Abstract
Main conclusion
Common duckweed Lemna minor was cultivated in 50% D2O to produce biomass with 50–60% deuterium incorporation containing cellulose with degree of polymerization close (85%) to that of H2O-grown controls.
The small aquatic plant duckweed, particularly the genus Lemna, widely used for toxicity testing, has been proposed as a potential source of biomass for conversion into biofuels as well as a platform for production of pharmaceuticals and specialty chemicals. Ability to produce deuterium-substituted duckweed can potentially extend the range of useful products as well as assist process improvement. Cultivation of these plants under deuterating conditions was previously been reported to require addition of kinetin to induce growth and was hampered by anomalies in cellular morphology and protein metabolism. Here, we report the production of biomass with 50–60% deuterium incorporation by long-term photoheterotrophic growth of common duckweed Lemna minor in 50% D2O with 0.5% glucose. L. minor grown in 50% D2O without addition of kinetin exhibited a lag phase twice that of H2O-grown controls, before start of log phase growth at 40% of control rates. Compared to continuous white fluorescent light, growth rates increased fivefold for H2O and twofold for 50% D2O when plants were illuminated at higher intensity with a metal halide lamp and a diurnal cycle of 12-h light/12-h dark. Deuterium incorporation was determined by a combination of 1H and 2H nuclear magnetic resonance (NMR) to be 40–60%. The cellulose from the deuterated plants had an average-number degree of polymerization (DPn) and polydispersity index (PDI) close to that of H2O-grown controls, while Klason lignin content was reduced. The only major gross morphological change noted was root inhibition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duckweeds of the genus Lemna are small, aquatic flowering plants that are found in fresh water world-wide. Various species, particularly L. minor and L. gibba, are widely used in toxicology tests due to their easy cultivation and quantification (Moody and Miller 2005; Brain and Solomon 2007). They are utilized as a high-protein feed crop both in the natural environment and in aquaculture and agriculture (Porath et al. 1979; Bergmann et al. 2000). More recently, large-scale cultivation to provide cellulosic biomass for conversion into biofuels has been proposed based on their rapid growth rates and low recalcitrance to enzymatic saccharification (Zhao et al. 2012, 2014). Typically, schemes for duckweed biomass production are combined with their established application in waste water treatment (Bergmann et al. 2000; Körner et al. 2003; Liu et al. 2017). Utilization of Lemna duckweed as a platform to produce biopharmaceutical products using recombinant DNA technology was proposed (Gasdaska et al. 2003; Stomp 2005) and production of recombinant antibodies has been reported (Cox et al. 2006; Firsov et al. 2018). Lemna spp. are also notable for their cultivation in D2O-enriched media to produce deuterium-labelled biomolecules and biomass (Cope et al. 1965; Trewavas 1970) and to investigate heterotrophic growth (Yakir and De Niro 1990). There is increasing interest in development of deuterium substitution for pharmaceutical applications (Halford 2016), particularly following the FDA approval of the first deuterated drugs (Schmidt 2017; DeWitt and Maryanoff 2018). Deuterium substitution is utilized to manipulate the scattering length density of a material, facilitating molecular structural analysis by neutron scattering and diffraction techniques that are increasingly being applied to understand biological systems (Langan et al. 2012). Partial deuteration of Lemna biomass (Cope et al. 1965) as well as production of deuterium-labeled DNA (Trewavas 1970) indicated these duckweed species could potentially be used to produce deuterium-labeled biomolecules. However, the initial investigations encountered complications related to toxicity and metabolic changes following transfer of the duckweed to 50% and higher concentrations of D2O. Since duckweed species of the genus Lemna can grow by vegetative reproduction and have simple roots, it was thought that these small plants would be able to adapt to high D2O concentrations similar to microalgae. Investigation of Lemna peruspilla and Lemna gibba for production of deuterated biomass found that duckweed could tolerate 50–60% D2O if grown under photoheterotrophic conditions with glucose as a carbon source in addition to CO2 in ambient air. D2O inhibition was synergistic with light intensity under the conditions used and addition of kinetin, a phytohormone that breaks dormancy, was needed to stimulate growth in 60% D2O. Glucose supplementation improved growth in 50–60% D2O while achieving fixed deuterium incorporation levels in the range of 32–56% in the whole biomass with protiated glucose and without addition of kinetin (Cope et al. 1965). Similar to the behavior of terrestrial plants in higher (> 30%) concentrations of D2O, growth was slower and root elongation was greatly inhibited. A subsequent heavy labeling study noted slower growth and shorter roots of L. minor plants grown in heavy isotope-labeling medium containing 2 mM calcium nitrate-15N, 5 mM potassium nitrate-15N, 10 mM sucrose, and 1 µM kinetin in 50% D2O under continuous illumination with warm white and daylight fluorescent lamps at an intensity of 1000 ft-c (Trewavas 1970). Despite these inhibitory effects, Lemna plants were later reported to adapt to heterotrophic growth in 50% D2O media over time. Membrane rearrangements of the tonoplast and chloroplast were noted in Lemna minor in the first 5 h following transfer to 50% D2O, but nearly complete recovery ensued after 24 h (Cooke et al. 1980). After 1 week, the cells appeared normal. Protein degradation rates increased and synthesis rates decreased after transfer, but returned to normal levels after 60 h (Cooke et al. 1979a). Protein degradation as a response to the isotopic stress of 50% D2O resembled that observed for other stressors such as nutrient deprivation (distilled water), nitrate deprivation, and osmotic shock with 0.5 M mannitol (Cooke and Davies 1980; Cooke et al. 1979b).
