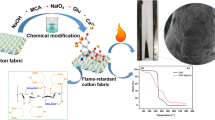
Abstract
In order to endow cotton fabric better water repellency and flame retardancy, ferrocene formaldehyde and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) were introduced into cotton fabric through facile chemical reaction. The specific pyrolysis products of Fe@DOPO cotton tested by TG-IR was reduced obviously compare with pristine cotton. More importantly, the peak heat release rate and total heat release of the modified cotton fabric were decreased by 48.9% and 19.2%, respectively. Through the analysis of combustion mechanism, it was indicated that ferrocene and DOPO have synergistic flame retardant effect. In addition to the flame retardant, the waterproof property of Fe@DOPO cotton was also greatly improved, and the contact angle was 138°. After 12 times of washing, the limiting oxygen index (LOI) of as-prepared cotton was still 24%, indicating Fe@DOPO cotton had a good washing resistance. These excellent properties were expected to make the modified cotton fabric applied in many fields.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the most important natural fabric, cotton fabric has many advantages, such as good air permeability, renewable, soft and so on (Feng et al. 2017; Vasiljević et al. 2015; Xu et al. 2019), thus it is widely used in sofa, home decoration and other industrial fields. However, the LOI value of pure cotton fabric is only 18%, making it very easy to burn (Feng et al. 2017; Wang et al. 2018), which limits its application. Consequently, in order to obtain flame retardant cotton fabric, flame retardant finishing was necessary.
In the past few years, many flame retardants containing halogen, nitrogen, phosphorus, silicon, boron and aluminum (Feng et al. 2017; Xie et al. 2013; Aksit et al. 2016; Alongi et al. 2014) have been used in the flame retardant finishing of cotton fabrics, and shown highly efficient flame retardant property. Previously, halogen compounds such as chlorine and bromine were widely used in flame retardant finishing of cotton fabrics. Although these flame retardants were very effective for flame retardant finishing of fabrics, they were harmful to health and environment (Yang et al. 2012). Besides, most of the flame retardant finishing of natural fiber fabrics were carried out by surface modification and the flame retardant fabrics have to be washed vigorously, so flame retardant cotton fabric together with excellent water repellent was necessary. Therefore, the flame retardant finishing of cotton fabric with environment friendly, high adhesion and excellent flame retardancy has been the goal pursued by industry and academia.
Compared with halogen containing flame retardants, phosphorus based flame retardants have attracted much attention due to their high efficiency and low toxicity (Malucelli et al. 2014; Zhao et al. 2017). During combustion, the phosphorous flame retardant can produce non-volatile metaphosphoric acid, which covers the surface of cotton fabric and promotes the dehydration of cotton fabric into char. This dense layer of carbon prevents heat from escaping and prevents oxygen from entering, thus preventing combustion (Abou-Okeil et al. 2013; He et al. 2018; Nguyen et al. 2012). The 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) was one of the most important phosphorus containing flame retardants. Compared with general phosphate flame retardants, DOPO showed higher thermal stability and flame retardant efficiency because of the phenanthrene ring and biphenyl structure in the molecular structure (Bai et al. 2014; Dong et al. 2013; Shan et al. 2017; Zhang et al. 2020a, b). Moreover, due to the existence of active P–H in DOPO, it can react with various chemical groups. (Qian et al. 2014; Xie et al. 2014). It has even been reported that DOPO can be grafted onto cotton directly by the reaction of Atherton-Todd, but total heat release (THR) was only reduced 10% by modification and char yield of as-prepared cotton was only 0.89% (Chen et al. 2019). This was due to the low grafting rate of DOPO on cotton fabric and the failure to give full play of its flame-retardant ability. Other studies have shown that it was difficult to achieve high efficiency by using DOPO alone on cotton fabrics (Hu et al. 2011; Chernyy et al. 2015; Liao et al. 2017). Therefore, it was urgent to find the materials and technology that could not only accelerate the catalytic flame retardancy of DOPO, but also loading or grafting more DOPO and better adhesion to cotton fabric.
In the past decades, ferrocene and its derivatives have gained great attraction because of their many advantages, such as electrochemistry, catalysis and magnetism (Liang et al. 2012). It is also reported that ferrocene has strong flame retardancy and smoke suppression properties, which can catalyze the formation of carbon (Kishore et al. 1991). At the same time, ferrocene could improve the thermodynamic stability of polymer materials (Hussein and Asiri 2012; Koshiba et al. 2012). Wen et al. reported the preparation of a novel material containing DOPO and ferrocene groups for fire retardant epoxy resin (Wen et al. 2018), only with 5 wt% addition of retardancy oligomer, the LOI value of epoxy resin increased to 32% and can pass V-0 rating.
Among all the studies, it was not found that ferrocene and DOPO were introduced into the flame retardant finishing of cotton fabric by chemical reaction at the same time. Moreover, the change of hydrophobic property of DOPO modified cotton fabric was not reported. The purpose of this paper is to introduce ferrocene and DOPO into the flame retardant finishing of cotton fabric simultaneously, to explore the synergistic flame-retardant properties of the two, as well as evaluating the hydrophobicity of the fabric surface. In this study, in order to have better adhesion on cotton fabric, the modified polyethyleneimine (PEI) cotton fabric (previously made by our research group) (Wang et al. 2019) was firstly reacted with ferrocene formaldehyde under appropriate conditions, and then DOPO was incorporated into the cotton fiber. To verify the success of the reaction, the chemical structure was characterized by X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR) and X-Ray Diffraction (XRD). By testing thermogravimetric analysis (TGA/DTG), thermogravimetry-FTIR (TG-IR), limit oxygen index (LOI) and cone calorimeter tests (CCT) of cotton fabric before and after modification, the synergistic flame retardant effect of ferrocene and DOPO on cotton fabric was studied. In particular, the surface water repellency of modified cotton fabric and the change of flame retardant property after washing were discussed.
