Abstract
A comparison of the thermal decomposition and combustion characteristics of raw and scoured cottons has demonstrated a mechanistic link caused by the presence of inorganic salts in raw cotton, which enhances resistance to heat and flame. Thermogravimetry, differential thermogravimetry, and microscale combustion calorimetry were used to examine the thermal decomposition kinetics and thermal stability of cotton. During pyrolysis, both raw cotton nonwoven and woven fabrics exhibited a slower decomposition with a larger initial weight loss and produced a greater char yield, as compared to the fabrics after scouring, which removes most inorganic components from cotton. The activation energy (E a ) values, calculated using the Kissinger method, the Flynn–Wall–Ozawa method, and the modified Coats–Redfern method, were consistently determined to be smaller for raw cotton than for scoured cotton. The analyses of cotton fabrics heated at elevated temperatures by 13C CP/MAS NMR and ATR-FTIR showed that trace quantities of inorganic components promoted the formations of oxygenated moieties at low temperatures and aliphatic intermediate char. In the combustion, raw cotton exhibited a much smaller heat release capacity and a smaller total heat release than scoured cotton, indicating enhanced thermal stability when the inorganic components are intact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton is most commonly scoured or scoured and bleached before being used for commercial purposes, but due to its propensity to burn, cotton’s use is limited. Flame retardant/resistant chemistry for cotton has been extensively researched over the last six decades, mostly triggered by the enforcement of regulations, and has broadened applications for cotton to uses in which resistance to heat and flame is required. Nevertheless, many questions on established flame retardants have been raised in terms of environmental sustainability, toxicology aspect, performance, and cost, thus in turn seeking innovative ways to replace them (Horrocks 2011).
The obstacles in exploiting desirable flame retardants are partly attributed to the difficulty of completely understanding the complex thermal reaction of cotton. Studies over many years suggest that the thermal degradation of cellulose, the main component of cotton, undergoes multiple steps (Antal et al. 1980; Bradbury et al. 1979; Radlein et al. 1991; Shafizadeh 1982). At low temperature (>300 °C), the decomposition of the glycosidic units occurs, producing water, carbon dioxide, carbon monoxide, and eventually char. At above 300 °C, two competitive processes occur: (1) depolymerization producing volatile levoglucosan, furan, and furan-derivatives and (2) dehydration producing char dominated by aliphatic moieties at ca. 350 °C, which is transformed into char composed primarily of aromatic functionality at about 400–600 °C. This accepted mechanism, however, is influenced by external and internal conditions that alter the thermal reaction routes of cellulose and consequently change an array of products formed upon decomposition. For example, heating rates influenced the thermal stability of char; less stable char was formed at a high heating rate (300 °C/min) (Alongi et al. 2013). At a low heating rate (2 °C/min), the pronounced dehydration was observed by FTIR before the onset of depolymerization reactions at higher temperatures, resulting in a reduced volatilization (Julien et al. 1991).
Special attention is given to the intriguing phenomena that a very small amount of inorganic impurities (<0.1 %) modifies the cellulose pyrolysis process. Natural inorganic components of woods apparently inhibit the formation of volatiles and increase the char yield (DeGroot and Shafizadeh 1984; Kelly et al. 1991; Piskorz et al. 1989; Shafizadeh 1968; Shafizadeh et al. 1979; Williams and Horne 1994). Piskorz et al. (1989) reported the influence of inorganic impurities on the pyrolysis products of wood cellulose: a significant increase in levoglucosan formation and decrease in glycolaldehyde formation were observed after removal of inorganic components. The inhibition mechanism of the naturally-occurring inorganic components, however, is unclear. An explanation proposed is that the formations of levoglucosan and hydroxyacetaldehyde result from parallel reactions of heterolytic and homolytic scissions, respectively (Richards 1987; Shafizadeh and Fu 1973). Metal ions act as catalysts for the decomposition of glycosidic units of cellulose, suppressing the equally selective pathway of depolymerization (Piskorz et al. 1989; Richards 1987). According to another explanation, the metal ions retard the unzipping reaction of depolymerization by capping chain ends (Basch and Lewin 1973; Halpern and Patai 1969). With respect to decreasing the levoglucosan formation, the type of metal ions present was found to be more influential than their concentration (Richards and Zheng 1991), while the char yield strongly depended on the concentration of metal ions (Mayer et al. 2012). The inorganic salts also influenced the nature of the char: Zn and Co ions were found to enhance the formation of aromatic char, whereas K increased aliphatic char (Soares et al. 1998).