In this study, we demonstrate the production of Lemna minor biomass with 50–60% deuterium incorporation containing cellulose with a similar molecular weight distribution (85%) as that found in H2O-grown controls. These results indicate that this protocol can be employed to prepare highly deuterated plant cellulose and, potentially, other components for experimental investigations and commercial applications.
Materials and methods
Cultivation of duckweed Lemna for deuteration experiments
The duckweed strains Lemna minor (UTCC490) and Lemna gibba (G3) were a generous gift from Biolex, Inc. (North Carolina, USA). The growth medium was Schenk and Hildebrandt’s basal salts (Phytotechnology Laboratories, Shawnee Mission, KA, USA). House-distilled water was further purified with a Milli-Q system (EMD Millipore, MA, USA) or with a Barnstead E-Pure system (ThermoFisher Scientific Massachusetts, USA). Deuterium oxide (D2O, 99.8%) was obtained commercially (Cambridge Isotope Laboratories, MA, USA).
For preliminary screening, the plants were grown at 23°°C under continuous illumination with white fluorescent light (Sylvania Daylight F75TBD/B 15 W) at 40 μmol m−2 s−1.
Carbon source experiments were set up with 50 ml of medium in 250-ml glass conical flasks closed with polyurethane foam stoppers. Each culture was inoculated with 10 fronds. Carbon sources were supplemented at 0.5% v/v. Sodium acetate and sodium succinate stocks were adjusted to pH 4.0 by addition of sodium hydroxide solution. The determination of optimal glucose concentration was carried out similarly, but with 0, 0.5, 1, 2, and 5.0% (0, 27, 55, 110 mM, respectively) glucose in the media.
For perfusion experiments, L. minor was grown in an in-house assembled culture system (Fig. 1). The culture system was assembled from 250-ml glass conical flasks fitted with rubber stoppers equipped with inlet and outlet connections. Glass tubing (1/4 inch O. D.) was used to connect the inlet tubing of each flask to a 4-port manifold made of the same tubing via 8-inch lengths of silicon tubing. The manifold was connected with silicone rubber tubing to an air source through an in-line 0.2-micron syringe filter to filter-sterilize the air stream. Air was supplied by a house airline stepped down to 100 ml/min with a flow gauge. The outlet tubing for each flask was a spiral water-trap made from ¼ inch glass tubing topped with a 0.2-micron syringe filter attached by silicone tubing to the vent end of the water trap tubing. The plants were grown in 50-ml medium per flask. Growth temperature was 25 °C.
For cultivation under higher light intensity with diurnal light–dark cycle, cultures of L. minor were also grown without perfusion in 100 ml of medium in 946-ml (32-oz) Phytocon™ plant growth containers with lids made of clarified polypropylene (Phytotechnology Laboratories, Kansas, USA) for 1–3 months (Fig. 2). These cultures were grown in 1X Schenk and Hildebrandt’s basal salts with 0.5% glucose in 50% D2O–H2O and in 100% H2O. The plants were illuminated with a metal halide lamp in a SunSystem2 fixture at an intensity of 130 μmol m−2 s−1 with a 12-h light/12-h dark diurnal cycle and temperature of 28 °C.
Plants were harvested by filtration on sterile Miracloth (Calbiochem, La Jolla, CA, USA) and washed with sterile water. Cultures were either immediately frozen and stored at − 20°°C, or dried for 2 days at 23 °C and 21 inches Hg in a vacuum oven. Dry biomass yields from 50-mL cultures grown under air perfusion and continuous illumination with white fluorescent lamps for 59 days were 0.2514 g for 50% D2O and 0.0521 g for 60% D2O. Dry biomass yields from 100-mL cultures inoculated with ten fronds and grown in 946-ml Phytocon™ containers for 38 days were comparable for duckweed grown in H2O (0.4283 g) and in 50% D2O (0.4226 g).