Experimental
Materials
Ferrocene formaldehyde, polyethyleneimine (PEI, Mn = 25,000), glycerol, sodium borohydride, tetrahydrofuran, sodium periodate, DOPO and methyl alcohol were supplied by Huaxia Chemical Industry Co.,Ltd (Chengdu, China). Cotton fabric was purchased from Dragon Clan (China) Co., LTD. (Fujian, China). Moreover, pristine cotton fabric was pre-treated by deionized (DI) water inultrasonic cleaning before use.
Sample preparation
Our research group has prepared PEI-grafted cotton fabric according to the requirements of the literature (Wang et al. 2019). The PEI-grated cotton fabric was immersed into methyl alcohol solution contained ferrocene formaldehyde (50 mg/mL) for 6 h at 60°C. After completion of the reaction, the Fe-grafted cotton fabric was washed with methyl alcohol and drying treatment. Then the processed fabric was immersed into 50 mg/ mL DOPO and methyl alcohol solution for 12 h at 60°C. Finally, the Fe@DOPO cotton fabric was rinsed in methyl alcohol solution and DI-water to remove the uncoated products and then fully dried in a vacuum oven at 80°C before used. Weight gain of Fe@DOPO cotton was determined to be 21.2% ± 0.6 compared with the pristine cotton. Schematic illustration of treatment process for construction of Fe@DOPO coating on cotton fabric is shown in Scheme 1. In order to analyze the mechanism better, DOPO grafted cotton was synthesized by direct reaction of PEI-grafted cotton with DOPO (under the similar reaction conditions) (DOPO-grafted cotton), and the weight gain was 22.3% ± 0.8.
Characterization
FT-IR and XPS tests were used with a Nicolet 560 FTIR spectrometer (Nicolet, United States) in the range of 400–4000 cm−1and XSAM 800 instrument (XSAM 800, UK), respectively. X-ray diffraction (XRD) was conducted to research the crystallization of samples by a Bruker D8 advance diffractometer in the range of 5° − 60°. Field emission transmission electron microscopy (FE-TEM, Tecnai G2 F20 S-TWIN, FEI, USA) analysis was applied to investigate the micromorphology of as-prepared cotton samples. The thermodynamic stability of cotton fabric was analyzed by TGA and DTG (TA Instruments, USA) from 50 to 800 °C. The test conditions were as follows: heating rate: 20°C/min; under N2 and air atmosphere. Cotton samples were tested by thermogravimetry-IR (TG-IR) spectroscopy for examination of combustion products by a sta8000 TG-IR spectrometer (PE, USA). Limit oxygen index (LOI) was measured by vertical combustion method with GB / T5454-1997 standard. The size is 150 mm (length) × 58 mm (width).The morphology of cotton fiber before and after modification as well as the char residues after CCT were characterized by SEM–EDX analyze (SEM, JSM-7500F, JEOL, Japan).). The standard of CCT combustion test method is ISO 5660–1 standard, using an FTT cone calorimeter (Grinstead, UK). The size is 100 mm (length) × 100 mm (width).The washing resistance of cotton fabric before and after modification is the AATCC 61–2006 standard. The water contact angles in the air (WCAs) were tested by the contact angle measuring device (DSA-25S, Kruss, Germany). 5 μl DI water were prepared as a testing liquid in this research.
The weight gain (WG) was calculated using the following formula:
where W0 is the weight of pristine cotton fabric and W1 is the weight of treated cotton fabric.
Results and discussion
Characterization
FT-IR spectra, XRD and XPS were characterized to analyze the composition of pristine cotton, PEI-grafted cotton and Fe@DOPO cotton to confirm whether the modification process was successful. As shown in Fig. 1, the broad peak at 3400 cm−1 was attributed to O–H stretching vibration, 1637 cm−1 could be regard as bending of –OH (Suryaprabha and Sethuraman 2018), and the relatively narrow peak at 2910 cm−1 was the C–H stretching vibration peak for the pristine cotton. Meanwhile, the peaks at 1054 cm−1, and 896 cm−1 corresponded to the C–O–C stretching vibration of pyranose ring of cellulose (Fang et al. 2016; Han et al. 2017). Compared to the pristine cotton, the peak at 1645 cm−1 corresponds to –NH– stretching vibration in PEI-grafted cotton, while the new characteristic peaks appeared at 1245 cm−1 and 1018 cm−1 for the Fe@DOPO cotton fabric attributed to the DOPO absorption of P = O and P–O–P stretching vibration, respectively.
