional properties that make these attractive for a wide range of applications. Because of their high porosity, specific surface area, remarkable mechanical strength, and thermal stability, cellulose aerogels have demonstrated substantial potential in various fields including energy storage, thermal insulation, absorption, and biomedical engineering. Although significant progress has been made in the synthesis and characterization of cellulose aerogels, further research is required to fully understand their properties and optimize their performance for specific applications. With regard to the selection of a solvent capable of dissolving cellulose, ionic liquids emerged as green-efficient solvents. Their high cost may be a challenge. However, the use of a co-solvent is an appropriate solution that requires further study. Among the recent developments is a new class of materials called hybrid aerogels. Herein, a combination of materials is integrated into cellulose aerogels. With continued advancements in this field, it is anticipated that cellulose aerogels will continue to play an increasingly important role in addressing the current societal challenges and facilitating sustainable development.
Future perspectives
Several properties of this topic are yet to be studied, such as the effect of the cellulose source and that of co-solvents systems in dissolving cellulose on the properties of aerogels. The cost of most complex solvents that are capable of dissolving cellulose is relatively high. Therefore, developing co-solvents systems that can reduce the cost of production for cellulose aerogel is a necessity. Although several solvents, such as ionic liquid, can be regenerated for reuse, the process of regeneration is time and energy consuming. On the other hand, certain abundant cellulose sources such as palm trees wood have not been examined for aerogel preparation. Moreover, the potential of cellulose aerogels for some applications such as catalysis needs to be further studied. In addition, due to high porosity, aerogels generally suffer weak mechanical performance. Therefore, enhancement and modification of the mechanical properties can be further improved. Improving the mechanical properties of cellulose aerogels will expand the field in which it can be utilized. For example, it can increase the service life in case it is used as a catalyst.
Data availability
Not applicable.
References
Aaltonen O, Jauhiainen O (2009) The preparation of lignocellulosic aerogels from ionic liquid solutions. Carbohydr Polym 75:125–129. https://doi.org/10.1016/j.carbpol.2008.07.008
Abdel-Hadi AK, Wafaa MH, Basta AH, El-Saied HU (1994) Metal chelates with some cellulose derivatives. Ii. Preparation and characterization of co(ii)-cmc complexes. Polym Plast Technol Eng 33:781–791. https://doi.org/10.1080/03602559408013108
Abdul Khalil HPS, Adnan AS, Yahya EB et al (2020) A review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers. https://doi.org/10.3390/polym12081759
Alothman OY, Kian LK, Saba N et al (2021) Cellulose nanocrystal extracted from date palm fibre: morphological, structural and thermal properties. Ind Crops Prod 159:113075. https://doi.org/10.1016/J.INDCROP.2020.113075
Basta AH, Lotfy VF (2023) Essential role of pulping route on thermal stability performance of rice straw-based nanocelluloses. Chem Eng Technol 46:837–847. https://doi.org/10.1002/ceat.202200431
Basta AH, Lotfy VF, Eldewany C (2021) Comparison of copper-crosslinked carboxymethyl cellulose versus biopolymer-based hydrogels for controlled release of fertilizer. Polym Plast Technol Mater 60:1884–1897. https://doi.org/10.1080/25740881.2021.1934017
Bendahou D, Bendahou A, Seantier B et al (2015) Nano-fibrillated cellulose-zeolites based new hybrid composites aerogels with super thermal insulating properties. Ind Crops Prod 65:374–382. https://doi.org/10.1016/j.indcrop.2014.11.012