Microscopy
Fresh plants were examined under the microscope and photographed at 100 and 400× without decolorization or staining. All images were taken with a Kodak DC290 Zoom Digital Camera using a Kodak MDS microscope attachment with a 7-mm spacer on a Leica Galen III light microscope with a blue filter to normalize coloring through the microscope adapter.
Chlorophyll assays
No size reduction was necessary for the Lemna samples. Chlorophyll was extracted from each sample by addition of 1 ml of methanol. Samples were then briefly vortexed, placed in a 60 °C water bath for 5 min, vortexed again, and centrifuged at 14,000 rpm for 15 min. The absorbance of the supernatants was measured and then examined with a Cary-Win UV-spectrophotometer at 652 nm, 665 nm, and 750 nm for scatter subtraction. Any samples that gave an absorbance of two or greater were diluted with methanol and rescanned. Total chlorophyll content in µg ml−1 was calculated using the coefficients 22.12 and 2.71 at 652 nm and 665 nm for chlorophyll a and b in methanol (Porra 2002, with subtraction of background absorbance at 750 nm according to the equation: (A652 − A750) (22.12) + (A665 − A750) (2.71).
NMR methods
Solid-state nuclear magnetic resonance (NMR) was carried out as described previously (Foston et al. 2012). Samples were lyophilized for 4 days to remove residual H2O and D2O. Ball-milled samples were loaded into 7-mm cylindrical ceramic MAS (magic angle spinning) rotors. Solid-state NMR measurements were carried out on a Bruker DSX-300 spectrometer operating at frequencies of 46.08 (2H) and 300.16 (1H) MHz in a Bruker double-resonance MAS probe head under non-spinning conditions. 2H NMR spectra were collected using a 90–90 solid-echo sequence for deuterium wide line observation accounting for the detector dead time delay, an echo delay of 50 μs, 16k data points, 250-kHz spectral width, 2-s recycle time, and 2k scans. 1H NMR spectra were measured with 4k data points, 44-kHz spectral width, 4-s recycle delay and 254 scans. Mixtures of various proportions of natural abundant glucose and glucose-6,6-d2 were used as standards.
Compositional analysis
Klason acid-insoluble lignin content was determined by an acid hydrolysis protocol based on Tappi method T-222 om-88 as described previously for annual ryegrass (Evans et al. 2014). The extractive-free samples were delignified using peracetic acid and cellulose was isolated from the delignified sample (holocellulose) by extraction with a 17.5% NaOH solution at 25 °C for 2 h. The mixture was diluted to 8.75% NaOH solution by addition of 5 mL of deionized water and repeated stirring at 25 °C for an additional 2 h. The isolated α-cellulose samples were then collected by centrifugation, washed with an excess of deionized water and air-dried.
Gel permeation chromatography (GPC) analysis of cellulose
Molecular weights of cellulose isolated from Lemna grown in 50% D2O and H2O-grown controls were determined by gel permeation chromatography (GPC) of the trianthranilate derivatives based on polystyrene calibration standards as described previously (Evans and Shah 2015, Evans et al. 2014, 2015). The weight-average molecular weights (Mw), defined as the first eluted statistical moment, and the number-average molecular weights (Mn), defined as the second eluted statistical moment, were used to calculate the degree of polymerization (DP) by dividing the Mw by the monomeric unit molecular weight after trianthranilate functionalization. The degree of polydispersity (DPI) was calculated by dividing Mw by the Mn to provide a measure of the range in molecular weights present in a particular cellulose sample.
Results
Comparison of Lemna species minor and gibba
Under the conditions used for cultivation, Lemna minor grew faster than Lemna gibba in both 50% D2O (data not shown) and in H2O solutions of Schenk and Hildebrandt’s basal salts (Table 1). Based on these results, L. minor was used for subsequent deuterium labeling experiments.
Effects of carbon source on growth of Lemna minor
The substrates acetate, glucose, glycerol, and succinate were initially screened as reduced carbon sources by addition at 0.5% w/v to the culture medium of plants grown under continuous illumination with cool white fluorescent lamps (Fig. 3 and Table 2). They were chosen based on availability in deuterated form and reported assimilation by photosynthetic organisms. As expected, addition of glucose increased the growth rate. The effect on growth of glucose was determined for concentrations from 0 to 5%. Optimal growth was observed at 0.5% glucose, while increase to 5% resulted in extreme growth inhibition (Fig. 4). Succinate at 0.5% decreased growth rate to 20% of control, while addition of acetate caused rapid bleaching and death, despite pH adjustment of the stock solutions to pH 4. Further investigation found an I25% of 0.87 mM for acetate (Fig. 5). Glycerol at 0.5% (54 mM) was inhibitory, reducing growth rate to 4% of controls (Fig. 3 and Table 2).
