Abstract
Production of deuterated cellulose is important from both theoretical and practical perspectives. In this study, cellulose fibers of cotton and Tencel fibers with exchange-resistant deuterium incorporation were prepared by hydrogen–deuterium exchange treatment. The effect of the micro-structure of cellulose crystallinity index as well as the reaction conditions including catalyst, reaction time, and temperature on the exchange-resistant deuterium incorporation process are reported. The ability of deuterated cellulose fibers to resist protium-exchange during H2O washing was also explored. The results found that higher crystallinity index is beneficial to stabilize the deuteration of cellulose fibers. Furthermore, alkaline catalysts such as sodium hydroxide or potassium carbonate and higher exchange temperature as well as longer reaction time contribute significantly to the stabilization of deuterium incorporation in the deuterated cellulose fibers. These observations revealed that the hydrogen–deuterium exchange treatment is effective to obtain exchange-resistant deuterium incorporated cellulose fibers. In addition, cotton fibers with several deuteration levels were produced and could be considered for the study of deuterium effect on cellulose properties.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is one of the most abundant polymers in the world and has received extensive attention as a viable, sustainable alternative to petroleum resources for a variety of bio-derived materials, chemicals, and fuels (Raghuwanshi et al. 2018). Meanwhile, there are now numerous ongoing studies directed at cellulose modification to expand the cellulose material application field (Chen et al. 2019). Therein, deuterated cellulose is one of the most interesting and insightful cellulose derivatization protocols that deserves more attention. Deuterated cellulose refers to modified cellulose with hydrogen atoms substituted by deuterium atoms, including alkyl hydrogens (which are bonded to carbon atoms and often termed "unexchangeable" hydrogens) and hydroxyl hydrogens (which are bonded to oxygen atoms and often termed "exchangeable" hydrogens). This can be accomplished by (1) a biological route including growth of plant material in heavy water (Evans et al. 2019) or cultivation of bacterial strain in a deuterated media (Bali et al. 2013) or (2) a chemical exchange process involving hydroxyl groups (Hishikawa et al. 2017), respectively. Since the hydrogen atom is changed to deuterium atom, many properties of the deuterated cellulose are altered, such as small angle neutron scattering (SANS) scattering length density (SLD) (Evans and Shah 2015), hydrolysis rate (Bhagia et al. 2018), and thermostability et al. (Budarin et al. 2010). In particular, replacing the hydrogen atoms (which have no neutron) with deuterium atoms (which has a neutron per atom) significantly enhances the potential utilization of SANS in cellulose characterization due to the fact that deuterium and hydrogen interact with the neutrons quite differently (Evans and Shah 2015). Through neutron studies, the deuterium incorporation into cellulose was able to reveal the cellulose biosynthesis (Zimmer 2015), cellulose dynamics (Garvey et al. 2019), and chemical and biochemical degradation (Garvey et al. 2019) process, etc. Thus, the production of deuterated cellulose materials is crucial but remains quite a challenge.