Berglund L, Forsberg F, Jonoobi M, Oksman K (2018) Promoted hydrogel formation of lignin-containing arabinoxylan aerogel using cellulose nanofibers as a functional biomaterial. RSC Adv 8:38219–38228. https://doi.org/10.1039/C8RA08166B
Borisova A, De Bruyn M, Budarin VL et al (2015) A sustainable freeze-drying route to porous polysaccharides with tailored hierarchical meso- and macroporosity. Macromol Rapid Commun 36:774–779. https://doi.org/10.1002/marc.201400680
Bowron DT, D’Agostino C, Gladden LF et al (2010) Structure and dynamics of 1-ethyl-3-methylimidazolium acetate via molecular dynamics and neutron diffraction. J Phys Chem B 114:7760–7768. https://doi.org/10.1021/JP102180Q/SUPPL_FILE/JP102180Q_SI_002.PDF
Buchtová N, Budtova T (2016) Cellulose aero-, cryo- and xerogels: towards understanding of morphology control. Cellulose 23:2585–2595. https://doi.org/10.1007/s10570-016-0960-8
Budtova T (2019) Cellulose II aerogels: a review. Springer, Netherlands
Cai J, Kimura S, Wada M et al (2008) Cellulose aerogels from aqueous alkali hydroxide-urea solution. ChemSusChem 1:149–154. https://doi.org/10.1002/cssc.200700039
Cai J, Liu S, Feng J et al (2012) Cellulose-silica nanocomposite aerogels by in situ formation of silica in cellulose gel. Angew Chem 124:2118–2121. https://doi.org/10.1002/ange.201105730
Cao J, Wei W, Gou G et al (2018) Cellulose films from the aqueous DMSO/TBAH-system. Cellulose 25:1975–1986. https://doi.org/10.1007/S10570-017-1639-5
Chai YD, Pang YL, Lim S et al (2022) Recent Progress on tailoring the biomass-derived cellulose hybrid composite photocatalysts. Polymers. https://doi.org/10.3390/polym14235244
Chatterjee S, Ke WT, Liao YC (2020) Elastic nanocellulose/graphene aerogel with excellent shape retention and oil absorption selectivity. J Taiwan Inst Chem Eng 111:261–269. https://doi.org/10.1016/J.JTICE.2020.04.020
Chen B, Zheng Q, Zhu J et al (2016) Mechanically strong fully biobased anisotropic cellulose aerogels. RSC Adv 6:96518–96526. https://doi.org/10.1039/c6ra19280g
Chen Y, Zhang L, Yang Y et al (2021) Recent progress on nanocellulose aerogels: preparation, modification, composite fabrication, applications. Adv Mater 33:2005569. https://doi.org/10.1002/ADMA.202005569
Chiaoprakobkij N, Seetabhawang S, Okhawilai M et al (2022) Multifunctional bacterial cellulose-gelatin containing mangosteen extract films with improved antibacterial and anticancer properties. Cellulose 29:6811–6830. https://doi.org/10.1007/S10570-022-04685-5/METRICS
Coma V, Sebti I, Pardon P et al (2003) Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr Polym 51:265–271. https://doi.org/10.1016/S0144-8617(02)00191-1
Dahlem MA, Borsoi C, Hansen B, Catto AL (2019) Evaluation of different methods for extraction of nanocellulose from Yerba mate residues. Carbohydr Polym 218:78–86. https://doi.org/10.1016/J.CARBPOL.2019.04.064
de Morais ZD, Battirola LC, Gonçalves M (2018) do C Chemically cross-linked aerogels based on cellulose nanocrystals and polysilsesquioxane. Cellulose 25:7225–7238. https://doi.org/10.1007/S10570-018-2090-Y/FIGURES/6
de Oliveira JP, Bruni GP, el Halal SLM et al (2019) Cellulose nanocrystals from rice and oat husks and their application in aerogels for food packaging. Int J Biol Macromol 124:175–184. https://doi.org/10.1016/J.IJBIOMAC.2018.11.205
Demilecamps A, Reichenauer G, Rigacci A, Budtova T (2014) Cellulose-silica composite aerogels from one-pot synthesis. Cellulose 21:2625–2636. https://doi.org/10.1007/s10570-014-0314-3
Demilecamps A, Beauger C, Hildenbrand C et al (2015) Cellulose-silica aerogels. Carbohydr Polym 122:293–300. https://doi.org/10.1016/j.carbpol.2015.01.022
Dilamian M, Noroozi B (2021) Rice straw agri-waste for water pollutant adsorption: relevant mesoporous super hydrophobic cellulose aerogel. Carbohydr Polym 251:117016. https://doi.org/10.1016/j.carbpol.2020.117016
Feng J, Nguyen ST, Fan Z, Duong HM (2015) Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem Eng J 270:168–175. https://doi.org/10.1016/j.cej.2015.02.034