Perfusion with air from an in-house line supplying compressed ambient air increased the growth rate of L. minor in medium supplemented with 0.5% glucose. For L. minor, initial growth rate was increased from 3.6 to 7.5 fronds d−1, while linear growth rate after 10 days post inoculation was increased from 21.5 to 32.5 fronds d−1 (Table 1).
Effects of deuteration on growth and morphology
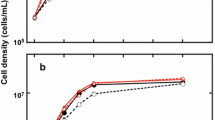
Growth of L. minor was screened for D2O concentrations of 50, 60, and 70% (Figs. 6 and 7). As had been reported previously for Lemna perpusilla (Scope et al. 1965), growth rate in 50% D2O was 40% of that in natural abundance water, while further increase to 60 and 70% D2O resulted in a precipitous drop in growth and increased mortality. Similar results were observed for the terrestrial monocot annual rye grass (Evans et al. 2014). Production of deuterium-labeled biomass was, therefore, carried out in 50% D2O with 0.5% glucose supplementation (Tables 3 and 4).
Comparison of growth rates of L. minor in H2O–D2O mixtures determined from the data presented in Fig. 5 showed that growth rates dropped precipitously beyond 50% D2O
The initial tests of D2O tolerance using continuous illumination with white fluorescent lamps found that, after a lag phase of approximately 10 days, L. minor started to grow in 50 and 60% D2O at rates one-half and one-third, respectively, of those of control plants (Fig. 7). Under more intense illumination in a diurnal cycle of 12-h light/12-h dark, the lag phase before onset of exponential growth in 50% D2O was approximately 20 days for growth in 50% D2O and 50 mM glucose (Fig. 8; Table 3). The increased illumination intensity and wavelength range of the metal halide lamp induced a twofold increase in the log phase growth rate of L. minor in 50% D2O compared to fivefold increase for the control in H2O media. The 50% D2O growth rate in both initial (lag) phase and log phase growth was approximately 40% of that measured for the control duckweed grown in H2O, similar to the reduction in growth rates observed previously for the terrestrial monocot annual ryegrass (Evans et al. 2014). The yields of dry biomass at 38 days were found to be comparable for the H2O and D2O cultures despite the slower growth initial and log-phase growth rates in 50% D2O. Once the cultures have reached confluence, the growth rates can be expected to slow due to crowding and shading.
Initial and log-phase growth rates were compared for L. minor grown in plant growth containers in H2O and in 50% D2O under illumination with a metal halide lamp and a diurnal cycle of 12-h light/12-h dark. Growth media were supplemented with 0.5% glucose. The rates determined by linear regression fitting are presented in Table 3
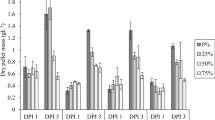
Inhibition of root elongation by 50% D2O was observed for L. minor (Fig. 9 and Table 5), consistent with results reported by earlier studies (Cope et al. 1965; Trewavas 1970). Control plants grew roots with average length 1.605 ± 0.904 cm, while plants grown in 50% D2O had an average root length of 0.31 ± 0.16 cm (p < 0.00001). In contrast to the increase in average frond surface area reported for L. perpusilla grown in 50% D2O with protiated glucose (Cope et al. 1965), fronds of L. minor were slightly smaller but heavier than those of controls grown in H2O (Table 5). Examination of cellular morphology under the light microscope (Fig. 10) did not detect any changes in cell wall dimensions or appearance in the fronds.
Biomass yields calculated as mg mL−1 d−1 of duckweed grown in 50% D2O were increased by 30% when cultures were grown at the higher light intensity under a diurnal cycle of 12-h light/12-h dark.
Deuterium incorporation
According to the results from the solid-state NMR analysis, deuterium substitution levels of 40–50% were achieved by cultivation in media containing 50% D2O and 0.50% glucose (Table 4). This level of partitioning of deuterium label from D2O in the growth media in the presence of a hexose carbon source is consistent with earlier studies of Lemna species grown photoheterotrophically in 50 and 60% D2O with glucose (Cope et al. 1965).