So far, production of deuterated cellulose is usually accomplished via biological routes, which could result in the substitution of non-exchangeable (i.e. alkyl hydrogens) and hydroxyl hydrogens that are not surface accessible (Putzbach et al. 2005; Raghuwanshi et al. 2016). However, replacing C–H with C–D is still quite challenging for some plants or bacteria because heavy water affects the cell growth and metabolism caused by the biological toxicity of D2O to plants or bacteria growing (Evans et al. 2014). Also, quite a large amount of time and efforts are needed to cultivate deuterium incorporated plants and bacteria and then extract deuterium incorporated cellulose. For other studies, there is a need to solely exchange the protio cellulose hydroxyl groups with deuterium. Currently, some of the generation of cellulose OD group in the published literature is focused on the easily exchangeable hydroxyl groups located primarily in the amorphous component of cellulose, where the re-hydration would occur readily. For example, D2O has been used to calculate the cellulose crystallinity and cellulose accessibility since only the amorphous area was believed to be accessible to D2O molecular (Reishofer and Spirk 2015). Also, the estimation of D2O content in cellulose thin films has opened the way to learn about cellulose hydration and adsorption during interaction with bio-molecules (Su et al. 2016; Yonenobu et al. 2009). There is relatively limited research that pays attention to the crystallite interior deuterated OH groups through hydrogen–deuterium exchange process, which were principally employed for crystalline cellulose interior structure revelation and hydrogen bonding related research (Agarwal et al. 2015; Russell 2015). For example, Nishiyama and Langan et al. deuterated alga Glaucocystis nostochinearum, tunicate cellulose and mercerized flax with all the crystalline cellulose hydroxyl groups being substituted by deuterium hydroxyl for the probing of structure and hydrogen bonding system in cellulose Iα, Iβ and cellulose II, respectively (Langan et al. 1999; Nishiyama et al. 2002; Nishiyama et al. 2003). However, replacing all the interior crystallite hydroxyl groups, namely "intracrystalline deuteration", requires a drastic condition (0.1 N NaOD in D2O at 210 °C for 1 h) (Jean et al. 2008), which may readily impair the original cellulose material properties. In summary, there is a need to prepare deuterated cellulose material with exchange-resistant cellulose OD hydroxyl groups through a relatively mild chemical exchange process for practical application such as security of high-value specialty paper or currency paper. Furthermore, the development of exchange-resistant deuterated cellulose material through hydrogen–deuterium exchange is especially attractive as it could be used as an important and useful tool for contrast variation SANS studies of cellulosic materials; and studies of cellulose dynamics etc. since the inclusion of heavy atoms is a powerful tool for the comparison theoretical calculations with vibrational spectroscopy (particularly low frequency motions) and solid state NMR spectroscopy (Müller et al. 2000; Russell 2015).
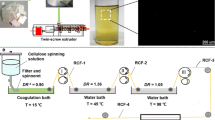
In this study, natural cotton fibers and regenerated Tencel fibers were deuterated by hydrogen–deuterium exchange treatment under high temperature with the addition of a catalyst (i.e., NaOH and K2CO3) (Lockley and Heys 2010). The influence of reaction conditions and cellulose crystalline information on deuterium exchanged fibers and the stability of R–O–D towards to exchange with H2O was investigated.
Experimental methods
Materials
Tencel (T) and cotton (C) fibers are provided by Dezhou Hengfeng Co., Ltd. Tencel fibers were produced by 4-methylmorpholine-N-oxide (NMMO) solution and regenerated into cellulose II fibers. All chemicals used were of reagent grade and used as received from Sigma Aldrich.
Cellulose fiber preparation
Prior to the exchange studies, cotton fibers were purified by refluxing with a 1% (w/v) NaOH solution to remove gum constituents (Dhillon et al. 2018). For comparison, the original cotton fibers were treated with cellulase from Trichoderma sp. at 35 °C for 72 h to obtain enzyme treated cotton fibers with decreased crystallinity index. The enzyme activity was 0.17 IU/mg, measured with Sigmacell cellulose as substrate at pH 5.0 and 37 °C (IU: international units) (Pu et al. 2006). The resultant fibers were washed with distilled water several times until the effluent was pH neutral.
Production of deuterated cellulose fibers with hydrogen–deuterium exchange treatment
Presoaking of cellulose fibers
The purified dry cotton fibers, Tencel fibers, and enzymatic treated cotton fibers (3.3 g) were individually immersed in 100 mL 99.98% deuterium oxide (D2O, 1:30, w/v) with stirring for 12 h at room temperature and these samples were then frozen at − 10 °C. The frozen samples were then freeze-dried to a constant weight.
Hydrogen–deuterium exchange treatment of cellulose fibers
The freeze-dried cellulose fiber samples were then placed in a 4560 series Parr reactor (Parr Instrument Company, USA) with 99.98% D2O (1:30, w:v) and then heated at 105 °C for 8 h. After the hydrogen–deuterium exchange treatment, D2O was used to wash the samples until the effluent was pH neutral. The resultant fibers were dried in the vacuum oven at 60 °C for 24 h. The dried fibers were stored at room temperature (25 ± 2 °C) and humidity (65 ± 5% RH, H2O moisture) for further analysis. In order to study the influence of parameters on deuteration rate, the experiments were repeated under the same conditions except for the addition of catalysts, either NaOH and K2CO3 (10% wt based on the cellulose sample). The reaction time and temperature for the hydrogen–deuterium exchange treatment were also varied according to Table 1. It should be noted that the left parallel experiments were run under the catalyst of K2CO3, the treating temperature of 105 °C and the reaction time of 8 h as fixed parameters. The chosen conditions are all referenced to the literature or pretest (Horikawa et al. 2009; Reishofer and Spirk 2015).