The crystal structures of the four kinds of cotton fibers were analyzed by XRD, which was clearly shown in Fig. 1(b). The peak values of pristine cotton fiber were at 2θ = 14.64°, 16.47°, 22.75° and 34.45° positions, corresponding to the crystal planes of cellulose (110), (110), (200) and (004), respectively (Kwak et al. 2015; Tian et al. 2019). The crystal structure of the modified cotton fiber was basically consistent with that of the original cotton cloth. The intensity of the peak decreased slightly. These structures indicate that a series of chemical modifications have not changed the crystal structure of cotton fiber.
As shown in Fig. 2a, the survey XPS of modified Fe@DOPO cotton demonstrated the coexistence of C, N, O, P and Fe elements compared with the characteristic peaks of C and Oof cotton fabric. In detail, then high-resolution XPS spectrum of N1s (399 eV) was a unique peak after PEI modification. It was the Schiff base reaction between oxidized cotton fabric and amino group on PEI, which also proved that PEI was successfully grafted to the cotton surface. Compared with the PEI-grafted (Fig. 2c), the N1s spectra of Fe-grafted (Fig. 2d) changed from 399.1 to 398.1 eV, confirming C = N bond formed and the Schiff base created between –NH2 of PEI-grafted cotton and aldehyde group of ferrocene formaldehyde, firmly anchored onto the surface of cotton fabrics (Wang et al. 2019). At position of about 708 eV, it was considered to be Fe2p in ferrocene and occurs simultaneously in Fe-grafted and Fe@DOPO cotton curves. 708.55 eV was the Fe 2p3/2 peak and 720.70 eV was the Fe 2p1/2 peak from ferrocene unit, which showed the existence of the ferrocene structure (Woodbridge 2000; Yun et al. 2017). In addition, two characteristic peaks of P were found in P2p (132 eV) and P2s (189 eV), respectively, only appeared after DOPO reacted with cotton (Wang et al. 2019).
The existence of these reactions was confirmed by XPS and FT-IR spectra characterization, as shown in Scheme 1. It was also proved that ferrocene formaldehyde and DOPO were successfully incorporated into the cotton surface.
Morphology analysis
The surface morphologies of the control and as-prepared cotton fabrics were measured by SEM magnified at different times and showed in Fig. 3.
From Fig. 3a, it could be seen that the surface of the original cotton fabric was very smooth, whilst there were slight wrinkles on the surface after PEI treatment (Fig. 3b). As shown in Fig. 3c, the cotton fabric fiber surface formed a rough structure proving that the ferrocene formaldehyde was grafted on the cotton. Finally, Fig. 3d showed the formation of a thick layer of translucent crystalline material on the surface of cotton fiber, and the roughness was very obvious. It should be explained that DOPO reacted with cotton fabric to form a coating. This was consistent with the previous characterization (Chen et al. 2019). Moreover, since roughness was a very important parameter affecting waterproof performance, the unique structure of cotton surface could explain its excellent waterproof performance.
We further used EDX mapping to investigate the element distribution of Fe@DOPO cotton fabric. It can be clearly seen that the elements of C, O, P, N and Fe were dispersed uniformly (Fig. 4). In addition, the chemical compositions on the surface of Fe@DOPO cotton fabric were listed in Fig. 5. In summary, the above results confirmed that ferrocene formaldehyde and DOPO were successfully grafted onto the cotton.
Thermogravimetric analysis (TGA)
The thermal degradation and stability of the material had important effects on the flame retardancy. Hence, we used TGA and DTG to test the thermal decomposition and oxidation process of cotton fabric before and after modification and the corresponding data were listed in Table 1.
As shown in Fig. 6a, the temperature at which the weight loss of cotton fabric was 5% (T5%) was 335°C, and the carbon residue at 800°C was only 8.25%, which may be caused by dehydration and carbonization pyrolysis in the crystallization zone. The T5% of Fe-grafted cotton fabric is 317°C, and the carbon residue increases to 18.9wt%, which is mainly due to the good catalytic carbonization ability of ferrocene (Hu et al. 2017; Tang et al. 2019; Tian et al. 2009). The T5% of Fe@DOPO cotton fabric decreased to 258°C, and the carbon residue rate increased to 46.8 wt%. This was because DOPO began to decompose under the catalysis of ferrocene at a lower temperature to form phosphate, which promoted the dehydration of cotton fiber into carbon, resulting in the increase of carbon residue rate (Chernyy et al. 2015). The improvement of carbon residue rate of modified cotton fabric indicated that Fe@DOPO cotton had better flame-retardant property. In particular, as shown in the DTG curve (Fig. 6b), the maximum mass loss rate (Rmax) of Fe@DOPO cotton was 8.1 wt%/min at 294°C, which was 35.6 wt%/min lower than that of control cotton fabric at 375°C. This indicates that the synergistic effect of ferrocene and DOPO greatly improves the thermal stability of the fabric.
TGA and DTG curves of cotton fabric in air atmosphere were shown in Fig. 6c and 6d, respectively. And their characteristic parameters were listed in Table 1. It can be seen that the T5% of pristine cotton fabric was 325°C, and the char residue at 800°C was only 7.8 wt%. As for Fe@DOPO cotton, the T5% weight loss was at 288°C, and the final carbon residue rate was 36.6%. The results were similar to those in nitrogen atmosphere. In addition, the peak value of DTG (Rmax) curve decreased after modification. The above results showed that the carbon layer was produced and the thermal stability was improved. The results of the study are consistent with those reported in the previous literature (Liao et al. 2017; Amer et al. 2012). The synergistic effect of ferrocene and DOPO could effectively inhibit the thermal oxidative degradation of cotton fabric, which was conducive to the flame retardancy of the fabric. The specific thermal degradation mechanism will be further elaborated.