Most studies on the effect of natural inorganics were conducted with woods by removing the inorganics via an acid-wash. Since the acid washing of cellulose reduces its degree of polymerization (DP), which is also known to affect the pyrolysis pathway, the sole effect of inorganics cannot be easily resolved or deduced. Apparently, the interrelationship between inorganic ions and DP was observed in the pyrolysis of woods (Julien et al. 1991). Therefore, it is necessary to study the roles of natural inorganics without disturbing DP. Another motivation is that most pyrolysis studies of cotton were conducted using scoured or scoured and bleached materials. As the use of unscoured raw cotton is receiving more attention, in particular for nonwoven applications such as batting for furniture and mattresses, the acoustic insulation of automobiles as well as various disposable products such as protective or medical garments, it is valuable to understand the thermal decomposition of raw cotton cellulose.
A goal of the present work was to compare the thermal decomposition and combustion characteristics of raw cotton before and after alkaline scouring, which removes inorganic ashes without changing the DP of cellulose. Several techniques, including thermogravimetry (TG), differential thermogravimetry (DTG), microscale combustion calorimetry (MCC), and solid-state NMR, were employed. Based on the TG/DTG data, activation energy (E a ) was calculated using three model-free methods. TG/DTG is an excellent tool to provide information pertaining to thermal processes, but other independent methods must be utilized to elucidate thermal decomposition kinetics and mechanism (White et al. 2011). MCC, which simulates combustion by pyrolyzing a sample at a constant heating rate and subsequently allowing a complete oxidation of the released gases, provided heat release parameters and estimated its fire hazard potential. 13C CP/MAS NMR and ATR-FTIR microscope gave information on the chemical changes of decomposed cellulose. Such combined results would allow better understanding of the thermal behavior of raw cotton cellulose with its inorganic components intact as an adjunct to the development of new methods for producing flame resistant cotton in general.
Experimental
Materials and sample preparation
Needle-punched raw cotton nonwoven (NW) fabric was fabricated in the pilot plant at the Southern Regional Research Center. The American upland raw cotton acquired from the national registry was mechanically cleaned without using water and chemicals through re-ginning and lint-cleaning processes. The resulting opened fiber was carded, cross-lapped, and subsequently needle-punched to produce a fabric (100 g/m2). Raw cotton plain woven (W) fabric (greige cotton duck, 237 g/m2) was purchased from the Testfabrics, Inc.
The alkaline scouring of raw NW and W fabrics was carried out as follows: using an overflow jet dyeing apparatus (Werner Mathis Inc.), fabric was immersed in an aqueous solution containing NaOH (1.8 g/L) and Triton® X-100 (0.2 g/L) with a liquid-to-fabric ratio of 22:1 and circulated at 100 °C for 60 min. After treatment, the fabric was washed in circulating water at 100 °C for 20 min, followed by cold water for 20 min. The scoured fabric was neutralized with a solution of acetic acid (0.25 g/L) in water for 10 min and then rinsed with cold water. The fabric was then passed through a continuous hot air dryer (Werner Mathis Inc.).
Elemental analysis
The elemental analysis was carried out by quadrupole inductively coupled plasma mass spectroscopy in the Elemental Analysis, Inc. in Lexington, KY. Six disk samples (2.5 cm in diameter) were randomly taken from each fabric and ground in a Wiley Mill (Arthur H. Thomas Co.) with a 40 mesh (0.42 mm). A 0.1 g of the sample was digested with 2 mL of nitric acid and 1 mL of hydrochloric acid in a closed-vessel microwave digestion system. The sample was diluted to 50 g with high-purity water, and internal standards were added.