Characterization of the cellulose fibers
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra of the original cellulose fibers and deuterated cellulose fibers were acquired with a Perkin Elmer Spectrum 100 FTIR spectrometer (Waltham, MA, USA) equipped with an ATR accessory. The spectral data was acquired over the wavelength range of 800–4000 cm−1 with a resolution of 2 cm−1 for 64 scans per spectrum.
The FTIR spectra of the deuterated cellulose fibers were baseline-corrected with the Origin Pro software. The peaks were manually picked over 3800–2300 cm−1 to compare the quantity of deuterium signals of different samples (Łojewska et al. 2007). In particular, the cellulose fibers’ FTIR spectra were baseline-corrected with reference to the wavelength numbers of 3670 cm−1, 3002 cm−1, 2636 cm−1 and 2320 cm−1, respectively. Since the CH/CH2 groups around 2890 cm−1 of cellulose fibers remained unchanged during the hydrogen–deuterium exchange treatment, it was selected as the reference to calculate the relative deuterium content. The OH groups were also selected for verifying the deuteration rate comparison. Thereby, the characteristic peaks of OD, OH and CH/CH2 groups were compared by their peak intensity.
In order to obtain the cellulose fibers with stable deuterium incorporation signal, one group of the deuterated cotton and Tencel cellulose fibers were stored in the constant temperature (25 ± 2 °C) and humidity air environment (65 ± 5% RH, H2O moisture) for 0.5, 3, 7, 14 and 30 days, respectively, prior to analysis. Then, the deuterated cellulose fibers were treated separately with different reaction conditions and analyzed for relative deuteration incorporation rate.
Moreover, to determine the environmental stability of the deuterated fibers, they were stirred in H2O (deionized water, 1:30, w/v) at room temperature for 0 to 30 days and then filtered before they were dried in the vacuum oven at 60 °C for 24 h. The FTIR spectra were then recorded as described above.
Crystalline information of the cellulose fibers
Cellulose crystallinity of the original cotton, Tencel and enzyme-treated cotton fibers were revealed by XRD diffraction patterns. Diffraction patterns were measured with a D8 Advanced X-ray diffractometer (Bruker, Germany). The 2θ range from 5° to 40° diffraction patterns were recorded with a scan rate of 2°/min at 40 kV and 50 mA, respectively. The crystallinity index (CrI) of the cellulose samples were calculated according to the Segal method equation previously reported (French 2014; French and Santiago Cintrón 2013).The equation was listed as follows:
I200/I020 refers to the maximum peak intensity of the 200/020 cellulose crystalline lattice diffraction (around 22.6° and 21.6° for native cellulose and regenerated cellulose, respectively), respectively (Nam et al. 2016). While, Iamorph is the minimum intensity near 2θ = 18° (cellulose I) and 16°(cellulose II), respectively, which is attributed to the amorphous area in native cellulose and regenerated cellulose, respectively (Azubuike et al. 2011; Song et al. 2018).
Results and discussion
FTIR spectra of the original cellulose fibers and deuterated cellulose fibers
FTIR spectra of the original cellulose fibers and deuterated cellulose fibers which were stored for 2 weeks in the H2O atmosphere are depicted in Fig. 1. As shown in Fig. 1, most of the cellulose characteristic peaks remained unaffected except for the decreasing of OH peak intensity and the emerging of OD stretching band after the hydrogen–deuterium exchange treatment. Segmental characteristic peaks that represent OH band were changed to lower wavelength with an absorption ratio averaging 1.34 to 1.35 due to the isotopic effect. Similar results were also reported in the literature (Hofstetter et al. 2006).
