Abstract
Organophosphate triesters tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate are widely used flame retardants (FRs) present in many products common to human environments, yet understanding of human exposure and health effects of these compounds is limited. Monitoring urinary metabolites as biomarkers of exposure can be a valuable aid for improving this understanding; however, no previously published method exists for the analysis of the primary TDCPP metabolite, bis(1,3-dichloro-2-propyl) phosphate (BDCPP), in human urine. Here, we present a method to extract the metabolites BDCPP and diphenyl phosphate (DPP) in human urine using mixed-mode anion exchange solid phase extraction and mass-labeled internal standards with analysis by atmospheric pressure chemical ionization liquid chromatography tandem mass spectrometry. The method detection limit was 8 pg mL−1 urine for BDCPP and 204 pg mL−1 for DPP. Recoveries of analytes spiked into urine ranged from 82 ± 10% to 91 ± 4% for BDCPP and from 72 ± 12% to 76 ± 8% for DPP. Analysis of a small number of urine samples (n = 9) randomly collected from non-occupationally exposed adults revealed the presence of both BDCPP and DPP in all samples. Non-normalized urinary concentrations ranged from 46–1,662 pg BDCPP mL−1 to 287–7,443 pg DPP mL−1, with geometric means of 147 pg BDCPP mL−1 and 1,074 pg DPP mL−1. Levels of DPP were higher than those of BDCPP in 89% of samples. The presented method is simple and sufficiently sensitive to detect these FR metabolites in humans and may be applied to future studies to increase our understanding of exposure to and potential health effects from FRs.

The flame retardant TDCPP is metabolized to BDCPP and detected in human urine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphate (OP) esters tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) are widely used as additive flame retardants (FRs) in polyurethane foams, which are commonly found in sofas, chairs, car upholstery, and related products [1–3]. Due to the recent phaseout of the commercial FR mixtures PentaBDE and OctaBDE in many regions, including the USA and Europe, production and use of alternative FRs, such as OPs, is likely to increase [2]. Recently, TDCPP and TPP were detected in 96% and 98%, respectively, of US house dust samples (n = 50) with geometric means of 1,890 and 7,360 ng g−1, respectively [3]. Interestingly, recent European values of TDCPP and TPP in house dust samples (n = 33) are lower, with means of 570 and 2,020 ng g−1 for TDCPP and TPP, respectively [4], indicating that the use of and the potential exposure to these OPs may vary geographically.
Given the high usage of OPFRs (organophosphate flame retardants) in human environments, there is a justified concern over the potential human health effects from exposure to these compounds. TPP can cause contact dermatitis in humans [5, 6]. In vitro studies have demonstrated that TPP has endocrine disrupting [7], hemolytic [8], and neurotoxic potentials [9]. Additional in vitro studies have indicated that TDCPP may be mutagenic [10], nephrotoxic and neurotoxic [11, 12]. Meeker and Stapleton observed associations between levels of TDCPP and TPP in house dust and reduced semen quality in men, suggesting endocrine disruption [13]. These authors also observed reduced free thyroxine associated with increased house dust TDCPP, suggesting this FR may also impair thyroid function. The mechanisms involved in the toxic effects associated with TPP and TDCPP, however, are not well understood.
Despite recent reports of high levels and detection frequencies of TDCPP and TPP in human environments, and given the potential toxicity of these compounds, there is very little understanding of human exposures and body burdens of these FRs, or of the levels of their metabolites in humans. Studies using rats and rat liver microsomes observed that TDCPP and TPP are metabolized to bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP), respectively [14–16]. In rat liver microsomes, incubation with NADPH yielded 91% transformation of TPP and 43% of TDCPP in 30 min. In rats, half lives of TDCPP varied with tissue from 1.5 to 5 h [14]. Mammalian studies with TPP are not available. These metabolites in human biological fluids, such as urine, may therefore serve as biomarkers of human exposure to their parent FRs. No methods currently exist to measure BDCPP in human biological fluids or tissues; however, there are a few published methods to measure DPP in human urine [17–19].