TG/DTG
Thermogravimetric analysis was carried out using a TGA Q500 thermal gravimetric analyzer (TA Instrument) under nitrogen atmosphere. The nitrogen flow into the furnace was maintained at a rate of 90 mL/min. About 5 mg of sample, which had been ground in a Wiley Mill (Arthur H. Thomas Co.) with a 40 mesh, were evenly placed at the bottom of an aluminum pan. The temperature was increased from 23 ± 3 to 1,000 °C. For the determination of E a , four different heating rates, 2, 5, 10 and 15 °C/min, were used. TG and DTG thermograms were analyzed using Universal Analysis 2000 software. Three runs were performed to obtain an average of the thermal decomposition parameter.
Ea determination
The E a values for the thermal decomposition of cotton fabrics were calculated from nonisothermal TGA data by using “model-free” methods: the Kissinger, the Flynn–Wall–Ozawa, and the modified Coats–Redfern methods. These methods are based on the following fundamental kinetic equation.
where α is the conversion fraction, (W 0 − W t )/(W 0 − W f ) (where W 0, W t , and W f are the initial, time t, and final weights of the sample, respectively), T is the absolute temperature, A is the pre-exponential factor, E a is the activation energy, R is the gas constant, and f(α) is the reaction model.
Introducing the heating rate, β = dT/dt, into Eq. (1) gives
Integration of Eq. (2) gives
The Kissinger method (Kissinger 1956) is based on taking the derivative of Eq. (2) and considering that the second derivative is zero at the maximum reaction rate. Since this method assumes a constant E a over the course of the reaction, it is not an isoconversional method. The Flynn–Wall–Ozawa method (Flynn and Wall 1966; Ozawa 1965), an isoconversional method, uses approximations of the integral form of Eq. (3) using the Doyle’s approximation (Doyle 1961). The modified Coats–Redfern method is also an isoconversional method transformed by model-fitting the Coats–Redfern method which utilizes Eq. (3). The isoconversional method has an advantage to produce E a at progressive α. The expressions of these three methods are summarized in Table 1.
MCC
Thermal combustion properties of raw and scoured cotton fabrics were examined using a microscale combustion calorimeter (MCC, FAA Micro Calorimeter, Fire Testing Technology Ltd.) according to ASTM D 7309-2007 (Method A). About 5 mg of sample were heated to 700 °C at a heating rate of 1 °C/s in a stream of nitrogen flowing at 80 cm3/min. The volatile thermal decomposition products forms in a pyrolyzer are swept by the gas stream of nitrogen and oxygen to a combustor where the decomposition products are completely oxidized at 900 °C for 10 s. Oxygen concentrations and flow rates of the combustion gases are used to determine the oxygen depletion involved in the combustion process and the heat release rate (HRR) is determined from these measurements. The obtained parameters include: specific HRR (W/g), obtained by dividing HRR by the initial sample mass; peak heat release rate (PHRR, W/g) which is the maximum specific HRR; temperature at PHRR (TPHRR, °C); heat release capacity (HRC, J/g K), obtained by dividing PHRR by the heating rate; total heat release (THR, J/g) which is the area under the specific HRR peaks; and char content, determined by weighing the sample before and after the pyrolysis. The average of duplicate measurements was presented.
13C CP/MAS NMR
13C CP/MAS NMR measurements were carried out on a Bruker Avance-400 NMR spectrometer equipped with a Bruker double-resonance MAS probe. The operating frequency of 13C is 100.55 MHz. Samples were heated in a bench furnace (Thermal Scientific Inc.) at seven different temperatures: 270, 320, 340, 350, 360, 380, and 400 °C, under nitrogen atmosphere. The flow rate of a nitrogen stream was 45 mL/min and the heating rate was 15 °C/min. The heated sample was ground in the same way used in the TG experiment and filled into a 4 mm ceramic MAS rotor. The sample was spun at a speed of 10 kHz at room temperature. The 13C CP/MAS spectra were acquired with 1H 90° pulse of 5 μs, a ramped H–H matched contact pulse of 1.5 ms, and a recycle delay of 4 s. Each free induction decay was acquired for 0.029 s, taking 2,048 data points.