Apparently, there are significant differences between the FTIR spectra changes of the deuterated cotton fibers and deuterated Tencel fibers upon hydrogen–deuterium exchange deuteration. Figure 2 presents the cellulose monosaccharide chemical structure. According to the literature, as summarized in Table 2, the OH characteristic peaks at 3340 cm−1 and 3290 cm−1 represent O3-H…O5 intramolecular hydrogen bond and O6–H…O3′ intermolecular hydrogen bond for natural cotton fibers, respectively (Altaner et al. 2014). After deuteration, the dominant hydrogen bond of OH groups were all partially substituted. The signal at 2530 cm−1 (shifted from 3405 cm−1) remained visible slightly above noise level and the 2480 cm−1 (shifted from 3340 cm−1) peak continued to exhibit the strongest intensity followed by the 2455 cm−1 (shifted from 3290 cm−1) peak (Fig. 1-C). Thus, it can be concluded that the O3H and O6H group (as shown in Fig. 2) were steadily substituted by OD for natural cotton fibers. Analysis of the deuterated Tencel fibers suggested that all the dominant types of hydroxyl groups were almost equally replaced by OD for regenerated fibers, even for the unremarkable hydrogen bonds from the original Tencel fibers such as the characteristic peaks at 3488 cm−1 and 3440 cm−1 (Fig. 1-T).
Chemical structure of cellulose (French 2017)
It is possible that some of the OD groups may be re-exchanged back into OH during storage, thus, the remaining OD characteristic peaks were considered “exchange-resistant” deuterium incorporation signals. As can be observed, besides the different situation of substitution position, the exchange-resistant relative deuterium incorporation content of Tencel fibers is significantly less than cotton fibers. It has been reported that the crystallinity index of cellulose remarkably influences the accessibility of cellulose hydroxyl groups (Fackler and Schwanninger 2011). It is possible that different deuteration rate in cotton and Tencel fibers might be caused by the fact that more amorphous cellulose exists in Tencel fibers. To validate the hypothesis, cellulose fibers with different CrI values were treated and tested.
Diffraction patterns of the cellulose fibers before deuteration and their FTIR spectra after deuteration
Figure 3 showed the crystalline information of the cellulose samples before deuteration. As we can see, the original cotton fibers and enzyme treated cotton fibers both exhibited cellulose I crystalline form, with the CrI of 87.33% and 78.01%, respectively, while the original Tencel fibers showed the crystalline form of cellulose II and CrI of 75.20%.
Figure 4 summarizes the FTIR spectral data for the deuterated cellulose fibers which were stored for 2 weeks in the H2O atmosphere. As shown from Fig. 3a, b, the enzymatic treatment only decreased the crystallinity index of cotton fibers while the crystalline form of cellulose remained cellulose I as to be expected (Song et al. 2018). However, the deuterium signal of enzymatic cotton fibers was significantly lower than the original cotton fibers (Fig. 4a, b) due to the lower crystalline index. It can be concluded that the higher crystalline index of cellulose fibers contributes to retaining the exchange-resistant deuterium signal in the hydrogen–deuterium exchanged fibers. It is because that higher crystalline cellulose possesses more deuterated hydroxyl groups in the crystalline area of cellulose, resulting in greater stable deuterium signal. Research have revealed that the deuterium incorporation of crystalline area is all exchange-resistant when treated with D2O liquid at 170 °C to 210 °C (Okajima and Kai 1972).
However, with the similar crystallinity index of enzyme-treated cotton fibers (Fig. 3b) and Tencel fibers (Fig. 3c), there is a notable difference of deuterium content (Fig. 4b, c). The deuterated enzyme treated cotton fibers (cellulose I) showed higher deuteration rates compared with the deuterated Tencel fibers (cellulose II). This result may be attributed to the difference of crystalline form or micro structure. There are literature that indicate the cellulose II hydrogen bonding is more stable than cellulose I hydrogen bond (Pushpamalar et al. 2006), which may cause the difficulty of K2CO3 swelling of cellulose interior structure which then hinders the hydrogen–deuterium exchange process. Also, the lumen inside of cotton fibers with greater specific surface is beneficial at incorporating and retaining deuterium incorporation signal. In conclusion, cellulose with higher crystallinity such as natural cotton and ramie fibers are suggested for the preparation of hydrogen–deuterium exchanged deuterated cellulose with a higher level of deuterium signal content.