Feng S, Deng J, Yu L et al (2020) Development of lightweight polypyrrole/cellulose aerogel composite with adjustable dielectric properties for controllable microwave absorption performance. Cellulose 27:10213–10224. https://doi.org/10.1007/s10570-020-03497-9
Fesmire JE (2006) Aerogel insulation systems for space launch applications. Cryogenics 46:111–117. https://doi.org/10.1016/j.cryogenics.2005.11.007
Filipova I, Fridrihsone V, Cabulis U, Berzins A (2018) Synthesis of nanofibrillated cellulose by combined ammonium persulphate treatment with ultrasound and mechanical processing. Nanomaterials 8:640. https://doi.org/10.3390/nano8090640
Fumagalli M, Ouhab D, Boisseau SM, Heux L (2013) Versatile gas-phase reactions for surface to bulk esterification of cellulose microfibrils aerogels. Biomacromolecules 14:3246–3255. https://doi.org/10.1021/bm400864z
Ganesamoorthy R, Vadivel VK, Kumar R et al (2021) Aerogels for water treatment: a review. J Clean Prod 329:129713. https://doi.org/10.1016/J.JCLEPRO.2021.129713
Garemark J, Yang X, Sheng X et al (2020) Top-down approach making anisotropic cellulose aerogels as universal substrates for multifunctionalization. ACS Nano 14:7111–7120. https://doi.org/10.1021/acsnano.0c01888
Gavillon R, Budtova T (2008) Aerocellulose: new highly porous cellulose prepared from cellulose-NaOH aqueous solutions. Biomacromolecules 9:269–277. https://doi.org/10.1021/bm700972k
Glińska K, Gitalt J, Torrens E et al (2021) Extraction of cellulose from corn stover using designed ionic liquids with improved reusing capabilities. Process Saf Environ Prot 147:181–191. https://doi.org/10.1016/j.psep.2020.09.035
Gnanaseelan M, Chen Y, Luo J et al (2018) Cellulose-carbon nanotube composite aerogels as novel thermoelectric materials. Compos Sci Technol 163:133–140. https://doi.org/10.1016/j.compscitech.2018.04.026
Gond RK, Gupta MK, Jawaid M (2021) Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym Compos 42:5400–5412. https://doi.org/10.1002/PC.26232
Gong C, Ni JP, Tian C, Su ZH (2021) Research in porous structure of cellulose aerogel made from cellulose nanofibrils. Int J Biol Macromol 172:573–579. https://doi.org/10.1016/j.ijbiomac.2021.01.080
Groult S, Buwalda S, Budtova T (2022) Tuning bio-aerogel properties for controlling drug delivery. Part 2: cellulose-pectin composite aerogels. Biomater Adv 135:212732. https://doi.org/10.1016/J.BIOADV.2022.212732
Gupta KM, Hu Z, Jiang J (2013) Cellulose regeneration from a cellulose/ionic liquid mixture: the role of anti-solvents. RSC Adv 3:12794–12801. https://doi.org/10.1039/C3RA40807H
Gupta P, Singh B, Agrawal AK, Maji PK (2018) Low density and high strength nanofibrillated cellulose aerogel for thermal insulation application. Mater Des 158:224–236. https://doi.org/10.1016/j.matdes.2018.08.031
Heath L, Thielemans W (2010) Cellulose nanowhisker aerogels. Green Chem 12:1448–1453. https://doi.org/10.1039/C0GC00035C
Hedlund A, Köhnke T, Hagman J et al (2019) Microstructures of cellulose coagulated in water and alcohols from 1-ethyl-3-methylimidazolium acetate: contrasting coagulation mechanisms. Cellulose 26:1545–1563. https://doi.org/10.1007/s10570-018-2168-6
Hermanutz F, Vocht MP, Panzier N, Buchmeiser MR (2019) Processing of Cellulose using ionic liquids. Macromol Mater Eng 304:1–8. https://doi.org/10.1002/mame.201800450
Hoepfner S, Ratke L, Milow B (2008) Synthesis and characterisation of nanofibrillar cellulose aerogels. Cellulose 15:121–129. https://doi.org/10.1007/s10570-007-9146-8
Hong HJ, Ban G, Kim HS et al (2021) Fabrication of cylindrical 3D cellulose nanofibril(CNF) aerogel for continuous removal of copper(Cu2+) from wastewater. Chemosphere 278:130288. https://doi.org/10.1016/J.CHEMOSPHERE.2021.130288
Illera D, Mesa J, Gomez H, Maury H (2018) Cellulose aerogels for thermal insulation in buildings: trends and challenges. Coatings 8:1–13. https://doi.org/10.3390/coatings8100345