ATR-FTIR
The ATR-FTIR spectra were measured using a Bruker Alpha FT-IR spectrometer. A diamond crystal plate was employed as a reflector. A resolution of 4 cm−1 and 32 scans were used. The IR-mapping was performed using a Bruker LUMOS FT-IR microscope. The integration of 1,714 cm−1 band was obtained at 49 spots of the sample (4 × 4 mm) using the ATR-mode with the automatic knife-edge aperture set to 120 × 120 μm. An acquisition time per measurement point was 20 s, and a spectral resolution was 4 cm−1.
Results and discussion
Inorganic components of cotton
Non-cellulosic components of cotton fiber, such as proteins, amino acids, wax, pectin, and ash, are located in the cuticle, primary cell wall, and lumen (Wakelyn et al. 2006). Among these components, the amount of ash, including inorganic salts and salts of organic acid, present in a typical mature cotton fiber is 1.2 % based on the dry weight of the fiber (McCall and Jurgens 1951). Generally, the amount of metals varies by fiber maturity, variety of cotton, and environmental conditions, and most of them can be removed by scouring (Wakelyn et al. 2006). Table 2 shows the results of the elemental analysis on cotton NW fabrics before and after scouring using quadrupole inductively coupled plasma mass spectrometry. Raw cotton contains noticeable amounts of K, Ca, Mg, and P. K is the most abundant metal in the cotton fiber. K, Mg, P, and Ca were mostly removed by scouring. Appreciable reductions in the amounts of Mn and Fe were also observed after scouring.
TG/DTG
The TG and DTG thermograms of raw and scoured W fabrics taken under nitrogen environment at a heating rate of 10 °C/min are presented in Fig. 1. Both fabrics show three stages of weight loss. The loss of water occurs near 100 °C, followed by the decomposition of cellulose with a high rate of weight loss. After the completion of the cellulose decomposition at ~ 400 °C, the decomposition of char proceeds with a much lower rate of weight loss until near 1,000 °C. The second stage was characterized by determining an onset temperature (T o ), an end decomposition temperature (T e ), and a maximum decomposition rate temperature (T m ). These values and corresponding weight losses are presented in Table 3 along with char yields measured at 600 °C. The T o of raw W fabric was found to be about 73 °C lower than the value of scoured W fabric. This difference could be ascribable to the decomposition of low-molecular-weight non-cellulosic components at low temperatures. However, the weight loss at the T o (WL o ) observed in this study is too large to consider the loss of non-cellulosic materials. Julien et al. (1991), who observed a similar difference in T o between untreated and acid-washed (ash-free) cellulose samples during vacuum pyrolysis, attributed to the thermal stability of glycosidic units enhanced by the metal ions. Concurrently, Williams and Horne (1994) also observed a marked decrease in the temperature of the initial weight loss by the addition of metal salts to the cellulose sample. In T m , WL m , T e , and WL e , there was no significant difference between raw and scoured W fabrics. However, it is noticed that scouring changed the shape of the DTG curves. Compared with raw W fabric, the DTG curve of scoured W fabric was deep and sharp, reflecting the occurrence of rapid and drastic thermal reactions. The char amount of raw W fabric was almost five times greater than that of scoured W fabric. As can be seen in Fig. 1, this difference was maintained up to 900 °C. The TG characteristic values of W fabric are consistent with those of NW fabric (Table 3).