Published methods for measuring urinary DPP rely on analysis of concentrated urine [17], or on solid phase extraction (SPE) with either a non-commercial molecularly imprinted polymer [19] or a commercially available cartridge containing a reversed-phase polymer with polar functionality [18]. Matrix effects causing difficulties in extraction and/or analysis were reported in the two methods that used liquid chromatography–mass spectrometry (LC/MS) [17]. Only one study on DPP relied on mass-labeled internal standards, which is the most reliable quantification approach [18]; however, this method required two SPE steps and chemical derivatization to facilitate gas chromatography–mass spectrometry (GC-MS) analysis. Quantification of DPP in the other published methods was achieved by standard addition [17] or by using dibutyl phosphate as an internal standard [19], which has recently been observed in human urine [17], making this compound unsuitable as an internal standard.
This study addresses the current need for approaches to evaluate the prevalence of OPFR metabolites in human urine. Our primary objective was to develop a method to extract BDCPP and DPP from human urine and to measure them by liquid chromatography–tandem mass spectrometry (LC/MS-MS). The sample preparation relies on mixed-mode weak anion-exchange SPE to target BDCPP and DPP, which have low estimated pK a values (1.18 and 1.12, respectively [20]) and are anions over typical urine pH range (pH 5–8). This method employs easy to use and commercially available SPE products, uses mass-labeled internal standards for quantification, and relies on LC/MS-MS thereby circumventing added sample workup necessary for chemical derivatization and GC-MS analysis. Our second objective was to apply the developed method to determine whether BDCPP and DPP would be detected in urine samples randomly collected from non-occupationally exposed adults.
Materials and methods
Chemicals
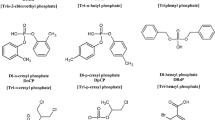
BDCPP (98%) was synthesized by Wellington Laboratories (Guelph, Ontario, Canada). DPP (99%), pyrrolidine (99%) and ammonium acetate (99%) were purchased from Isotec (Miamisburg, OH, USA), Alfa Aesar (Ward Hill, MA, USA), and Fluka (St. Louis, MI, USA), respectively. Mass-labeled internal standards included deuterated BDCPP (d10-BDCPP) and DPP (d10-DPP), which were synthesized by Dr. Vladimir Belov (Max Planck Institute for Biophysical Chemistry, Goettingen, Germany). 13C12-labeled bisphenol A (13C-BPA) was purchased from Cambridge Isotope Laboratories (Andover, MA, USA) and used to quantify recovery of the phosphate diester internal standards. All stock solutions were prepared in HPLC-grade methanol (MeOH; Honeywell, NJ, USA) and stored under refrigeration in the dark. LC/MS-grade water and MeOH (Honeywell) were used for the LC/MS-MS mobile phase. HPLC-grade water (JT Baker, Philipsburg, NJ, USA), MeOH (EMD, Gibbstown, NJ, USA) and acetonitrile (ACN; BDH, West Chester, PA, USA) were used in the SPE procedure described below. Structures of the parent compounds, the phosphate diester metabolites, and respective internal standards are provided in Fig. 1.
Urine collection and characterization
Nine urine samples from non-occupationally exposed adult volunteers in North America were collected in amber glass jars and frozen at −20 °C until use. For three donors, urine was also collected at the same time in polypropylene specimen cups to evaluate effects of collection cup material. Equal volumes (10 mL) of all nine individual urine samples were combined for a pooled urine sample for use in matrix spike tests. Urine was characterized for pH, specific gravity, total creatinine, and total protein. Specific gravity was measured using a digital handheld refractometer (Atago, Bellevue, WA, USA). Creatinine was measured using an enzymatic assay (Diazyme, Poway, CA, USA) with a colorimetric endpoint analyzed on a plate reader (FLUOStar Optima). Total protein was measured colorimetrically on a plate reader (FLUOStar Optima) using the Bradford Assay (Pierce Coomassie Plus, Rockford, IL, USA).