Compositional characterization
The degree of polymerization (DP) and polydispersity index (DPI), are parameters used to compare polymers such as cellulose, hemicellulose, and lignin from different sources (Foston and Ragauskas 2010), between deuterated and control bacterial cellulose (Bali et al. 2013), and between deuterated and control plants (Evans and Shah 2015, Evans et al. 2014, 2015). Cellulose isolated from L. gibba plants grown in 50% D2O had a substantially lower DPw that was 63% of that of controls grown in H2O. The DPW of cellulose isolated from L. minor grown in 50% D2O was 85% of the DPw of H2O-grown controls (Table 5). This is consistent with the results reported previously for the terrestrial species annual ryegrass (Evans et al. 2014) and switchgrass (Evans et al. 2015), which found that cellulose isolated from plants grown in 50% D2O had DP close to that of control plants grown in H2O (Table 6).
Determination of Klason lignin found that growth in 50% D2O decreased lignin content from 18 to 8% dry weight (Table 7). Previously published studies have generally found the lignin content of Lemna duckweed species to be much lower. When the low yields of about 10% for the alkaline cupric hydroxide method are taken into account, a content of around 2.5% derived from H and G units can be estimated from previously reported determinations (Blazey and McClure, 1968). Those results are consistent with the lignin content of 2.4% dry weight determined as Klason lignin for L. minor harvested from the wild in Great Britain reported later (Zhao et al. 2014). In a report surveying the phenolic constituents of the Lemnaceae, L. minor lignin was once again reported to be composed of p-coumaryl and coniferyl (H and G) units (McClure 1975). However, a Klason lignin content of 12% was reported for Lemna perpusilla collected in Calcutta, India (Chandra et al. 1991), indicating possible variance in lignin content dependent on purification protocols, assay choice, and growth conditions. The lower lignin content of L. minor grown in 50% D2O resembles the results reported earlier for annual ryegrass (Evans et al. 2014), while switchgrass grown hydroponically in 50% D2O-exhibited higher lignin content than H2O-grown hydroponic switchgrass (Evans et al. 2015).
Discussion
The growth inhibition observed for supplementation with 0.5% glycerol (approximately 54 mM) could be due to sensitivity of the duckweed to osmotic stress induced by polyols. Mannitol, a six-carbon polyol, is used at concentrations of 100–400 mM to induce osmotic stress in plant experiments (Butt et al. 2017; Singh et al. 2015). A standard method for assay of drought tolerance utilizes 5% polyethylene glycol 6000 (Joshi et al. 2017). Sodium acetate was found to be rapidly toxic to L. minor with an I25% of 0.89 mM. As salinity from sodium chloride has been reported to inhibit growth of L. minor at concentrations greater than 25 mM over the course of 3 days, it appears unlikely that the sodium counter ions were responsible for this rapid toxic effect (Liu et al. 2017).
Inhibition of root elongation by 50% D2O in Lemna is consistent with earlier studies for this genus as well as terrestrial plants. In this study, common duckweed Lemna minor was found to adapt better to growth in 50% D2O than L. gibba based on the growth rates and the properties of the isolated cellulose. Cellulose isolated from L. gibba had a DPw more than twice that of L. minor cellulose. The differences in cellulose chain length may be correlated with the morphology of L. gibba which is distinguished from that of L. minor by larger frond size and the presence of a vascular structure called a nerve. Both species exhibited cellulose PDI values, whether grown in H2O or in 50% D2O, approximately threefold higher than those determined for annual ryegrass and for switchgrass grown under similar conditions (Evans et al. 2014, 2015).
Analysis of sequentially extracted fractions of Lemna minor biomass found a composition typical of primary cell walls, being largely composed of cellulose and pectin, with relatively small amounts (around 3%) of hemicellulose and lignin (Zhao et al. 2014). Celluloses extracted from primary cell walls are reported to have average degrees of polymerization in the range of 2000–6000 glucose residues, while secondary cell walls contain longer cellulose molecules with DPs as high as 10,000 (Reid 1997).