Ea for thermal decomposition
To investigate the effect of the inorganics on the kinetics of the cellulose decomposition, the activation energy (E a ) was calculated from the dynamic TG data, which were obtained using four different heating rates. The E a values of W and NW fabrics were found to be very close, so their average value was reported. By the Kissinger method, the E a was determined from the slope of a linear plot of ln(β/T 2p ) against 1/T p (Table 1). The calculated E a values are 170.1 ± 4.3 kJ/mol for raw cotton fabrics and 182.5 ± 6.4 kJ/mol for scoured fabrics. This result is consistent with the decreases in E a observed when various metal salts were incorporated into cellulosic materials (Tang and Neil 1964; Williams and Horne 1994). The Kissinger method produces a single E a based on T m . The variation of E a in the course of the cellulose decomposition was further examined using two isoconversional methods, the Flynn–Wall–Ozawa (F–W–O) method and the modified Coats–Redfern (C–R) method. The isoconversion plots of log β versus 1/T by the F–W–O method and of ln(β/T 2) versus 1/T by the modified C–R method are presented in Fig. 2a, b, respectively. All samples exhibited straight lines (r > 0.99) in the entire conversion level studied (α = 0.1–0.9). The average E a values for W and NW fabrics are plotted as a function of conversion level in Fig. 3. The E a values calculated from the two isoconversional methods are in excellent agreement. The E a of raw cotton fabrics slightly increases with increasing conversion level and becomes constant above 0.5, whereas the E a of scoured fabrics remains constant over the entire conversion level. The constant behavior of E a for cellulose materials has been observed elsewhere (Cabrales and Abidi 2010). Considering that the cellulose thermal decomposition is a multistep process involving competitive reactions (Kilzer and Broido 1965; Shafizadeh 1985), the constant tendency of E a indicates that the measured E a is governed by the slowest step (Vyazovkin et al. 2007). Agreeing with the result by the Kissinger method, the overall E a of raw cotton fabrics was smaller than that of scoured fabrics. The difference in E a between these two sets of fabrics was greater at low conversion levels. Such catalytic action of inorganics on the thermal reactions of cellulose has also been observed for other lignocellulosic materials (rice straw and rice hulls), whose apparent activation energies increased in the absence of inorganic salts (Nassar 1999; Teng and Wei 1998).
MCC
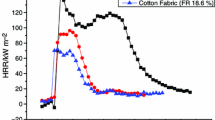
The quantitative results from TG/DTG analyses provided a comparison of the thermal decomposition processes of raw and scoured cotton fabrics, but they may not predict the flammability. MCC, which simulates combustion by pyrolyzing a sample at a constant heating rate and subsequently conducting a complete oxidation of the gases released from the pyrolyzer, provides heat release related parameters that are important in determining the hazard in a fire. Several studies have demonstrated that the MCC parameters have a good correlation with conventional flammability test values (Lyon et al. 2007; Yang et al. 2009; Yang and He 2012). Typical plots of specific heat release rate versus temperature for raw and scoured cotton NW fabrics are presented in Fig. 4. The HRR plot of cotton cellulose is characterized as one single peak. Raw cotton fabric starts to decompose and release gases at a lower temperature than scoured fabric; the onset temperatures for raw and scoured cotton fabrics are 293 and 313 °C, respectively. The heat release of raw cotton fabric ends at about 12 °C lower than that of scoured fabric (393 °C). The maximum rate in heat release (TPHRR) for raw cotton is 349 °C, which is same as its T m measured in the TG analysis, whereas the TPHRR value of scoured cotton is 355 °C, which is greater than its T m (348 °C). This shift of TPHRR to a higher temperature for scoured cotton suggests that the low heating rate (1 °C/min) used in the pyrolysis stage of MCC may allow scoured cotton cellulose to recrystallize and thus require a higher temperature to reach the maximum rate in heat release. The increased crystalline cellulose for scoured cotton was also detected in the 13C CP/MAS NMR spectra of scoured cotton later.