Urine extraction
In the course of developing a method to extract urinary BDCPP and DPP, we have evaluated several SPE products including Varian Bond Elut Plexa (Agilent, Santa Clara, CA, USA), SampliQ (Agilent), Oasis WAX (Waters Inc., Milford, MA, USA), Bond Elut DEA, Bond Elut PSA, Bond Elut NH2, and StrataX-AW (Phenomenex, Torrance, CA, USA). Of all these SPEs, the StrataX-AW weak anion exchange cartridge proved most effective. For extraction using StrataX-AW (60 mg, 3 mL), 5 mL of urine was spiked with 10 μL each of d10-BDCPP (2.3 μg mL−1) and d10-DPP (1.21 μg mL−1), diluted 1:1 (v/v) with HPLC-grade water and acidified to pH 6.5 with 0.1 M acetic acid if the sample was above pH 6.5. SPE cartridges were conditioned with 2 mL of methanol followed by 2 mL HPLC-grade water. The sample was passed through the SPE cartridge at a flow rate no greater than 1 mL min−1. The cartridge was then washed with 2 mL HPLC-grade water and dried under vacuum. Each cartridge was eluted with 2 mL ACN containing 5% pyrrolidine, concentrated to dryness under N2 at 45 °C, reconstituted in 500 μL of 4:1 HPLC-grade water/MeOH, and filtered through a 0.2-μm nylon membrane. Blank extractions were conducted using 5 mL of HPLC-grade water. All standards were prepared in of 4:1 HPLC-grade water/MeOH.
LC/MS-MS analysis
BDCPP and DPP extracted from urine was analyzed by LC/MS-MS on an Agilent 1200 series LC connected to an Agilent 6410B triple quadrupole MS detector with multimode source. Chromatographic separation of the extracts (5 μL injection) was performed on a Kinetex XBC18 column (100 × 2.1 mm; 2.6 μm; Phenomenex) maintained at 45 °C. The mobile phases consisted of LC/MS-grade water and MeOH and the flow rate was 0.3 mL min−1. Analytes were separated over a gradient of 20% to 100% MeOH from 0 to 6 min and held at 100% MeOH for 2 min. Between injections, the column was re-equilibrated at 20% MeOH for 10 min. BDCPP and DPP were detected by atmospheric pressure chemical ionization (APCI) operating in negative ionization mode using multiple reaction monitoring under the conditions described in Table 1. In the ion source, gas (N2) temperature was 350 °C, vaporizer temperature was 200 °C, gas flow was 10 mL min−1, nebulizer pressure was 345 kPa (50 psi), the capillary voltage was −2,500 V and the corona charge was 4 μA. Standards curves were linear from 194 to 194,000 pg mL−1 for BDCPP and 406 to 406,000 pg mL−1 for DPP.
Assessment of method performance
Several criteria were used to evaluate method performance using the SPE extraction and LC/MS-MS analysis described above. Analyte recoveries were determined from triplicate extractions of the pooled urine sample spiked with BDCPP and DPP at three concentrations (78, 1,458, and 7,760 pg mL−1 for BDCPP; 238, 4,462, and 23,202 pg mL−1 for DPP). Quantification of the mass-labeled internal standards was calculated using 13C12-BPA as an internal standard added just prior to LC/MS-MS analysis. Method repeatability was evaluated in triplicate extractions of all urine samples. Matrix effects were evaluated by comparing areas observed for analytes spiked into pooled urine extract at three levels to corresponding areas measured in spiked 4:1 water/MeOH, as recommended by Matuszewski et al. [21]. The instrumental detection limit (IDL) was calculated as three times the average baseline noise in the quantitative MRM signal for each analyte and standard. Method detection limits (MDLs) were calculated for each analyte as 3 × standard deviation of the blanks normalized to 5 mL of urine.
Results and discussion
Early method development
Early method development targeted only BDCPP, which was initially our primary interest. Both BDCPP and the urine matrix presented challenges in the development of both the extraction and LC/MS-MS analysis. Initial trials using reversed-phase SPE (Varian Bond Elut Plexa; Agilent SampliQ) and liquid–liquid extractions yielded low analyte recoveries (e.g., <20%), possibly because of the highly polar character of BDCPP and its low pK a (estimated pK a, 1.18 [20]), which may have made it difficult to fully protonate BDCPP and facilitate partitioning. Because BDCPP is likely an anion at pHs commonly observed in urine (e.g., pH 6–7), subsequent trials focused on ion-exchange SPE using Oasis WAX (pK a, ∼6.5), StrataX-AW (pK a, 9), and Bond Elut NH2 (pK a, 9), DEA (pK a, 10.7), PSA (pK as, 10.1 and 10.9) and SAX (permanently positively charged). A summary of the most successful extractions using these products is provided in Table 2. No BDCPP was recovered from the strong anion exchange phase (Bond