The phenomenon of growth inhibition by D2O at 50% and higher is likely to be the cumulative result of specific impacts on multiple metabolic pathways. A correlation between germination and growth with cold tolerance and the differences in the physical properties of D2O (higher viscosity, higher melting point, and higher temperature of maximum density) had been noted in earlier studies (Siegel et al. 1964; Blake et al. 1968). Both growth rate and metabolism of L. minor were observed to change in response to water temperature. Within a temperature range of 8–31 °C, temperatures lower than 25 °C result in slower growth and higher ratios of carbohydrate to protein in the plant biomass (Bornkamm 1966). Increase in growth temperature may improve growth in 50% D2O, as had been reported for winter grain rye (Siegel et al. 1964). The membrane potential of Lemna species increases in response to light, believed to be mediated by phytochrome (Löppert et al. 1978) and to hexoses in the media (Novacky et al. 1978). Uptake of both hexoses and amino acids from growth media has been shown to be coupled to proton transport. Lemna species are reported to grow in the pH range of 4.5–7.2 (Stomp 2005). Previously reported studies of cultivation of Lemna spp. in 50% D2O have used media at pH 5.0, observing reduced growth rate and shortened roots (Trewavas 1970).The medium formulation published by Hutner, with a pH approximately 4.8, as well as Hoagland’s medium with pH were used in studies of Lemna perpusilla in 50–63% D2O (Scope et al. 1965), while Cooke and co-workers (Cooke et al. 1979a, b; 1980) used the medium published by Trewavas (1970). The lower pH of 4.2 employed in this study may have partially ameliorated the decrease in membrane potential due to the effects of 50% D2O, which can be expected to include both 50% slower transport of deuterons compared to protons (De Coursey and Cherny 1997) as well as the disturbance of the phytochrome equilibrium (Sarkar and Song 1981; Borucki et al. 2005). Differences in results from these reported studies may also stem from variation in the type of illumination used for cultivation. Inhibition of L. minor by phenylalanine was previously shown to be correlated with intensity and spectral characteristics of illumination (Evans et al. 2017).
Growth in 50% D2O had been previously reported to result in morphological changes to the cellular structure of fronds of Lemna perpusilla (Cope et al. 1965). Enlargement of cells, decrease in size of air spaces, and disorganization of cellular arrangement in frond tissues were visible at 150× magnification. Changes to the ultrastructure of the tonoplast and chloroplast membranes of Lemna minor during initial exposure to 50% D2O, followed by recovery and adaptation after 24 h, were observed by electron microscopy (Cooke et al. 1980). Protein fractions from the isotopically stressed L. minor were more susceptible to protease digestion, similar to those from nitrate-stressed plants. However, examination of seedlings of winter rye (Secale cereale) germinated in 99.8% D2O by electron microscopy found no major differences in the cellular ultrastructure compared to H2O-germinated controls (Waber and Sakai 1974).
The decrease in the yield of Klason lignin determined for the duckweed grown in 50% D2O could result from the known kinetic isotope effects of D2O on phytochrome equilibria (Sarkar and Song 1981). The enzyme phenylalanine ammonia lyase (PAL) is known to be induced through a phytochrome-activated pathway. L. minor produces PAL and tyrosine ammonia lyase (TAL) in synchrony with the light cycle used for cultivation, and induction by illumination with red light has been demonstrated. Photoactivation and cycling of phytochromes have been shown to be perturbed by D2O with relatively large solvent kinetic isotope effects (Sarkar and Song 1981).
In conclusion, the results of this study indicate that production of 40–50% deuterated biomass can be carried out by cultivation of Lemna minor duckweed at increased growth rates under higher illumination levels with diurnal period. Plants eventually adapt to growth in 50% D2O without addition of growth hormones but continue to exhibit root stunting and slower growth rates than H2O-grown controls. Cell wall appearance and cellulose degree of polymerization resembled those of H2O-grown controls, indicating that duckweed could be used to produce deuterium-enriched carbohydrates.
Author contribution statement
BRE carried out plant cultivation experiments with assistance of DTR, CSR, KMcG, and HO’N. MF and GB carried out characterization by NMR, FTIR, and other methods at Georgia Tech under supervision of AJR. BD coordinated and led the research project.