Innerlohinger J, Weber HK, Kraft G (2006) Aerocellulose: aerogels and aerogel-like materials made from cellulose. In: Macromolecular symposia, vol 244, pp 126–135. https://doi.org/10.1002/masy.200651212
Isogai A, Zhou Y (2019) Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: nanonetworks, nanofibers, and nanocrystals. Curr Opin Solid State Mater Sci 23:101–106. https://doi.org/10.1016/j.cossms.2019.01.001
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85. https://doi.org/10.1039/c0nr00583e
Jackcina Stobel Christy E, Gopi S, Pius A (2020) Chitin and chitosan-based aerogels. In: Handbook of Chitin and Chitosan: Volume 1: preparation and properties, pp 285–334. https://doi.org/10.1016/B978-0-12-817970-3.00010-9
Jiang F, Hsieh Y, Lo (2014a) Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing-thawing. J Mater Chem A Mater 2:350–359. https://doi.org/10.1039/c3ta13629a
Jiang F, Hsieh Y, Lo (2014b) Amphiphilic superabsorbent cellulose nanofibril aerogels. J Mater Chem A Mater 2:6337–6342. https://doi.org/10.1039/c4ta00743c
Jiang F, Hsieh Y, Lo (2017) Cellulose nanofibril aerogels: synergistic improvement of hydrophobicity, strength, and thermal stability via cross-linking with diisocyanate. ACS Appl Mater Interfaces 9:2825–2834. https://doi.org/10.1021/acsami.6b13577
Jiang S, Zhang M, Li M et al (2020) Cellulose nanofibril (CNF) based aerogels prepared by a facile process and the investigation of thermal insulation performance. Cellulose 27:6217–6233. https://doi.org/10.1007/s10570-020-03224-4
Jin H, Nishiyama Y, Wada M, Kuga S (2004) Nanofibrillar cellulose aerogels. Colloids Surf a Physicochem Eng Asp 240:63–67. https://doi.org/10.1016/j.colsurfa.2004.03.007
Khan MN, Rehman N, Sharif A et al (2020) Environmentally benign extraction of cellulose from dunchi fiber for nanocellulose fabrication. Int J Biol Macromol 153:72–78. https://doi.org/10.1016/J.IJBIOMAC.2020.02.333
Kistler SS (1931) Coherent expanded aerogels and jellies. Nature 127:741. https://doi.org/10.1038/127741a0
Lavoine N, Bergström L (2017) Nanocellulose-based foams and aerogels: processing, properties, and applications. J Mater Chem A Mater 5:16105–16117. https://doi.org/10.1039/c7ta02807e
Le KA, Sescousse R, Budtova T (2012) Influence of water on cellulose-EMIMAc solution properties: a viscometric study. Cellulose 19:45–54. https://doi.org/10.1007/s10570-011-9610-3
Le KA, Rudaz C, Budtova T (2014) Phase diagram, solubility limit and hydrodynamic properties of cellulose in binary solvents with ionic liquid. Carbohydr Polym 105:237–243. https://doi.org/10.1016/j.carbpol.2014.01.085
Lee S, Kang KY, Jeong MJ et al (2017) Evaluation of supercritical CO2 dried cellulose aerogels as nano-biomaterials. J Korean Phys Soc 71:483–486. https://doi.org/10.3938/jkps.71.483
Lei C, Gao J, Ren W et al (2019) Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr Polym 205:35–41. https://doi.org/10.1016/j.carbpol.2018.10.029
Li M, Jiang H, Xu D, Yang Y (2017) A facile method to prepare cellulose whiskers–silica aerogel composites. J Solgel Sci Technol 83:72–80. https://doi.org/10.1007/s10971-017-4384-1
Liang L, Zhang S, Goenaga GA et al (2020) Chemically cross-linked cellulose nanocrystal aerogels for effective removal of cation dye. Front Chem 8:570. https://doi.org/10.3389/FCHEM.2020.00570/BIBTEX
Liao Y, Pan X (2021) Self-indicating and high-capacity mesoporous aerogel-based biosorbent fabricated from cellulose and chitosan via co-dissolution and regeneration for removing formaldehyde from indoor air. Environ Sci Nano 8:1283–1295. https://doi.org/10.1039/d1en00122a
Liao Y, Pang Z, Pan X (2019) Fabrication and mechanistic study of aerogels directly from whole Biomass. ACS Sustain Chem Eng 7:17723–17736. https://doi.org/10.1021/acssuschemeng.9b04032
Lin WH, Jana SC (2021) Analysis of porous structures of cellulose aerogel monoliths and microparticles. Microporous Mesoporous Mater 310:110625. https://doi.org/10.1016/j.micromeso.2020.110625