Elut SAX). For the weak anion exchange cartridges (i.e., all but Bond Elut SAX), trials included testing dilution and pH adjustment of the sample, compositions of the wash step (e.g., deionized (DI) water, ammonium formate, and acetate buffers) and elution step (e.g., volume and selection of organic solvent and base additive). Because the pK a of the Oasis WAX cartridge falls within the pH range of urine, pH adjustment of the sample with 10-mM ammonium acetate buffer at pH 5 was necessary. For all other cartridges, fresh DI water yielded best results for dilution of the sample and for the wash step. For the elution step, MeOH and ACN were tried with addition of up to 10% NH4OH (pK a, 9.25 for ammonium ion [22]) and pyrrolidine (pK a, 11.31 [22]). Recoveries ≥70% were observed with Oasis WAX, StrataX-AW and NH2 cartridges. Overall, however, extraction using StrataX-AW was most promising, because high recoveries were matched with low matrix interferences (e.g., low-ion suppression in post-extraction spikes) when eluting with ACN containing 5% pyrrolidine. This additive likely provided better recoveries of BDCPP at least in part because its pK a (11.3) is sufficiently above the sorbent pK a (9) to neutralize most of the sorbent positive charge, reducing ionic interactions between the sorbent and analyte and facilitating analyte removal from the SPE. Other studies investigating the use of SPE for recovery of phosphate diesters found success using the mixed-mode polymeric SPE cartridges Isolute ENV+ (Biotage; [18]) and non-commercially available molecularly imprinted polymers [19]. Interestingly, Schindler et al. [18] observed poor recoveries of phosphate diesters using anion exchange SPE, however, these authors did not elaborate on the methods attempted using anion exchange. Similar to our results, Moller et al. [19] noted lack of recovery of DPP from strong anion exchangers.
Selection of an analytical column was also critical in the method development. Analysis using a traditional C18 column (Thermo Keystone Hypersil BDS (100 × 4.6 mm; 5 μm) was complicated by a BDCPP elution near the injection front and by co-eluting matrix interferences. The elution profile was improved by using hydrophilic interaction liquid chromatography (HILIC) on a Waters XBridge Amide column (50 × 2.1 mm; 3.5 μm) and a Phenomenex Kinetex HILIC column (100 × 2.1 mm; 2.6 μm). Both columns contain polar functionality that likely increased retention of the polar anionic phosphate diesters. Analysis on the Kinetex HILIC column, however, was problematic for several urine samples, as evidenced by relative retention time shifts for BDCPP of up to 28% compared with reference standards. Interestingly, use of the Phenomenex Kinetex XBC18 (100 × 2.1 mm; 2.6 μm), another reversed-phase column, eliminated retention time shifting problems, while allowing sufficient retention for elution of BDCPP well past the injection front. Chromatograms of BDCPP and DPP in standards and urine extracts analyzed on the XBC18 column are shown in Fig. 2. Differences in column stationary phase characteristics may explain the different behaviors of BDCPP on the Kinetex XBC18 and Hypersil columns. According to the manufacturers’ descriptions of the columns, the Kinetex XBC18, compared with the Hypersil, contains smaller particles of silica that allows for increased theoretical plates. Furthermore the silica particles have a fused core as opposed to the fully porous particles in the Hypersil column. The fused core particles improve the efficiency of analyte mass transfer in the stationary phase thereby reducing band broadening. Additionally, although both are essentially reversed-phase C18 columns, there may be differences in the manufacturing process and starting materials that result in differences in the chemistry of the particle surface, which ultimately can affect analyte retention.
Example of LC/MS-MS chromatograms of (a) bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and (b) diphenyl phosphate (DPP) in a standard and a urine extract separated on Phenomenex Kinetex XBC18 (100 × 2.1 mm; 2.6 μm). Concentrations of BDCPP standard and sample were 9,700 and 7,170 pg mL−1, respectively. Concentrations of DPP standard and sample were 4,060 and 2,504 pg mL−1, respectively. The volume of extract analyzed was 0.5 mL
Analysis of BDCPP and DPP by MS was evaluated using both negative electrospray ionization (ESI(−)) and APCI(−). While both compounds ionized under both ionization modes, APCI(−) was selected because preliminary investigations indicated fewer matrix effects using APCI(−).