FTIR spectra of the deuterated cellulose fibers stored in H2O atmosphere
Deuterated cotton and Tencel fibers that were treated with aqueous NaOH treatment at 105 °C for 8 h were subsequently maintained at constant temperature (25 °C) and atmospheric humidity for a period of 0.5 to 30 days. The deuterium incorporated cellulose fiber samples were then analyzed by FTIR and the results are illustrated in Fig. 5.
It is apparent that the relative deuterium incorporation rate decreased quickly in the initial several days when they were exposed to the air with ambient humidity. These changes can be attributed to the fact that some of the active deuterium incorporation from surface accessible cellulose were exchanged with hydrogen when exposed to the air with H2O. The phenomenon that the deuterium signal content from the Tencel fibers with more amorphous area decreased more rapidly supports this claim. Fortunately, the dramatic decreasing of deuterium signal content became very minimal after being stored for 2 weeks. This proved that the deuterium signal remained after exposed to air for 2 weeks are exchange-resistant to some extent. Thereby, the resistant deuteration rate of fiber treated by different reaction conditions could be comparable after 2 weeks exposing to the air atmosphere.
FTIR spectra of the deurated cellulose fibers with varying reaction conditions of catalyst, exchange temperature and time
According to the results of Fig. 5, all the samples for comparison were analyzed after equilibrium at constant temperature 25 °C and atmospheric humidity for 2 weeks.
FTIR spectra of deuterated cellulose fibers with varying catalyst
The influence of catalyst on the stability of the deuterium incorporation of hydrogen–deuterium exchanged cellulose fibers were conducted under the reaction time of 8 h and a reaction temperature of 105 °C. The FTIR spectra of the sample fibers with different catalyst charge were shown in Fig. 6. Compared with the cellulose fibers treated by solely D2O, the exchange-resistant deuteration rate was greatly improved with the addition of catalysts, for both natural cotton and regenerated Tencel fibers. It can be explained that on one hand, the catalyst (K2CO3 or NaOH) accelerated and increased the exchange of cellulose R–O–H groups. On the other hand, the alkaline reagent helped to swell the cellulose interior structure, which made the exchange occur easily because the accessibility was improved (Alves et al. 2015). There is an increase of deuterium signals of cellulose fibers treated with NaOH as a catalyst compared with K2CO3, in D2O. This effect was due to the stronger alkalinity of NaOH, which contributed to the exchange of R–O–H groups. Pönni et al. also reported that the alkaline environment enhanced the exchanging of OH and OD groups for cellulose (Pönni et al. 2014). However, NaOH is believed to damage the cellulose molecular chain under high temperature and thus destroy the fiber crystallinity, strength etc. as a strong alkaline chemical (Song et al. 2018). Therefore, catalyst with mild alkalinity such as K2CO3 or Na2CO3 is suggested for the exchange experiment.
FTIR spectra of deuterated cellulose fibers with varying reaction temperature
The effect of temperature on the exchange-resistant deuterium signals of hydrogen–deuterium exchanged cellulose fibers were examined with 10% weight of K2CO3 as a catalyst and a reaction time of 8 h in D2O. Figure 7 exhibits the FTIR results of the sample fibers treated at different temperatures.
With the elevation of temperature, the deuterium incorporation content increased obviously. There are two mechanisms that can explain this phenomenon. Firstly, the higher temperature accelerated the exchange of R-O–H groups. Secondly, the higher pressure caused by higher temperature helped the D2O molecule to penetrate into the cellulose crystalline bundles, which promotes the exchanging of hydrogen and deuterium (Matthews et al. 2011). It has also been reported that all the protio hydroxyl groups of crystalline cellulose could be substituted with deuterium for ramie at 210 °C with the 0.1 mol NaOD (Yuan et al. 2005). However, these severe conditions will degrade the cellulose molecule chain and then result in the loss of strength for cellulose material (Chen et al. 2018). Thereby, 105 °C was selected for the following exchange studies.
FTIR spectra of deuterated cellulose fibers with varying reaction time
The influence of treatment time on the exchange-resistant deuterium signals for hydrogen–deuterium exchanged cellulose fibers were carried out with the addition of 10% weight of K2CO3 and a treatment temperature of 105 °C in D2O. The FTIR spectra with different treatment times are summarized in Fig. 8.