References
Bali G, Foston MB, O’Neill HM, Evans BR, He J, Ragauskas AJ (2013) The effect of deuterium incorporation on the structure of bacterial cellulose. Carbohydr Res 374:82–88
Bergmann BA, Cheng J, Classen J, Stomp A-M (2000) In vitro selection of duckweed geographical isolates for potential use in swine lagoon effluent renovation. Bioresour Technol 73:13–20
Blake MI, Crane FA, Uphaus RA, Katz JJ (1968) Effect of heavy water on the germination of a number of species of seeds. Planta 78:35–38
Blazey EB, McClure JW (1968) The distribution and taxonomic significance of lignin in the Lemnaceae. Am J Bot 55:1240–1245
Bornkamm R (1966) A seasonal rhythm of growth in Lemna minor L. Planta 69:178–186
Borucki B, von Stetten D, Seibeck S, Lamparter T, Michael N, Mroginski MA, Otto H, Murgida DH, Heyn MP, Hildebrandt P (2005) Light-induced proton release of phytochrome is coupled to the transient deprotonation of the tetrapyrrole chromophore. J Biol Chem 280:34358–34364
Brain RA, Solomon KR (2007) A protocol for conducting 7-day daily renewal tests with Lemna gibba. Nat Protoc 2:979–987
Butt HI, Yang Z, Gong Q, Chen E, Wang X, Zhao G, Ge X, Zhang X, Li F (2017) GaMYB85, an R2R3 MYB gene, in transgenic Arabidopsis plays an important role in drought tolerance. BMC Plant Biol 17:142. https://doi.org/10.1186/s12870-017-1078-3
Chandra S, Bhaduri SK, Sardar D (1991) Chemical characterization of pressed fibrous residues of four aquatic weeds. Aquat Bot 42:81–85
Cooke RJ, Davies DD (1980) General characteristics of normal and stress-enhanced protein degradation in Lemna minor (duckweed). Biochem J 192:499–506
Cooke RJ, Grego S, Oliver J, Davies DD (1979a) The effect of deuterium oxide on protein turnover in Lemna minor. Planta 146:229–236
Cooke RJ, Oliver J, Davies DD (1979b) Stress and protein turnover in Lemna minor. Plant Physiol 64:1109–1113
Cooke RJ, Grego S, Roberts K, Davies DD (1980) The mechanism of deuterium oxide-induced protein degradation in Lemna minor. Planta 148:374–380
Cope BT, Bose S, Crespi HL, Katz JJ (1965) Growth of Lemna in H2O–D2O mixtures: enhancement by kinetin. Bot Gaz 126:214–221
Cox KM, Sterling JD, Regan JT, Gadaska JR, Frantz KK, Peele CG, Black A, Passmore D, Moldovan-Loomis C, Srinivasan M, Cuison S, Cardarelli PM, Dickey LF (2006) Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 24:1591–1597
De Coursey TE, Cherny VV (1997) Deuterium isotope effects on permeation and gating of proton channels in rat alveolar epithelium. J Gen Physiol 169:415–434
DeWitt SH, Maryanoff BE (2018) Deuterated drug molecules: focus on FDA-approved deutetrabenazine. Biochemistry 57:472–473
Evans BR, Shah R (2015) Development of approaches for deuterium labeling in plants. In: Kelman Z (ed) Methods in enzymology: volume 565 isotope labeling of biomolecules. Elsevier, Oxford, pp 213–243
Evans B, Bali G, Reeves D, O’Neill H, Sun Q, Shah R, Ragauskas A (2014) Effect of D2O on growth properties and chemical structure of annual ryegrass (Lolium multiflorum). J Agric Food Chem 62:2592–2604
Evans BR, Bali G, Foston M, Ragauskas AJ, O’Neill H, Shah R, McGaughey J, Reeves D, Rempe CS, Davison BH (2015) Production of deuterated switchgrass by hydroponic cultivation. Planta 242:215–222
Evans BR, Bali G, Ragauskas A, Shah R, O’Neill H, Howard C, Lavenhouse F, Ramirez D, Weston K, Ramey K, Cangemi V, Kinney B, Partee C, Ware T, Davison B (2017) Alleopathic effects of exogenous phenylalanine: a comparison of four monocot species. Planta 246:673–685
Firsov A, Tarasenko I, Mitiouchkina T, Shaloiko L, Kozlov O, Vinokurov L, Rasskazova E, Murashev A, Vainstein A, Dolgov S (2018) Expression and immunogenicity of M2e peptide of avian influenza virus H5N1 fused to ricin toxin B chain produced in duckweed plants. Front Chem 6:22. https://doi.org/10.3389/fchem.2018.00022
Foston MB, McGaughey J, O’Neill H, Evans BR, Ragauskas AJ (2012) Deuterium incorporation in biomass cell wall components by NMR analysis. Analyst 137:1090–1093
Foston M, Ragauskas AJ (2010) Changes in lignocellulosic supramolecular and ultrastructure during dilute acid pretreatment of populus and switchgrass. Biomass Bioenergy 34(12):1885–1895
Gasdaska J, Spencer D, Dickey L (2003) Advantages of therapeutic protein production in the aquatic plant Lemna. Bioprocess J 3:50–56
Halford B (2016) The deuterium switcheroo. Chem Eng News 94:32–36
Joshi R, Anwar K, Das P, Singla-Pareek SL, Pareek A (2017) Overview of methods for assessing salinity and drought tolerance of transgenic wheat lines. In: Bhalla PL, Singh MB (eds) Wheat biotechnology. Springer, New York, pp 83–95