Lindman B, Medronho B, Alves L et al (2017) The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys Chem Chem Phys 19:23704–23718. https://doi.org/10.1039/C7CP02409F
Liu X, Jiang Y, Qin C et al (2018) Enzyme-assisted mechanical grinding for cellulose nanofibers from bagasse: energy consumption and nanofiber characteristics. Cellulose 25:7065–7078. https://doi.org/10.1007/S10570-018-2071-1/TABLES/3
Liu H, Du H, Zheng T et al (2021a) Cellulose based composite foams and aerogels for advanced energy storage devices. Chem Eng J 426:130817. https://doi.org/10.1016/J.CEJ.2021.130817
Liu X, Xiao Y, Zhang Z et al (2021b) Recent progress in Metal-Organic Frameworks@Cellulose hybrids and their applications. Chin J Chem 39:3462–3480. https://doi.org/10.1002/CJOC.202100534
Liu Z, Zhang S, He B et al (2021c) Synthesis of cellulose aerogels as promising carriers for drug delivery: a review. Cellulose 28:2697–2714. https://doi.org/10.1007/s10570-021-03734-9
Long LY, Weng YX, Wang YZ (2018) Cellulose aerogels: synthesis, applications, and prospects. Polymers 8:1–28. https://doi.org/10.3390/polym10060623
Ma H, Wang S, Meng F et al (2017) A hydrazone-carboxyl ligand-linked cellulose nanocrystal aerogel with high elasticity and fast oil/water separation. Cellulose 24:797–809. https://doi.org/10.1007/s10570-016-1132-6
Medronho B, Lindman B (2015) Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv Colloid Interface Sci 222:502–508. https://doi.org/10.1016/j.cis.2014.05.004
Mehanny S, Abu-El Magd EE, Ibrahim M et al (2021a) Extraction and characterization of nanocellulose from three types of palm residues. J Mater Res Technol 10:526–537. https://doi.org/10.1016/J.JMRT.2020.12.027
Mehanny S, Abu-El Magd EE, Sorbara S et al (2021b) Spanish poplar biomass as a precursor for nanocellulose extraction. Appl Sci 11:6863. https://doi.org/10.3390/APP11156863
Mi QY, Ma SR, Yu J et al (2016) Flexible and transparent cellulose aerogels with Uniform Nanoporous structure by a controlled regeneration process. ACS Sustain Chem Eng 4:656–660. https://doi.org/10.1021/acssuschemeng.5b01079
Mussana H, Yang X, Tessima M et al (2018) Preparation of lignocellulose aerogels from cotton stalks in the ionic liquid-based co-solvent system. Ind Crops Prod 113:225–233. https://doi.org/10.1016/j.indcrop.2018.01.025
Nang An V, Chi Nhan HT, Tap TD et al (2020) Extraction of high crystalline nanocellulose from biorenewable sources of Vietnamese agricultural wastes. J Polym Environ 28:1465–1474. https://doi.org/10.1007/S10924-020-01695-X/TABLES/2
Navard P, Wendler F, Meister F et al (2013) Preparation and Properties of Cellulose Solutions. In: The European Polysaccharide Network of Excellence (EPNOE): research initiatives and results, pp 91–152. https://doi.org/10.1007/978-3-7091-0421-7_5
Nguyen ST, Feng J, Ng SK et al (2014) Advanced thermal insulation and absorption properties of recycled cellulose aerogels. Colloids Surf A Physicochem Eng Asp 445:128–134. https://doi.org/10.1016/j.colsurfa.2014.01.015
Novak BM, Auerbach D, Verrier C (1994) Low-density, mutually interpenetrating organic-inorganic composite materials via supercritical drying techniques. Chem Mater 6:282–286. https://doi.org/10.1021/CM00039A006/ASSET/CM00039A006.FP.PNG_V03
Onwukamike KN, Lapuyade L, Maillé L et al (2019) Sustainable approach for cellulose aerogel preparation from the DBU-CO 2 switchable solvent. ACS Sustain Chem Eng 7:3329–3338. https://doi.org/10.1021/acssuschemeng.8b05427
Östlund Å, Lundberg D, Nordstierna L et al (2009) Dissolution and gelation of cellulose in TBAF/DMSO solutions: the roles of fluoride ions and water. Biomacromolecules 10:2401–2407. https://doi.org/10.1021/BM900667Q
Pierre AC, Pajonk GM (2002) Chemistry of aerogels and their applications. Chem Rev 102:4243–4265. https://doi.org/10.1021/cr0101306
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:6712–6728. https://doi.org/10.1021/cr9001947