Once an approach for the extraction and analysis of BDCPP in urine was established, it was evaluated for extraction of DPP in preliminary experiments by comparing recoveries of DPP spiked into urine to values in spiked 4:1 water:MeOH following subtraction of background levels. Additionally, some pre-extraction sample treatments were tested. Protein precipitation prior to extraction was evaluated using acetone or ACN, followed by centrifugation and evaporation of the solvent from the supernatant, but this treatment did not improve analyte recovery. Because many metabolites in urine are present in a conjugated form, enzymatic treatment of the urine with glucuronidase and sulfatase enzymes, including bovine β-glucuronidase B-10, Eschericia coli glucuronidase type VII-A, and Helix pomatia glucuronidase were evaluated. These treatments did not improve recovery and in many trials reduced recovery. This does not necessarily imply that conjugated BDCPP or DPP forms do not occur in urine. Commercially available glucuronidase and sulfatase enzyme preparations, however, can vary considerably in substrate preference, and it is possible that another enzyme preparation may be more specific for BDCPP and DPP conjugates.
Sample characterization
Urine was collected in early 2011 from nine donors in the U.S. Donors were aged 23–46 years and included five females and four males. Urine pH (5.32–7.21; average, 6.35 ± 0.67), specific gravity (1.0028–1.0238; average, 1.0105 ± 0.0066), creatinine (119–2,112 μg mL−1; average, 756 ± 621 μg mL−1), and total protein (12–264 μg mL−1; average, 57 ± 5 μg mL−1) generally fell within levels considered normal [23–25]. Because some samples had slightly alkaline pH, acidification to pH 6.5 with 0.1 M acetic acid was necessary to ensure pH of the sample loaded onto the SPE was well below the sorbent pK a (9.0).
Method performance
Method performance data for the extraction of BDCPP and DPP from urine using anion exchange SPE are presented in Table 3. Recoveries were assessed at three concentrations of each analyte in triplicate in a pooled urine sample comprised of equal volumes of all nine individual urine samples, and were determined after background levels of metabolites in the urine were subtracted. For BDCPP, recovery at the lowest spike level (78 pg mL−1) was 82 ± 10%. Higher BDCPP recoveries of 91 ± 4% and 95 ± 2% were observed at the medium (1,458 pg mL−1) and high (7,760 pg mL−1) spike levels, respectively. Recoveries of 71 ± 12%, 76 ± 8% and 97 ± 3% were observed for DPP added at the low (238 pg mL−1), medium (4,462 pg mL−1), and high (23,202 pg mL−1) spike levels, respectively. Recoveries for DPP are comparable to those reported by Moller et al. [19] (78%), but lower than that reported by Schindler et al. (99.3%) [18]. The current study is the first to present a method for extracting BDCPP from urine and therefore previously reported recovery values for BDCPP are unavailable. Recoveries of the internal standards averaged 90 ± 19% for d10-BDCPP and 86 ± 16% for d10-DPP. Method repeatability was assessed in triplicate extractions of all urine samples. For BDCPP, relative standard deviations for triplicate extractions ranged from 4% to 23% and averaging 13% across all samples. Triplicate extractions of DPP yielded relative standard deviations ranging from 9% to 31% and averaging 18% across all samples. In eight samples, including the pooled urine sample, greater variability was observed for extracted DPP than for BDCPP for reasons that are unclear.
The IDL, defined as three times the average baseline noise, was lower for BDCPP (0.3 pg/5 μL injection) than for DPP (1.7 pg/5 μL injection). The IDL observed for DPP is notably lower than values reported in previously published methods developed for analysis of urinary DPP: 75 pg per injection [18] and 5,000 pg per injection [19]. MDLs, calculated as the 3× standard deviation of the blanks, were 8 pg BDCPP mL−1 and 204 pg DPP mL−1 in urine. Comparison of our MDL value for DPP to results from previously published methods is confounded due to differences in the validation criteria employed for each method. Criteria provided by Moller et al. [19] and Schindler et al. [18] allowed us to calculate an MDL value for DPP according to our definition described above. MDL for our method is lower than that calculated from the validation criteria reported by Moller et al. (64,103 pg mL−1) [19] but higher than that of Schindler et al. (15 pg mL−1) [18]. Because no analyte-free urine was available, blank extractions were conducted using HPLC-grade water acidified with 0.1 M acetic acid. No BDCPP was observed in blank extractions, however they did contain traces of DPP at levels of 79 ± 41 pg mL−1 (Table 3). No source for the background DPP could be found. However, scientific literature demonstrates the use of DPP in metal lubrication and protection [26, 27], in plastics [28], and it is sold in products used in coating applications (e.g., IsleChem, LLC, phenyl acid phosphate, http://www.islechem.com/pdfs/papmsds.pdf). It is also a product of degradation from cellulose acetate films [29]. Investigation into the source of the background DPP levels indicated that the SPE cartridges contributed little to no DPP to the samples (data not shown). Despite the background levels of DPP observed, the detection limits were sufficiently sensitive to quantify DPP in all samples.