TG-IR analysis
The TG-IR techniques have been widely used to study the thermal degradation mechanism of flame retardant materials. As seen from Fig. 7 . 3D (three-dimensional) TG-IR image of the pyrolysis products of pristine cotton (a) and Fe@DOPO cotton (b) under nitrogen atmosphere would be used to study the gases released after combustion of the two materials. The Fe@DOPO cotton fabric did not appear new peak after combustion, which indicated that no new toxic gas was produced. The shape of other peaks was similar to that of pristine cotton fabric, but the absorbance value of some peaks was obviously lower than that of pristine cotton fabric. In order to further compare the changes of typical pyrolysis products of pristine cotton and Fe@DOPO cotton, the intensity of characteristic peaks of pyrolysis products (H2O, hydrocarbons, CO2, CO, carbonyl and NH3) were compared in Fig. 8. The absorption peak of decomposition products of Fe@DOPO cotton was weaker than that of pristine cotton, which was supposed to be due to the change of decomposition process and mechanism. The peak at 2960 cm−1 should be hydrocarbons (–CH2– and –CH3) vibration and –OH groups as clearly seen at 3586 cm−1 (Zhao et al. 2017). The signal of CO2 stretching vibration can be observed at 2352 cm−1; moreover, the weak peak at 2184 cm−1 was considered to be the stretching vibration of CO produced by carbon decomposition. (Qian et al. 2017). The peak at 1742 cm−1 was considered to be the C = O vibration of carbonyl compounds (Chen et al. 2017). The characteristic peak at 920 cm−1 was attributed to NH3 emerged from the carbon–nitrogen bond (–N–C or –N = C–). According to the literature reports (Hu et al. 2017; Tian et al. 2009), ferrocene had a strong smoke suppression effect. In the early stage of smoke generation, some iron compounds precipitate on the surface of the substrate to form condensation nuclei, which reduces the efficiency of smoke generation. Soot was preferentially condensed on the surface of iron oxide to reduce emission, so as to achieve the purpose of suppressing smoke and promoting carbon.
Cone calorimetry tests
In order to further demonstrate the combustion behavior of cotton fabric before and after modification in real fire, cone calorimetry tests (CCT) was used to test the combustion parameters, as shown in Fig. 9 and Table 2. The heat release rate (HRR) and total heat release (THR) curves of cotton cloth were shown Fig. 9a and Fig. 9b, respectively. The peak heat release rate (PHRR) and THR for pristine was 280.7 kW/m2 and 4.7 MJ/m2, respectively. While the values of PHRR and THR was decreased to 145.9 kW/m2 and 3.8 MJ/m2 after treating by Fe@DOPO. The following Eq. (2) was the fire growth rate (FGR) of the fire to assess the material hazard, and t was the time to PHRR (Zhang et al. 2020a, b).
Generally speaking, the greater the FGR value is, the more harmful the fire is, which indicate the worse flame retardancy of the material (Shao et al. 2014). The FGR value before modification was 10.8 kW/(m2 s), almost twice as much as that after modification (6.08 kW/(m2 s)). These results showed that in case of fire, the flame retardant properties of these materials gave many people time to escape and reduce the loss of life and property.
It can also be seen from the Table 2 that the ratio of CO2–CO also decreased from 102 to 15, indicating that Fe@DOPO cotton fabric cannot be fully burned due to the fire-retardancy of Fe@DOPO (Duquesne et al. 2004; Zheng et al. 2016). The residue of Fe@DOPO and DOPO-grafted cotton fabric was 21.5 wt% and 16.5 wt%, respectively, while that of the original cotton fabric was only 5.6 wt%, indicating that DOPO was very helpful to increase the carbon residue, and it can be obtained higher carbon residue by synergism of DOPO and ferrocene. This result is consistent with the thermal analysis. Besides, the total smoke release (TSR) of the fabric was increase from 3.4 m2/m2 to 42.9 m2/m2 after treated by Fe@DOPO. This result may be ascribed to the vapour-phase action of DOPO that can interrupt cotton combustion by entrapping radical H• and OH• species generated by its thermo-oxidation (Jiang et al. 2019; Vasiljević et al. 2015; Zhao et al. 2017). These results also indicate that the cotton fabrics release much more volatile products diluting the concentration of flammable gas after treating with Fe@DOPO, which means that Fe@DOPO can act as gas-phase flame retardant mechanism as well as char-formation capability (Jiang et al. 2019). In addition, it could be seen from Table 2 that the TSR of DOPO-grafted cotton was 91.5 m2/m2, which was twice as much as that of Fe@DOPO cotton fabric, indicating that the addition of ferrocene can indeed inhibit the smoke. The same conclusion can be seen in the CO2 / CO ratio. This is consistent with many reported literatures, that is to say ferrocene could accelerate graphitization during combustion and greatly suppress smoke (Kishore et al. 1991; Carty et al. 1996; Hu et al. 2017; Lawson 1976). Possible pyrolytic route of Fe@DOPO cotton fabric during thermal degradation was shown in Scheme 2 (Liao et al. 2017; Li et al. 2020; Wen et al. 2018). With the increasing of temperature, the C–P = O bond was firstly dissociated; decomposing into two parts of ferrocene-based group and DOPO (Li et al. 2020). Subsequently, ferrocene-based group decomposes into ferrocene and cotton fiber. In the gas phase, with increasing of the temperature, DOPO decomposed into PO· and PO2· possessing strong trapping ability, capturing H· and OH· to form inert molecules in air, thus destroying the normal process of combustion reaction(Rakotomalala et al. 2010; Ma et al. 2016). Meanwhile, a lot of non-flammable gases generated to prevent combustion (Chen et al. 2019; Linteris et al. 2000). Then ferrocene can suppress smoke by inhibiting soot nucleation and growth (Carty et al. 1996). In the condensed phase, on the one hand, DOPO decomposed phosphoric acid possessing strong dehydration and carbonization ability, and formed a protective film on the surface of the fiber to prevent further combustion; on the other hand, ferrocene has strong catalytic dehydration and char-forming reaction ability which may prevent levoglucosan formation and accelerate the formation of a protective char layer by catalyzing the dehydration of cellulose macromolecules (Carty et al. 1996; Lawson 1976; Liao et al. 2017). Therefore, the existence of ferrocene can promote the flame retardancy of DOPO and the system composed of ferrocene and DOPO has a good effect on the flame retardancy of cotton fabric.