Although the relative deuteration rate increased with the prolonged treating time, it seemed to be less pronounced when it reached 8 h. There is a very limited increase in the R-O-D signal for the 8 h to 12 h treatment time for the cellulose fibers, especially for the natural cotton fibers. This may be caused by a saturation point under the same treating temperature. It was reported that the accessibility of cellulose to liquid D2O occurs quickly and there was a minimal increase from 4 h to 1 week (Reishofer and Spirk 2015). Thus, treating for 8 h at 105 °C is sufficient for the cellulose hydrogen–deuterium exchange treatment.
In conclusion, the alkaline catalyst and treating temperature as well as treating time contribute markedly to influence the exchange-resistant deuteration incorporation rate.
FTIR spectra of the deuterated cellulose with H2O washing treatment
The FTIR spectra of the deuterated cellulose fibers after washing with H2O are presented in Fig. 9.
It can be observed that both the deuterated cotton and Tencel fibers were partially re-protonated after the washing treatment with H2O. This is because the deuterium incorporation from cellulose amorphous area was exchanged with the hydrogen from H2O during washing. The initial drastic drop of Tencel fibers with lower CrI could also prove this claim. However, there are deuterium signals from the deuterated cellulose that were resistant to washing treatment for even 1 month. There were about 33% (based on the original OH group) of deuterium content remained for cotton fibers and only 10% for Tencel fibers after washing for 30 days. Hence, the deuterated cotton fibers were more exchange-resistant to H2O washing compared to Tencel fibers, which is also caused by a higher crystallinity of cotton fibers that retained the deuterium incorporation better. These results illustrate that deuterated cellulose fibers produced by hydrogen–deuterium exchange treatment could resist protium-exchange during H2O washing for at least 30 days.
Conclusion
The deuterated cellulose fibers of cotton and Tencel were prepared with hydrogen–deuterium exchange treatment. The higher CrI was beneficial for the hydroxyl deuteration of cellulose with an exchange-resistant deuterium incorporation. Also, alkaline catalysts such as NaOH or K2CO3 and higher temperature as well as longer reaction time contribute significantly to the exchange-resistant deuteration of cellulose. The deuterated fibers possess stable deuterium signal that are resistant to H2O washing treatment for at least 30 days. Furthermore, deuterated cotton fibers perform better at retarding protium exchanging than Tencel fibers. These observations could be instrumental in improving cellulose properties and understanding of cellulose structure. What’s more, deuterated cotton fibers of different deuteration rate levels could be produced based on these results and they could be utilized for the investigation of deuterium effect on cellulose properties as well as expand the application field of cellulose material.
References
Agarwal UP, Ralph SA, Reiner RS, Stark NM (2015) Formation of irreversible H-bonds in cellulose materials. In: Proceedings of the 18th ISWFPC (international symposium on wood, fiber, and pulping chemistry) held in Vienna (Sept 9–11, 2015), pp 18–21
Altaner CM, Thomas LH, Fernandes AN, Jarvis MC (2014) How cellulose stretches: synergism between covalent and hydrogen bonding. Biomacromol 15:791–798. https://doi.org/10.1021/bm401616n
Alves L, Medronho B, Antunes FE, Topgaard D, Lindman B (2015) Dissolution state of cellulose in aqueous systems. 1. Alkaline Solvents Cellul 23:247–258. https://doi.org/10.1007/s10570-015-0809-6
Azubuike CP, Rodríguez H, Okhamafe AO, Rogers RD (2011) Physicochemical properties of maize cob cellulose powders reconstituted from ionic liquid solution. Cellulose 19:425–433. https://doi.org/10.1007/s10570-011-9631-y