To ensure the analytes did not degrade under the basic conditions necessary for their elution from the SPE cartridge, a 4-h stability experiment at room temperature of BDCPP and DPP in the elution solution (ACN with 5% pyrrolidine) was executed. Concentrations of both compounds measured at 0, 1, and 4 h were not statistically different, indicating the compounds can be regarded stable under these conditions. Additionally, the concentrations of analytes in the urine extracts stored at −20 °C for 20 days were found to be within 6% of original measurements, indicating that extracted analytes are stable over this time period.
Matrix effects were evaluated by standard addition tests. Extracts of the pooled urine as well as 4:1 water/MeOH were spiked at three levels of BDCPP (522, 988, and 9,931 pg per extract) and DPP (1,139, 2,423, and 21,245 pg per extract). Peak areas of BDCPP and DPP measured in unspiked pooled urine extract was subtracted from corresponding peak areas observed in the spiked extracts. The observed peaks areas of spiked analytes in the urine extract were compared with the expected areas measured in spiked water/MeOH after subtraction of peak areas of background DPP in unspiked water/MeOH. Observed peak areas in the spiked extracts ranged from 107 ± 5% to 117 ± 9% of expected areas for BDCPP, and 98 ± 15% to 128 ± 7% of expected values for DPP. Overall, these results suggest that some minor matrix-dependent ion enhancement may occur for both analytes (Table 3). Linear regression of the analyte response by the expected total analyte concentration yielded intercepts not significantly different than 0 (ANOVA, p < 0.05), providing further support that matrix interferences were minimal. Because urine samples may vary considerably with regard to types and concentrations of solutes, it is not surprising that some matrix effects may occur. In the most ideal situation, matrix effects may be accounted for by preparing standards in the same matrix as the sample. In this case, however, no urine tested was found free of BDCPP and DPP (results presented below), hence, the analysis relied on the use of mass-labeled internal standards that compensate for the occurring matrix effects.
Levels of BDCPP and DPP in urine
Levels of BDCPP and DPP in nine random urine samples are reported in Table 4 and Fig. 3 with and without normalization to specific gravity to account for differences in hydration levels across donors. Concentrations of BDCPP and DPP in urine were normalized to urine specific gravity as follows [30]:
where P is the measured analyte concentration, SG is urine specific gravity, and P n is analyte concentration normalized to SG. Normalization of the concentrations to creatinine levels, often used as a means to account for hydration levels and overall urine concentration, was not employed since creatinine production may vary with sex, age, and health condition [23]. Both BDCPP and DPP were observed in all urine samples. Non-normalized BDCPP levels ranged from 46 to 1,662 pg mL−1 with a median of 83 pg mL−1. In 89% of samples, levels of DPP (non-normalized) were higher than those of BDCPP, ranging from 287 to 7,443 pg mL−1, with a median of 803 pg mL−1. Concentrations of both BDCPP and DPP were log-normally distributed (Shapiro–Wilk test, p < 0.05; Kolmogorov–Smirnov test on log-transformed values, p > 0.05). Geometric means of non-normalized BDCPP and DPP were 148 and 1,074 pg mL−1, respectively. When analytes were normalized to specific gravity, BDCPP ranged from 88 to 3,469 pg mL−1 with a geometric mean of 410 pg mL−1 and DPP ranged from 569 to 63,796 pg mL−1 with a geometric mean of 1,810 pg mL−1.
Bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in nine individual urine samples and pooled urine sample (composite of equal volumes of individual samples) presented as concentrations normalized to urine specific gravity. Error bars are standard deviations of triplicate extractions
Urinary DPP and BDCPP concentrations were not correlated with each other or significantly related to sex, age, or any measured urine characteristic. This result is not surprising given the small number of samples evaluated. Additional studies evaluating urinary phosphate diesters is necessary to understand how BDCPP and DPP concentrations vary within the population and how variable measurements may be over time within individuals. For the three urine samples collected in both glass and plastic, BDCPP and DPP values were higher in urine collected in glass, with the exception of DPP in sample 1 (Fig. 4). However, this comparison was only significant (ANOVA on log-transformed values and non-transformed values, p < 0.05) for BDCPP in samples 1 and 2. Plastic specimen cups are commonly used to collect urine samples; however, these results suggest that some urinary BDCPP and DPP may adhere to the plastic container. Therefore, glass containers are recommended for use whenever possible.