Char residues analysis
In order to further clarify the synergistic flame-retardant ability of ferrocene and DOPO, the morphology of carbon residue after CCT combustion of pristine cotton samples and Fe@DOPO cotton were studied by SEM. From the high-definition photo after CCT shown in Fig. 10, it can be seen that there was only a little residue left after the combustion of large pieces of original cotton fabric, while there was a large carbon layer left after the combustion of modified cotton fabric. The paste substance can be seen at the bottom of the carbon layer, which should be the protective carbon layer material caused by the expansion of DOPO combustion. This result was similar to the previous TGA characterization. The carbon ashes were further tested by SEM. As shown in Fig. 11a, it can be seen that the carbon ash from the combustion of the original cotton fabric it is very fluffy, and it will burst when it is touched. Figure 11b shown that the modified cotton charcoal ash has a protective layer of 100-300 nm, which should be a carbon layer of phosphate catalyzed by ferrocene.
EDX was then adopted to testify the element composition of Fe@DOPO cotton after CCT, as shown in Fig. 12. As for Fe@DOPO cotton, the content of carbon and phosphorus increased to 65.46% and 5.72% respectively, indicating that the carbonization rate of modified cotton fabric was greatly increased. In addition, 1.68% Fe was found in the residue, which should be the residue of ferrocene combustion. It could be inferred from the flame retardant mechanism that DOPO is first degraded into phosphoric acid under the catalysis of ferrocene at low temperature after ignition, which makes cotton fiber dehydrated into carbon and prevents further combustion (Jin et al. 2017; Shi et al. 2018).
Water repellency
In order to further evaluate the wash-ability of the fabric, the water contact angle of the cotton fabric before and after modification was tested, and the dates were shown in Fig. 13. We were surprised to find that the water contact angle of the cotton fabric modified by ferrocene formaldehyde and DOPO could reach 138°, and the data did not change much after 5 min of storage (132°). This was due to the fact that DOPO and ferrocene formaldehyde were hydrophobic groups, forming a similar superhydrophobic structural model, so the hydrophobicity was very strong. The water repellency of the fabric should be favorable for washing fastness. Therefore, the modified cotton fabric should have good washing resistance.
LOI test and washing resistance
In order to explore the washing resistance of the cotton fabric modified by ferrocene and DOPO, the modified cotton fabric was repeatedly washed by home washing method (GB/T17595-1998). Figure 14 was a high-definition image of the vertical combustion experiment after different washing times. The char length was also shown in the picture. For clearer analysis, the weight change, PHRR and LOI of the fabric after several washing cycles were also listed in Table 3. It can be seen from Fig. 14 that the LOI value of Fe-grafted cotton (23%) was slightly higher than that of pristine cotton fabric, which means that ferrocene has slight flame retardant effect when used alone, which was consistent with the previous conclusion. In order to carry out the comparative experiment, we did the sample of DOPO grafting with cotton fabric, and the weight gain was basically similar, but the LOI value was only 26.5%. What's more, the LOI value decreased rapidly after washing, and there was no flame retardant effect after less than four times. It was estimated that DOPO used alone, adhesion was not good enough. While after 12 times of washing, the LOI value of the modified cotton fabric showed a downward trend, and the weight gain of the fabric also decreases, but very slowly. PHRR values were maintained at a certain value, with little change. The char length was similar, which indicated that the modified cotton had better wash resistance and good flame retardancy than pristine cotton. The main reason should be that ferrocene formaldehyde and DOPO were successfully incorporated into the cotton fabric. Through the chemical bond link, the washing resistance was greatly increased. More importantly, a water repellent layer was formed on the surface of the cotton fabric, which made it difficult for water to penetrate into the fabric, which improved the washing resistance.