Pircher N, Fischhuber D, Carbajal L et al (2015) Preparation and reinforcement of dual-porous biocompatible cellulose scaffolds for tissue Engineering. Macromol Mater Eng 300:911–924. https://doi.org/10.1002/MAME.201500048
Pircher N, Carbajal L, Schimper C et al (2016) Impact of selected solvent systems on the pore and solid structure of cellulose aerogels. Cellulose 23:1949–1966. https://doi.org/10.1007/S10570-016-0896-Z/TABLES/5
Qi H, Liu J, Pionteck J et al (2015) Carbon nanotube-cellulose composite aerogels for vapour sensing. Sens Actuators B Chem 213:20–26. https://doi.org/10.1016/j.snb.2015.02.067
Rahmanian V, Pirzada T, Wang S, Khan SA (2021) Cellulose-based hybrid aerogels: strategies toward design and functionality. Adv Mater 33:1–26. https://doi.org/10.1002/adma.202102892
Rashid S, Dutta H (2020) Characterization of nanocellulose extracted from short, medium and long grain rice husks. Ind Crops Prod 154:112627. https://doi.org/10.1016/J.INDCROP.2020.112627
Raza M, Abu-Jdayil B (2022) Cellulose nanocrystals from lignocellulosic feedstock: a review of production technology and surface chemistry modification. Springer, Amsterdam
Raza M, Abu-Jdayil B, Banat F, Al-Marzouqi AH (2022) Isolation and characterization of cellulose nanocrystals from date Palm Waste. ACS Omega 7:25366–25379. https://doi.org/10.1021/acsomega.2c02333
Rostamitabar M, Seide G, Jockenhoevel S, Ghazanfari S (2021) Effect of cellulose characteristics on the properties of the wet-spun aerogel fibers. Appl Sci 11:1–16. https://doi.org/10.3390/app11041525
Rudaz C (2013) Cellulose and pectin aerogels: towards their nano-structuration. Ecole Nationale Supérieure des Mines de Paris
Rudaz C, Courson R, Bonnet L et al (2014) Aeropectin: fully biomass-based mechanically strong and thermal superinsulating aerogel. Biomacromolecules 15:2188–2195. https://doi.org/10.1021/BM500345U
Seantier B, Bendahou D, Bendahou A et al (2016) Multi-scale cellulose based new bio-aerogel composites with thermal super-insulating and tunable mechanical properties. Carbohydr Polym 138:335–348. https://doi.org/10.1016/j.carbpol.2015.11.032
Sehaqui H, Zhou Q, Berglund LA (2011) High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos Sci Technol 71:1593–1599. https://doi.org/10.1016/j.compscitech.2011.07.003
Sen S, Singh A, Bera C et al (2022) Recent developments in biomass derived cellulose aerogel materials for thermal insulation application: a review. Cellulose 29:4805–4833. https://doi.org/10.1007/s10570-022-04586-7
Sescousse R, Budtova T (2009) Influence of processing parameters on regeneration kinetics and morphology of porous cellulose from cellulose-NaOH-water solutions. Cellulose 16:417–426. https://doi.org/10.1007/s10570-009-9287-z
Sescousse R, Gavillon R, Budtova T (2011) Aerocellulose from cellulose-ionic liquid solutions: preparation, properties and comparison with cellulose-NaOH and cellulose-NMMO routes. Carbohydr Polym 83:1766–1774. https://doi.org/10.1016/J.CARBPOL.2010.10.043
Shaheed N, Javanshir S, Esmkhani M et al (2021) Synthesis of nanocellulose aerogels and Cu-BTC/nanocellulose aerogel composites for adsorption of organic dyes and heavy metal ions. Sci Rep 11:1–11. https://doi.org/10.1038/s41598-021-97861-9
Shahnaz T, Vishnu Pridiyan V, Panan S, Narayanasamy S (2021) Use of nanocellulose extracted from grass for adsorption abatement of ciprofloxacin and diclofenac removal with phyto, and fish toxicity studies. Environ Pollut 268:115494. https://doi.org/10.1016/j.envpol.2020.115494
Shamsuri AA, Siti Nurul SNA, Abdan K (2022) Nanocellulose extraction using ionic liquids: syntheses, processes, and Properties. Front Mater 9:919918. https://doi.org/10.3389/FMATS.2022.919918/BIBTEX
Shi J, Lu L, Guo W et al (2013) Heat insulation performance, mechanics and hydrophobic modification of cellulose-SiO2 composite aerogels. Carbohydr Polym 98:282–289. https://doi.org/10.1016/j.carbpol.2013.05.082
Singh R, Shukla A, Tiwari S, Srivastava M (2014) A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew Sustain Energy Rev 32:713–728. https://doi.org/10.1016/J.RSER.2014.01.051