Bis(1,3-dichloro-2-propyl) phosphate (BDCPP) (a) and diphenyl phosphate (DPP) (b) in three urine samples collected in glass and plastic containers. Values are concentrations normalized to urine specific gravity. Error bars are standard deviations of triplicate extractions. *p < 0.05 (ANOVA), samples for which values are significantly different between glass and plastic containers
There are no previously published reports of BDCPP levels in human urine, and only limited information on the urinary levels of other dialkyl and/or diaryl OPFR metabolites. Schindler et al. reported DPP levels in 30 non-occupationally exposed residents of southern Germany [18]. Values were presented as concentrations in urine not adjusted for specific gravity or any other parameter. DPP was not detected in 70% of the samples, and the median level was below the detection limit (15 pg mL−1). The highest value reported was 4,100 pg mL−1, approximately half of the highest value observed in the current study, suggesting that geographical differences may factor into human exposure to TPP. However, recently, Reemtsma et al. reported a median of 1,300 pg mL−1 and 95% percentile of 28,600 pg mL−1 DPP in human urine in Germany [17]. These results vary considerably from those found by Schindler et al. [18]. Several studies published in the last few years have reported high levels of TDCPP and TPP, the parent OPs of BDCPP and DPP, respectively, in polyurethane foams and in dust from houses, cars, and workplaces [1–3]. Given the high frequency of occurrence of these OPFRs in areas where human exposure is likely, it is not surprising that most samples contained one or both diester metabolites.
Conclusions
The method presented here for analysis of OPFRs in human urine is the first published for BDCPP and one of few methods available for DPP. Advantages over previously published methods for urinary DPP analysis include use of readily available SPE products, use of mass-labeled internal standards, no need for chemical derivatization, and, in some cases, lower detection limits. Method development highlighted the difficulties in extracting and analyzing soluble anionic compounds from urine, but also emphasized the advances made in SPE technology and chromatography stationary phases. Although we evaluated these metabolites in urine from nine volunteers, additional research examining a greater number of donors is needed to characterize levels of BDCPP and DPP within a population. Additional research may also focus on adaptation of this method to other biological matrices such as serum or to other OPFRs.
References
Marklund A, Andersson B, Haglund P (2003) Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 53(9):1137–1146
Reemtsma T, Quintana JB, Rodil R, García-López M, Rodríguez I (2008) Organophosphorus flame retardants and plasticizers in water and air I. Occurrence and fate. Trends Anal Chem 27(9):727–737
Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF (2009) Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol 43(19):7490–7495
Van den Eede N, Dirtu AC, Neels H, Covaci A (2011) Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int 37(2):454–461
Kanerva L, Jolanki R, Estlander T (1997) Allergic and irritant patch test reactions to plastic and glue allergens. Contact Derm 37(6):301–302
O’Driscoll JB, Marcus R, Beck MH (1989) Occupational allergic contact dermatitis from triphenyl phosphite. Contact Derm 20(5):392–393
Honkakoski P, Palvimo JJ, Penttilä L, Vepsäläinen J, Auriola S (2004) Effects of triaryl phosphates on mouse and human nuclear receptors. Biochem Pharm 67(1):97–106
Sato T, Watanabe K, Nagase H, Kito H, Niikawa M, Yoshioka Y (1997) Investigation of the hemolytic effects of various organophosphoric acid triesters (OPEs) and their structure–activity relationship. Toxicol Environ Chem 59:305–313
WHO (1991) Environmental Health Criteria 111: triphenyl phosphate. World Health Organization, Geneva
Gold MD, Blum A, Ames BN (1978) Another flame-retardant, tris-(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science 1978(4343):785–787