Conclusions
In conclusion, ferrocene formaldehyde and DOPO were successfully introduced into cotton fabric through facile chemical reaction to simultaneously endow cotton fabric (Fe@DOPO cotton) better water repellency and flame retardancy. TGA test showed that the thermal stability of the modified cotton fabric was significantly improved. The specific pyrolysis products of Fe@DOPO cotton tested by TG-IR was reduced obviously compare with pristine cotton. More importantly, the PHRR and THR of the modified cotton fabric were reduced by 48.9% and 19.2%, respectively. Through the comparison of LOI values, it was found that Fe-grafted cotton fabric had a little flame retardant effect. The LOI value of DOPO-grafted was 26% before washing, but after washing, the flame retardant effect was lost rapidly. Therefore it was indicated that ferrocene and DOPO have synergistic flame retardant effect. In addition to the flame retardant, the waterproof property of the modified cotton fabric was also greatly improved, and the contact angle was 138°. After 12 times of washing, the flame retardancy of the modified cotton fabric remains unchanged, indicating Fe@DOPO cotton had a good washing resistance. These excellent properties were expected to make the modified cotton fabric applied in many fields.
References
Abou-Okeil A, El-Sawy SM, Abdel-Mohdy FA (2013) Flame retardant cotton fabrics treated with organophosphorus polymer. Carbohyd Polym 92:2293–2298. https://doi.org/10.1016/j.carbpol.2012.12.008
Aksit A, Onar N, Kutlu B, Sergin E, Yakin I (2016) Synergistic effect of phosphorus, nitrogen and silicon on flame retardancy properties of cotton fabric treated by sol-gel process. Int J Cloth Sci Tech 28:319–327. https://doi.org/10.1108/IJCST-03-2016-0029
Alongi J et al (2014) Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym Degrad Stabil 99:111–117. https://doi.org/10.1016/j.polymdegradstab.2013.11.016
Amer WA, Wang L, Yu H, Amin AM, Wang Y (2012) Synthesis and properties of a ferrocene-based metallomesogenic polymer containing Bis(4-hydroxyoctoxyphenyl)sulfone. J Inorg Organomet 22:1229–1239
Bai Z, Jiang S, Tang G, Hu Y, Song L, Yuen RKK (2014) Enhanced thermal properties and flame retardancy of unsaturated polyester-based hybrid materials containing phosphorus and silicon. Polym Advan Technol 25:223–232. https://doi.org/10.1002/pat.3227
Carignan CC et al (2013) Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int 55:56–61. https://doi.org/10.1016/j.envint.2013.02.004
Carty P, Grant J, Metcalfe E (1996) Flame-retardancy and Smoke-suppression Studies on Ferrocene Derivatives in PVC. Appl Organomet Chem 10:101–111. https://doi.org/10.1002/(SICI)1099-0739(199603)10:2%3c101::AID-AOC484%3e3.0.CO;2-7
Chen T et al (2019) Superhydrophobic and flame retardant cotton modified with DOPO and fluorine-silicon-containing crosslinked polymer. Carbohyd Polym 208:14–21. https://doi.org/10.1016/j.carbpol.2018.12.023
Chen X, Wang W, Jiao C (2017) A recycled environmental friendly flame retardant by modifying para-aramid fiber with phosphorus acid for thermoplastic polyurethane elastomer. J Hazard Mater 331:257–264. https://doi.org/10.1016/j.jhazmat.2017.02.011
Chernyy S, Ulah S, Sørensen G, Tordrup SW, Pedersen PB, Almdal K (2015) DOPO-VTS-based coatings in the realm of fire retardants for cotton textile J Appl Polym Sci 132: https://doi.org/10.1002/app.41955
Cooper EM, Covaci A, van Nuijs ALN, Webster TF, Stapleton HM (2011) Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 401:2123–2132. https://doi.org/10.1007/s00216-011-5294-7
Dong Q et al (2013) Synergistic effect of DOPO immobilized silica nanoparticles in the intumescent flame retarded polypropylene composites. Polym Advan Technol 24:732–739. https://doi.org/10.1002/pat.3137
Duquesne S, Lefebvre J, Seeley G, Camino G, Delobel R, Le Bras M (2004) Vinyl acetate/butyl acrylate copolymers. Polym Degrad Stabil 85:883–892. https://doi.org/10.1016/j.polymdegradstab.2004.04.004
Fang F et al (2016) Boron-containing intumescent multilayer nanocoating for extinguishing flame on cotton fabric. Cellulose 23:2161–2172. https://doi.org/10.1007/s10570-016-0928-8
Feng Y, Zhou Y, Li D, He S, Zhang F, Zhang G (2017) A plant-based reactive ammonium phytate for use as a flame-retardant for cotton fabric. Carbohyd Polym 175:636–644. https://doi.org/10.1016/j.carbpol.2017.06.129
Han J et al (2017) Effects of nanocellulose on the structure and properties of poly(vinyl alcohol)-borax hybrid foams. Cellulose 24:4433–4448. https://doi.org/10.1007/s10570-017-1409-4
He P et al (2018) Preparation and flame retardancy of reactive flame retardant for cotton fabric. J Therm Anal Calorim 132:1771–1781. https://doi.org/10.1007/s10973-018-7057-6