Song Y, Jiang W, Zhang Y et al (2018) A novel process of nanocellulose extraction from Kenaf Bast. Mater Res Express 5:085032. https://doi.org/10.1088/2053-1591/AAC80D
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellose with ionic liquids. J Am Chem Soc 124:4974–4975. https://doi.org/10.1021/ja025790m
Szlapak T, Potulski DC, Viana LC et al (2019) Nanocellulose obtained from residues of peach palm extraction (Bactris gasipaes). Carbohydr Polym 218:8–19. https://doi.org/10.1016/j.carbpol.2019.04.035
Trifol Sillard D, Plackett P, Szabo J, Bras AE, Daugaard JC (2017) Chemically extracted nanocellulose from sisal fibres by a simple and industrially relevant process. Cellulose 24:107–118. https://doi.org/10.1007/s10570-016-1097-5
Voon LK, Pang SC, Chin SF (2017) Porous cellulose beads fabricated from regenerated cellulose as potential drug delivery carriers. J Chem 2017:1. https://doi.org/10.1155/2017/1943432
Wan C, Jiao Y, Wei S et al (2019) Functional nanocomposites from sustainable regenerated cellulose aerogels: a review. Chem Eng J 359:459–475. https://doi.org/10.1016/j.cej.2018.11.115
Wang Z, Liu S, Matsumoto Y, Kuga S (2012) Cellulose gel and aerogel from LiCl/DMSO solution. Cellulose 19:393–399. https://doi.org/10.1007/s10570-012-9651-2
Wang H, Shao Z, Bacher M et al (2013) Fluorescent cellulose aerogels containing covalently immobilized (ZnS)x(CuInS2)1-x/ZnS (core/shell) quantum dots. Cellulose 20:3007–3024. https://doi.org/10.1007/s10570-013-0035-z
Wang C, Xiong Y, Fan B et al (2016a) Cellulose as an adhesion agent for the synthesis of lignin aerogel with strong mechanical performance, sound-absorption and thermal insulation. Sci Rep 6:1–9. https://doi.org/10.1038/srep32383
Wang X, Zhang Y, Jiang H et al (2016b) Fabrication and characterization of nano-cellulose aerogels via supercritical CO2 drying technology. Mater Lett 183:179–182. https://doi.org/10.1016/j.matlet.2016.07.081
Wang X, Zhang Y, Jiang H et al (2017) Tert-butyl alcohol used to fabricate nano-cellulose aerogels via freeze-drying technology. In: IOP science
Wang Z, Song L, Wang Y et al (2019) Lightweight UiO-66/cellulose aerogels constructed through self-crosslinking strategy for adsorption applications. Chem Eng J 371:138–144. https://doi.org/10.1016/j.cej.2019.04.022
Wong JCH, Kaymak H, Tingaut P et al (2015) Mechanical and thermal properties of nanofibrillated cellulose reinforced silica aerogel composites. Microporous Mesoporous Mater 217:150–158. https://doi.org/10.1016/j.micromeso.2015.06.025
Wu T, Dong J, Gan F et al (2018) Low dielectric constant and moisture-resistant polyimide aerogels containing trifluoromethyl pendent groups. Appl Surf Sci 440:595–605. https://doi.org/10.1016/J.APSUSC.2018.01.132
Wu G, Bing N, Li Y et al (2022) Three-dimensional directional cellulose-based carbon aerogels composite phase change materials with enhanced broadband absorption for light-thermal-electric conversion. Energy Convers Manag 256:115361. https://doi.org/10.1016/j.enconman.2022.115361
Xu A, Wang J, Wang H (2010) Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem 12:268–275. https://doi.org/10.1039/b916882f
Yang Y, Zhang Y, Dawelbeit A et al (2017) Structure and properties of regenerated cellulose fibers from aqueous NaOH/thiourea/urea solution. Cellulose 24:4123–4137. https://doi.org/10.1007/s10570-017-1418-3
Yang Y, Chen Z, Zhang J et al (2019) Preparation and applications of the cellulose nanocrystal. Int J Polym Sci. https://doi.org/10.1155/2019/1767028
Yu H, Qin Z, Liang B et al (2013) Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J Mater Chem A Mater 1:3938–3944. https://doi.org/10.1039/c3ta01150j
Yu S, Sun J, Shi Y et al (2021) Nanocellulose from various biomass wastes: its preparation and potential usages towards the high value-added products. Environ Sci Ecotechnol 5:100077. https://doi.org/10.1016/j.ese.2020.100077