Körner S, Vermaat JE, Veenstra S (2003) The capacity of duckweed to treat wastewater: ecological consideration for a sound design. J Environ Qual 32:1583–1590
Langan P, Evans BR, Foston M, Heller WT, O’Neill HM, Petridis L, Pingali SV, Ragauskas AJ, Smith JC, Davison B (2012) Neutron technologies for bioenergy research. Ind Biotechnol 8:209–216
Liu C, Dai Z, Sun H (2017) Potential of duckweed (Lemna minor) for removal of nitrogen and phosphorus from water under salt stress. J Environ Manag 187:497–503
Löppert H, Kronberger W, Kandeler R (1978) Phytochrome-mediated changes in the membrane potential of subepidermal cells of Lemna paucicostata 6746. Planta 138:133–136
McClure JW (1975) The applicability of polyphenolic data to systematic problems in the Lemnaceae. Aquat Bot 1:395–405
Moody M, Miller J (2005) Lemna minor growth inhibition test. In: Blaise C, Férard J-F (eds) Small scale freshwater toxicity investigations. Springer, Amsterdam, pp 271–298
Novacky A, Ullrich-Eberius CI, Lüttge U (1978) Membrane potential changes during transport of hexoses in Lemna gibba G1. Planta 138:263–270
Porath D, Hepher B, Koton A (1979) Duckweed as an aquatic crop: evaluation of clones for aquaculture. Aquat Bot 7:273–278
Porra R (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Reid GSG (1997) Carbohydrate metabolism: structural carbohydrates. In: Dey PM, Harborne JB (eds) Plant biochemistry. Academic Press, San Diego, CA, USA, London, UK, pp 205–235
Sarkar HK, Song PS (1981) Phototransformation and dark reversion of phytochrome in deuterium oxide. Biochemistry 20:4315–4320
Schmidt C (2017) First deuterated drug approved. Nat Biotechnol 35:493–494
Siegel SM, Halpern LA, Giumaro C (1964) Germination and seedling growth of winter rye in deuterium oxide. Nature 201:1244–1245
Singh A, Jha SK, Bagri J, Pandey GK (2015) ABA inducible rice protein phosphatase 2C confers ABA insensitivity and abiotic stress tolerance in arabidopsis. PLoS One 10(4):e0125168. https://doi.org/10.1371/journal.pone.0125168
Stomp A-M (2005) The duckweeds. A valuable plant for biomanufacturing. Biotechnol Annu Rev 11:69–99
Trewavas A (1970) The turnover of nucleic acids in Lemna minor. Plant Physiol 45:742–751
Waber J, Sakai WS (1974) Effect of growth in 99.8% deuterium oxide on ultrastructure of winter rye. Plant Physiol 53:128–130
Yakir D, De Niro MJ (1990) Oxygen and hydrogen isotope fractionation during cellulose metabolism in Lemna gibba L. Plant Physiol 23:325–332
Zhao X, Elliston A, Collins SRA, Moates GK, Coleman MJ, Waldron KW (2012) Enzymatic saccharification of duckweed (Lemna minor) biomass without thermophysical pretreatment. Biomass Bioenerg 47:352–361
Zhao X, Moates GK, Wellner N, Collins SRA, Coleman MJ, Waldron KW (2014) Chemical characterization and analysis of the cell wall polysaccharides of duckweed (Lemna minor). Carbohydr Polym 111:410–418
Acknowledgements
This research was supported by the U. S. Department of Energy, Office of Science, through the Genomic Science Program, Office of Biological and Environmental Research, under Contract FWP ERKP752. The research at Oak Ridge National Laboratory’s Center for Structural Molecular Biology (CSMB) was supported by the U. S. Department of Energy, Office of Science, through the Office of Biological and Environmental Research under Contract FWP ERKP291, using facilities supported by the Office of Basic Energy Sciences, U. S. Department of Energy. Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for the U. S. Department of Energy under Contract DE-AC05-00OR22725. D. Reeves was supported by a U. S. Department of Energy Higher Education Research Experience internship managed by Oak Ridge Institute of Science and Education. C. Rempe was supported by a Department of Energy Science Undergraduate Laboratory Internship and Higher Education Research Experience internship managed by Oak Ridge Institute of Science and Education. K. McGrath was supported by the DOE Academies Creating Teacher Scientists (ACTS) summer 2010 program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been authored by UT-Battelle, LLC, under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Rights and permissions
About this article
Cite this article
Evans, B.R., Foston, M., O’Neill, H.M. et al. Production of deuterated biomass by cultivation of Lemna minor (duckweed) in D2O. Planta 249, 1465–1475 (2019). https://doi.org/10.1007/s00425-019-03097-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03097-3