Soederlund EJ, Dybing E, Holme JA, Hongslo JK, Rivedal E, Sanner T, Nelson SD (1985) Comparative genotoxicity and nephrotoxicity studies of the two halogenated flame retardants tris(1,3-dichloro-2-propyl) phosphate and tris(2,3-dibromopropyl) phosphate. Acta Pharmacol Toxicol 56:20–29
Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, Stapleton HM (2011) Is the PentaBDE replacement, tris(1,3-dichloro-2-propyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharm, doi:10.1016/j.taap.2011.01.005
Meeker JD, Stapleton HM (2010) House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect 118(3):318–323
Nomeir AA, Kato S, Matthews HB (1981) The metabolism and disposition of tris(1,3-dichloro-2-propyl) phosphate (fyrol fr-2) in the rat. Toxicol Appl Pharm 57(3):401–413
Lynn RK, Wong K, Garviegould C, Kennish JM (1981) Disposition of the flame-retardant, tris(1,3-dichloro-2-propyl) phosphate, in the rat. Drug Metab Disposition 9(5):434–441
Sasaki K, Suzuki T, Takeda M, Uchiyama M (1984) Metabolism of phosphoric-acid triesters by rat-liver homogenate. B Environ Contam Tox 33(3):281–288
Reemtsma T, Lingott J, Roegler S (2011) Determination of 14 monoalkyl phosphates, dialkyl phosphates and dialkyl thiophosphates by LC-MS/MS in human urinary samples. Sci Tot Environ 409(10):1990–1993
Schindler BK, Forster K, Angerer J (2009) Determination of human urinary organophosphate flame retardant metabolites by solid-phase extraction and gas chromatography-tandem mass spectrometry. J Chromatogr B 877(4):375–381
Moller K, Crescenzi C, Nilsson U (2004) Determination of a flame retardant hydrolysis product in human urine by SPE and LC-MS. Comparison of molecularly imprinted solid-phase extraction with a mixed-mode anion exchanger. Anal Bioanal Chem 378(1):197–204
Chemical Abstracts Service: Columbus, OH, 2011; RN 115-86-6 (diphenyl phosphate) and RN 13674-87-8 (tris(1,3-dichloro-2-propyl) phosphate; pK a values, calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02. (2011)
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal Chem 75(13):3019–3030
Haynes WM (2011) (ed) CRC handbook of chemistry and physics, 91st edn (Internet Version 2011). CRC Press, Boca Raton
Simerville JA, Maxted WC, Pahira JJ (2005) Urinalysis: a comprehensive review. Am Fam Physician 71(6):1153–1162
Encyclopedia ADAMME (2009) Atlanta (GA): A.D.A.M., Inc.; ©2005. Creatinine-urine (updated 2009 Aug 7). Available from: http://www.nlm.nih.gov/medlineplus/ency/article/003610.htm. Accessed 2 Apr 2011
Encyclopedia ADAMME Atlanta (GA): A.D.A.M., Inc.; ©2005. Protein-urine (updated 2009 Aug 7). Available from: http://www.nlm.nih.gov/medlineplus/ency/article/003580.htm. Accessed 2 Apr 2011
Markley TA, Forsyth M, Hughes AE (2007) Corrosion protection of AA2024-T3 using rare earth diphenyl phosphates. Electrochim Acta 52:4024–4031
Yu T, Lin C-T (1997) Performance of in-situ phosphatizing reagents in solvent-borne paints. Ind Eng Chem Res 36:368–374
Camacho W, Karlsson S (2000) Quality-determination of recycled plastic packaging waste by identification of contaminants by GC-MS after microwave assisted extraction (MAE). Polym Degrad Stab 71:123–134
Shinagawa Y, Murayama M, Sakaino Y (1992) Investigation of the archival stability of cellulose triacetate film: the effect of additives to CTA support. Spec Publ - R Soc Chem 105:138–150
Meeker JD, Yang T, Ye X, Calafat AM, Hauser R (2010) Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect 119(2):252–257. doi:10.1289/ehp.1002238
Acknowledgments
The authors thank Alex Konstantinov and Wellington Labs, Inc. for synthesis and donation of BDCPP, and Dr. Vladimir N. Belov at Max Planck Institute for Biophysical Chemistry, Göttingen, Germany, for synthesis of d10-BDCPP and d10-DPP. The authors also acknowledge funding support from NIEHS (grants RO1ES016099 and RO1ES015829). Alexander van Nuijs and Adrian Covaci are financially supported by Ph.D. and postdoctoral fellowships, respectively, from the Research Scientific Foundation-Flanders (FWO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cooper, E.M., Covaci, A., van Nuijs, A.L.N. et al. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 401, 2123–2132 (2011). https://doi.org/10.1007/s00216-011-5294-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5294-7