Bali G, Foston MB, O’Neill HM, Evans BR, He J, Ragauskas AJ (2013) The effect of deuteration on the structure of bacterial cellulose. Carbohydr Res 374:82–88
Bhagia S et al (2018) Ultrastructure and enzymatic hydrolysis of deuterated switchgrass. Sci Rep 8:1–9
Budarin VL, Clark JH, Lanigan BA, Shuttleworth P, Macquarrie DJ (2010) Microwave assisted decomposition of cellulose: A new thermochemical route for biomass exploitation. Biores Technol 101:3776–3779. https://doi.org/10.1016/j.biortech.2009.12.110
Chen D, Gao A, Cen K, Zhang J, Cao X, Ma Z (2018) Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers Manag 169:228–237. https://doi.org/10.1016/j.enconman.2018.05.063
Chen X, Xin D, Wang R, Qin Y, Wen P, Hou X, Zhang J (2019) Factors affecting hydrolytic action of xylanase during pennisetum saccharification: Role of cellulose and its derivatives. Ind Crops Prod 130:49–56. https://doi.org/10.1016/j.indcrop.2018.12.077
Dhillon A, Rajulapati V, Goyal A (2018) Bio-scouring of cotton fabric and enzymatic degumming of jute fibres by a thermo-alkaline recombinant rhamnogalacturonan lyase, ctrglf fromClostridium thermocellum. Can J Chem Eng 97:1043–1047. https://doi.org/10.1002/cjce.23342
Evans BR, Bali G, Reeves DT, O'Neill HM, Sun Q, Shah R, Ragauskas AJ (2014) Effect of D2O on growth properties and chemical structure of annual ryegrass (Lolium multiflorum). J Agric Food Chem 62:2595–2604. https://doi.org/10.1021/jf4055566
Evans BR, Shah R (2015) Development of approaches for deuterium incorporation in plants. Methods Enzymol 565:213–243. https://doi.org/10.1016/bs.mie.2015.07.014
Evans BR et al (2019) Production of deuterated biomass by cultivation of Lemna minor (duckweed) in D2O. Planta 249:1465–1475
Fackler K, Schwanninger M (2011) Accessibility of hydroxyl groups of brown-rot degraded spruce wood to heavy water. J Near Infrared Spectrosc 19:359–368. https://doi.org/10.1255/jnirs.943
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
French AD (2017) Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 24:4605–4609. https://doi.org/10.1007/s10570-017-1450-3
French AD, Santiago Cintrón M (2013) Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 20:583–588. https://doi.org/10.1007/s10570-012-9833-y
Garvey CJ, Simon GP, Whittaker AK, Parker IH (2019) Moisture-activated dynamics on crystallite surfaces in cellulose. Colloid Polym Sci 297(4):521–527
Hishikawa Y, Togawa E, Kondo T (2017) Characterization of individual hydrogen bonds in crystalline regenerated cellulose using resolved polarized FTIR spectra. ACS Omega 2(4):1469–1476
Hofstetter K, Hinterstoisser B, Salmén L (2006) Moisture uptake in native cellulose – the roles of different hydrogen bonds: a dynamic FT-IR study using deuterium exchange. Cellulose 13(2):131–145
Horikawa Y, Clair B, Sugiyama J (2009) Varietal difference in cellulose microfibril dimensions observed by infrared spectroscopy. Cellulose 16:1–8
Jean B, Dubreuil F, Heux L, Cousin F (2008) Structural details of cellulose nanocrystals/polyelectrolytes multilayers probed by neutron reflectivity and AFM. Langmuir 24:3452–3458
Langan P, Nishiyama Y, Chanzy H (1999) A revised structure and hydrogen-bonding system in cellulose ii from a neutron fiber diffraction analysis. J Am Chem Soc 121(43):9940–9946
Lockley WJS, Heys JR (2010) Metal-catalysed hydrogen isotope exchange labelling: a brief overview. J Label Compd Radiopharm 53:635–644. https://doi.org/10.1002/jlcr.1851
Łojewska J, Missori M, Lubańska A, Grimaldi P, Ziȩba K, Proniewicz LM, Congiu Castellano A (2007) Carbonyl groups development on degraded cellulose correlation between spectroscopic and chemical results. Appl Phys A 89:883–887. https://doi.org/10.1007/s00339-007-4220-5
Müller M, Czihak C, Schober H, Nishiyama Y, Vogl G (2000) All disordered regions of native cellulose show common low-frequency dynamics. Macromolecules 33:1834–1840