Hu C, Li W, Lin Q, Cheng X, Huang Q, Zhang H, Wang Z (2017) Effects of ferrocene on flame temperature, formation of soot particles and growth of polycyclic aromatic hydrocarbons. J Energy Inst 90:893–901. https://doi.org/10.1016/j.joei.2016.08.005
Hu S, Hu Y, Song L, Lu H (2011) Effect of modified organic–inorganic hybrid materials on thermal properties of cotton fabrics. J Therm Anal Calorim 103:423–427. https://doi.org/10.1007/s10973-010-1093-1
Hussein MA, Asiri AM (2012) Organometallic ferrocene- and phosphorus-containing polymers: synthesis and characterization. Des Monomers Polym 15:207–251. https://doi.org/10.1163/156855511X615650
Jiang Z, Xu D, Ma X, Liu J, Zhu P (2019) Facile synthesis of novel reactive phosphoramidate siloxane and application to flame retardant cellulose fabrics. Cellulose 26:5783–5796. https://doi.org/10.1007/s10570-019-02465-2
Jin X et al (2017) Preparation of a novel intumescent flame retardant based on supramolecular interactions and its application in polyamide 11. Acs Appl Mater Inter 9:24964–24975. https://doi.org/10.1021/acsami.7b06250
Kishore K, Kannan P, Iyanar K (1991) Synthesis, characterization, and fire retardancy of ferrocene containing polyphosphate esters. J Polym Sci Part A: Polym Chem 29:1039–1044. https://doi.org/10.1002/pola.1991.080290711
Koshiba Y, Takahashi Y, Ohtani H (2012) Flame suppression ability of metallocenes (nickelocene, cobaltcene, ferrocene, manganocene, and chromocene). Fire Safety J 51:10–17. https://doi.org/10.1016/j.firesaf.2012.02.008
Kwak W, Oh MH, Gong M (2015) Preparation of silver-coated cotton fabrics using silver carbamate via thermal reduction and their properties. Carbohyd Polym 115:317–324. https://doi.org/10.1016/j.carbpol.2014.08.070
Lawson DF (1976) Investigation of the mechanistic basis for ferrocene activity during the combustion of vinyl polymers. J Appl Polym Sci 20:2183–2192. https://doi.org/10.1002/app.1976.070200813
Li et al (2020) Novel eco-friendly flame retardants based on nitrogen-silicone schiff base and application in cellulose. Acs Sustain Chem Eng 8:290–301. https://doi.org/10.1021/acssuschemeng.9b05338
Liang C, Jing L, Shi X, Zhang Y, Xian Y (2012) Magnetically controlled bioelectrocatalytic system based on ferrocene-tagged magnetic nanoparticles by thiol-ene reaction. Electrochim Acta 69:167–173. https://doi.org/10.1016/j.electacta.2012.02.089
Liao D et al (2017) Ferrocene-based nonphosphorus copolymer: synthesis, high-charring mechanism, and its application in fire retardant epoxy resin. Ind Eng Chem Res 56:12630–12643. https://doi.org/10.1021/acs.iecr.7b02980
Linteris GT, Rumminger MD, Babushok V, Tsang W (2000) Flame inhibition by ferrocene and blends of inert and catalytic agents. P Combust Inst 28:2965–2972. https://doi.org/10.1016/S0082-0784(00)80722-5
Ma C, Yu B, Hong N, Pan Y, Hu W, Hu Y (2016) Facile synthesis of a highly efficient, halogen-free, and intumescent flame retardant for epoxy resins: thermal properties, combustion behaviors, and flame-retardant mechanisms. Ind Eng Chem Res 55:10868–10879. https://doi.org/10.1021/acs.iecr.6b01899
Malucelli G et al (2014) Biomacromolecules as novel green flame retardant systems for textiles: an overview. Rsc Adv 4:46024–46039. https://doi.org/10.1039/c4ra06771a
Nguyen TD, Chang S, Condon B, Uchimiya M, Fortier C (2012) Development of an environmentally friendly halogen-free phosphorus-nitrogen bond flame retardant for cotton fabrics. Polym Advan Technol 23:1555–1563. https://doi.org/10.1002/pat.3029
Qian L, Qiu Y, Liu J, Xin F, Chen Y (2014) The flame retardant group-synergistic-effect of a phosphaphenanthrene and triazine double-group compound in epoxy resin. J Appl Polym Sci. https://doi.org/10.1002/app.39709
Qian Y, Li S, Chen X (2017) Preparation of mesoporous silica-LDHs system and its coordinated flame-retardant effect on EVA. J Therm Anal Calorim 130:2055–2067. https://doi.org/10.1007/s10973-017-6508-9
Rakotomalala M, Wagner S, Döring M (2010) Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 3:4300–4327. https://doi.org/10.3390/ma3084300
Shan G, Jia L, Zhao T, Jin C, Liu R, Xiao Y (2017) A novel DDPSi-FR flame retardant treatment and its effects on the properties of wool fabrics. Fiber Polym 18:2196–2203. https://doi.org/10.1007/s12221-017-7244-2
Shao Z, Deng C, Tan Y, Chen M, Chen L, Wang Y (2014) An efficient mono-component polymeric intumescent flame retardant for polypropylene: preparation and application. Acs Appl Mater Inter 6:7363–7370. https://doi.org/10.1021/am500789q
Shi X, Xu Y, Long J, Zhao Q, Ding X, Chen L, Wang Y (2018) Layer-by-layer assembled flame-retardant architecture toward high-performance carbon fiber composite. Chem Eng J 353:550–558. https://doi.org/10.1016/j.cej.2018.07.146