Zaman A, Huang F, Jiang M et al (2020) Preparation, Properties, and applications of natural cellulosic aerogels: a review. Energy Built Environ 1:60–76. https://doi.org/10.1016/j.enbenv.2019.09.002
Zhang H, Wu J, Zhang J, He J (2005) 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38:8272–8277. https://doi.org/10.1021/ma0505676
Zhang H, Li Y, Xu Y et al (2016) Versatile fabrication of a superhydrophobic and ultralight cellulose-based aerogel for oil spillage clean-up. Phys Chem Chem Phys 18:28297–28306. https://doi.org/10.1039/c6cp04932j
Zhang J, Wu J, Yu J et al (2017) Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends. Mater Chem Front 1:1273–1290. https://doi.org/10.1039/c6qm00348f
Zhang L, Liao Y, Wang YC et al (2020) Cellulose II aerogel-based triboelectric nanogenerator. Adv Funct Mater 30:1–9. https://doi.org/10.1002/adfm.202001763
Zhang Z, Tan J, Gu W et al (2020) Cellulose-chitosan framework/polyailine hybrid aerogel toward thermal insulation and microwave absorbing application. Chem Eng J 395:125190. https://doi.org/10.1016/J.CEJ.2020.125190
Zhao S, Malfait WJ, Guerrero-Alburquerque N et al (2018) Biopolymer aerogels and foams: chemistry, properties, and applications. Angew Chem Int Edn 57:7580–7608. https://doi.org/10.1002/anie.201709014
Zheng Q, Cai Z, Gong S (2014) Green synthesis of polyvinyl alcohol (PVA)–cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J Mater Chem A Mater 2:3110–3118. https://doi.org/10.1039/C3TA14642A
Zhou J, Hsieh YL (2020) Nanocellulose aerogel-based porous coaxial fibers for thermal insulation. Nano Energy 68:104305. https://doi.org/10.1016/J.NANOEN.2019.104305
Zhou S, Wang M, Chen X, Xu F (2015) Facile template synthesis of microfibrillated cellulose/polypyrrole/silver nanoparticles hybrid aerogels with electrical conductive and pressure responsive properties. ACS Sustain Chem Eng 3:3346–3354. https://doi.org/10.1021/ACSSUSCHEMENG.5B01020/ASSET
Zhou T, Cheng X, Pan Y et al (2019) Mechanical performance and thermal stability of polyvinyl alcohol–cellulose aerogels by freeze drying. Cellulose 26:1747–1755. https://doi.org/10.1007/s10570-018-2179-3
Zhuo H, Hu Y, Chen Z, Zhong L (2019) Cellulose carbon aerogel/PPy composites for high-performance supercapacitor. Carbohydr Polym 215:322–329. https://doi.org/10.1016/j.carbpol.2019.03.101
Zhu C, Koutsomitopoulou AF, Eichhorn SJ et al (2018) High stiffness cellulose fibers from low molecular weight microcrystalline cellulose solutions using DMSO as co-solvent with ionic liquid. Macromol Mater Eng 303:1800029. https://doi.org/10.1002/MAME.201800029
Zhu G, Chen Z, Wu B, Lin N (2019) Dual-enhancement effect of electrostatic adsorption and chemical crosslinking for nanocellulose-based aerogels. Ind Crops Prod 139:111580. https://doi.org/10.1016/J.INDCROP.2019.111580
Acknowledgments
Not applicable.
Funding
This work was supported by the National Water and Energy Centre, United Arab Emirates University (Project # 12R122).
Author information
Authors and Affiliations
Contributions
Hyder Al Abdallah: methodology, formal analysis, investigation, writing—original draft preparation. Joy Tannous: methodology, validation, and writing—review and editing. Basim Abu-Jdayil: Conceptualization, methodology, validation, formal analysis, resources, writing—review and editing, supervision, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The authors confirm that the prepared manuscript was approved by all authors.
Consent for publication
The authors confirmed here that the submitted manuscript has not been published previously and it is not under consideration for publication elsewhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al Abdallah, H., Tannous, J.H. & Abu-Jdayil, B. Cellulose and nanocellulose aerogels, their preparation methods, and potential applications: a review. Cellulose 31, 2001–2029 (2024). https://doi.org/10.1007/s10570-024-05743-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-05743-w