Matthews JF, Bergenstrahle M, Beckham GT, Himmel ME, Nimlos MR, Brady JW, Crowley MF (2011) High-temperature behavior of cellulose I. J Phys Chem B 115:2155–2166. https://doi.org/10.1021/jp1106839
Nam S, French AD, Condon BD, Concha M (2016) Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr Polym 135:1–9
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082
Nishiyama Y, Sugiyama J, Chanzy H, Langan P (2003) Crystal structure and hydrogen bonding system in cellulose I β from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 125:14300–14306
Okajima S, Kai A (1972) Deuteration behaviour of ramie cellulose. Sen'i Gakkaishi 28:387–391
Pönni R, Rautkari L, Hill CAS, Vuorinen T (2014) Accessibility of hydroxyl groups in birch kraft pulps quantified by deuterium exchange in D2O vapor. Cellulose 21:1217–1226. https://doi.org/10.1007/s10570-014-0166-x
Pu Y, Ziemer C, Ragauskas AJ (2006) CP/MAS 13C NMR analysis of cellulase treated bleached softwood kraft pulp. Carbohydr Res 341:591–597. https://doi.org/10.1016/j.carres.2005.12.012
Pushpamalar V, Langford SJ, Ahmad M, Lim YY (2006) Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr Polym 64:312–318. https://doi.org/10.1016/j.carbpol.2005.12.003
Putzbach K, Krucker M, Albert K, Grusak MA, Tang G, Dolnikowski GG (2005) Structure determination of partially deuterated carotenoids from intrinsically labeled vegetables by HPLC-MS and 1H NMR. J Agric Food Chem 53:671–677
Raghuwanshi VS, Cohen Y, Garnier G, Garvey CJ, Russell RA, Darwish T, Garnier G (2018) Cellulose dissolution in ionic liquid: ion binding revealed by neutron scattering. Macromolecules 51:7649–7655. https://doi.org/10.1021/acs.macromol.8b01425
Raghuwanshi VS, Su J, Garvey CJ, Holt SA, Garnier G (2016) Bio-deuterated cellulose thin films for enhanced contrast in neutron reflectometry. Cellulose 24:1–10
Reishofer D, Spirk S (2015) Deuterium and cellulose: a comprehensive review. Adv Polym Sci 271:1–22
Russell RA, Garvey CJ, Darwish TA, Foster LJR, Holden PJ (2015) Biopolymer deuteration for neutron scattering and other isotope-sensitive techniques. Methods Enzymol 565:97–121. https://doi.org/10.1016/bs.mie.2015.06.015
Song Y, Jiang W, Zhang Y, Ben H, Han G, Ragauskas AJ (2018) Isolation and characterization of cellulosic fibers from kenaf bast using steam explosion and Fenton oxidation treatment. Cellulose 25:4979–4992. https://doi.org/10.1007/s10570-018-1916-y
Su J, Raghuwanshi VS, Raverty W, Garvey CJ, Garnier G (2016) Smooth deuterated cellulose films for the visualisation of adsorbed bio-macromolecules. Sci Rep 6:36119
Yonenobu H, Tsuchikawa S, Sato K (2009) Near-infrared spectroscopic analysis of aging degradation in antique washi paper using a deuterium exchange method. Vib Spectrosc 51:100–104. https://doi.org/10.1016/j.vibspec.2008.11.001
Yuan H, Nishiyama Y, Kuga S (2005) Surface esterification of cellulose by vapor-phase treatment with trifluoroacetic anhydride. Cellulose 12:543–549. https://doi.org/10.1007/s10570-005-7136-2
Zimmer J (2015) A molecular description of cellulose biosynthesis. Biophys J 108:499a
Acknowledgments
This work was supported by the National Science Foundation of China (51706044 and 51903131), the Natural Science Foundation of the Shandong of China (ZR2019QEM007), the Natural Science Foundation of the Jiangsu of China (BK20170666), Special Foundation of “Taishan Scholar” Construction Program and the Recruitment Program for Young Professionals in China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, Y., Jiang, W., Ben, H. et al. The production of hydrogen–deuterium exchanged cellulose fibers with exchange-resistant deuterium incorporation. Cellulose 27, 6163–6174 (2020). https://doi.org/10.1007/s10570-020-03230-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03230-6