Suryaprabha T, Sethuraman MG (2018) Fabrication of superhydrophobic and enhanced flame-retardant coatings over cotton fabric. Cellulose 25:3151–3161. https://doi.org/10.1007/s10570-018-1757-8
Tang X, Wang C, Zhang F, Wang Q, Wang J, Seifert S, Winans RE (2019) Effect of nickel acetylacetonate addition on soot inception and growth in an ethylene flame studied by using in situ small-angle X-ray scattering. Combust Flame 206:390–399. https://doi.org/10.1016/j.combustflame.2019.05.021
Tian C, Wang C, Ren X, Hong L (2019) Synthesis of silane-modified polyphosphate esters and its application in transparent flame-retardant coatings. J Appl Polym Sci 136:47199. https://doi.org/10.1002/app.47199
Tian K et al (2009) Influence of ferrocene addition to a laminar premixed propene flame: laser diagnostics, mass spectrometry and numerical simulations. P Combust Inst 32:445–452. https://doi.org/10.1016/j.proci.2008.05.056
Vasiljević J et al (2015) Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose 22:1893–1910. https://doi.org/10.1007/s10570-015-0599-x
Wang D, Zhong L, Zhang C, Li S, Tian P, Zhang F, Zhang G (2018) Eco-friendly synthesis of a highly efficient phosphorus flame retardant based on xylitol and application on cotton fabric. Cellulose (London) 26:2123–2138. https://doi.org/10.1007/s10570-018-2193-5
Wang S, Du X, Deng S, Fu X, Du Z, Cheng X, Wang H (2019) A polydopamine-bridged hierarchical design for fabricating flame-retarded, superhydrophobic, and durable cotton fabric. Cellulose (London) 26:7009–7023. https://doi.org/10.1007/s10570-019-02586-8
Wen Y et al (2018) A novel oligomer containing DOPO and ferrocene groups: Synthesis, characterization, and its application in fire retardant epoxy resin. Polym Degrad Stabil 156:111–124. https://doi.org/10.1016/j.polymdegradstab.2018.08.010
Woodbridge CM (2000) DLPR HREELS and XPS Studies of Ferrocene on Ag(100). J Phys Chem B 104:3085–3093. https://doi.org/10.1021/jp993235
Xie C, Zeng B, Gao H, Xu Y, Luo W, Liu X, Dai L (2014) Improving thermal and flame-retardant properties of epoxy resins by a novel reactive phosphorous-containing curing agent. Polym Eng Sci 54:1192–1200. https://doi.org/10.1002/pen.23642
Xie K, Gao A, Zhang Y (2013) Flame retardant finishing of cotton fabric based on synergistic compounds containing boron and nitrogen. Carbohyd Polym 98:706–710. https://doi.org/10.1016/j.carbpol.2013.06.014
Xu F, Zhong L, Xu Y, Zhang C, Zhang F, Zhang G (2019) Highly efficient flame-retardant and soft cotton fabric prepared by a novel reactive flame retardant. Cellulose 26:4225–4240. https://doi.org/10.1007/s10570-019-02374-4
Yang Z, Wang X, Lei D, Fei B, Xin JH (2012) A durable flame retardant for cellulosic fabrics. Polym Degrad Stabil 97:2467–2472. https://doi.org/10.1016/j.polymdegradstab.2012.05.023
Yun J, Chen L, Zhang X, Zhao H, Wen Z, Zhang C (2017) The effects of silicon and ferrocene on the char formation of modified novolac resin with high char yield. Polym Degrad Stabil 139:97–106. https://doi.org/10.1016/j.polymdegradstab.2017.03.018
Zhang F, Lu Y, Wan C, Tian P, Liu M, Zhang G (2020a) A bio-resourced mannitol phospholipid ammonium reactive flame retardant for cotton with efficient antiflaming and durability. Cellulose 27:4803–4815. https://doi.org/10.1007/s10570-020-03064-2
Zhang Z, Dong C, Liu J, Kong D, Sun L, Lu Z (2020b) Preparation of a synergistic reactive flame retardant based on silicon, phosphorus and nitrogen and its application to cotton fabrics. Cellulose 27:1799–1815. https://doi.org/10.1007/s10570-019-02900-4
Zhao B, Liu Y, Zhang C, Liu D, Li F, Liu Y (2017) A novel phosphoramidate and its application on cotton fabrics: synthesis, flammability and thermal degradation. J Anal Appl Pyrol 125:109–116. https://doi.org/10.1016/j.jaap.2017.04.011
Zheng D, Zhou J, Zhong L, Zhang F, Zhang G (2016) A novel durable and high-phosphorous-containing flame retardant for cotton fabrics. Cellulose 23:2211–2220. https://doi.org/10.1007/s10570-016-0949-3
Acknowledgments
This work was funded by National Natural Science Foundation of China (NO. 51773129, 51903167), Support Plan of Science and Technology Department of Sichuan Province, China (2019YFG0257, 2020YFG0071), International Science and Technology Cooperation Program of Chengdu (2019-GH02-00021-HZ), Miaozi Project in Science and Technology Innovation Program of Sichuan Province (20-YCG045). We also appreciate Mi Zhou and Sha Deng for her assistance with the experimental test.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Y., Wang, S., Du, X. et al. Durable flame retardant and water repellent cotton fabric based on synergistic effect of ferrocene and DOPO. Cellulose 28, 1809–1826 (2021). https://doi.org/10.1007/s10570-020-03636-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03636-2