The average values of MCC data for raw and scoured W and NW fabrics, including heat release capacity (HRC), peak heat release rate (PHRR), temperature at PHRR (TPHRR), total heat release (THR), and char yield, are presented in Table 4. These parameters are good indicators of the flammability of materials (Walters and Lyon 2003). The PHRR and THR of raw cotton fabric are 147 W/g and 8.1 kJ/g, equivalent to 56 and 64 % of the PHRR and THR of scoured fabric, respectively. The reduction in PHRR and THR implies that, in the presence of inorganics, fewer amounts of combustible products were generated during combustion. Hu et al. (2011) reported the decrease of flammable decomposition products, such as carbonyl compounds, hydrocarbons, and methanol, in association with a lower value of PHRR obtained by the organic–inorganic hybrid coating on cotton fabric. The char yield of raw cotton fabric was measured to be three times greater than that of scoured fabric. The formed char insulates the substrate underneath to prevent the release of heat, oxygen, and combustible gases. The MCC results clearly show that very low concentrations of inorganic components significantly improve the thermal stability of cotton cellulose.
Chemical changes of pyrolyzed cotton
The kinetic data along with TG and MCC results showed that natural inorganics catalytically acted to lower the onset temperature of cellulose decomposition and promote char-forming reactions; however, kinetic models currently available have some limitations in elucidating heterogeneous thermal reactions of solid materials, and thus it is necessary to analyze changes in the chemical composition and/or structure of the material upon decomposition using other analytical techniques (White et al. 2011). In this study, cotton was examined at elevated temperatures using 13C CP/MAS NMR and ATR-FTIR. 13C CP/MAS NMR is a technique well-suited to characterize chemical changes occurring in highly insoluble material systems such as pyrolyzed cellulosic samples (David et al. 2009). 13C CP/MAS NMR spectra of scoured and raw cotton NW fabrics heated under nitrogen environment at elevated temperatures are shown in Fig. 5a, b, respectively. The spectrum of cotton has four distinct resonances representing the carbons within the monomeric glycosidic ring unit (C-1; C-4; C-2, 3, 5; and C-6) in the chemical shift ranges of 102–108, 80–92, 67–80, and 57–67 ppm, respectively (Atalla and Vanderhart 1984, 1999). For scoured cotton, the glycosidic carbon peaks primarily remained intact until 360 °C and suddenly disappeared at 380 °C. This rapid decomposition behavior of scoured cotton agrees well with the TG/DTG result. A careful observation shows that the spectrum of scoured cotton heated at 270 °C has slight changes in chemical shift and significant modification to resonance line-shapes, specifically becoming sharper and resembling overlapping multiplets rather than broad Gaussian shapes. These alterations suggest a narrowing in the distribution of magnetically non-equivalent carbons (i.e., carbons which are chemically equivalent but have different chemical shifts because the conformation of surrounding nuclei cause those carbons to become magnetically non-equivalent; for example, the chemical shift of the C-1 in cellulose I and II is different) and may indicate a reduction in the proportion of amorphous and/or para-crystalline cellulose between 270 and 360 °C. At 350 °C, the aliphatic and aromatic peaks centered at around 35 and 130 ppm, respectively, start to develop, but only aromatic carbon peaks remain at 380 °C, indicating that most decomposed products of scoured cotton converted into aromatic compounds containing C–H, C–R, and C–OR bonds.
In contrast with scoured cotton, raw cotton exhibits more gradual changes in the chemical structure as a function of temperature. The magnified spectrum of raw cotton heated at 270 °C shows the appearance of new broad resonances centered near 132 and 154 ppm for carbon–carbon double bonds, 175 ppm for acid and/or ester carbons, and 212 ppm for aldehyde and/or ketone carbons, which were not observed for scoured cotton. These peaks diminish on further heating to 340 °C. Verification that both the peaks at 175 and ~200 ppm were not due to spinning side bands (which would appear at 170 ppm if spun at 10 kHz) was accomplished via re-acquisition at a different spinning rate (data not shown).
The chemical change in raw cotton occurred at low temperatures was further evidenced by ATR-FTIR spectra in Fig. 6a. The absorptions at 1,690–1,725 and 1,612–1,562 cm−1 corresponding to carbonyl groups and carbon–carbon double bonds, respectively, appear for raw cotton at 270 °C but do not appear for scoured cotton at the same temperature. This comparison suggests that raw cotton underwent the dominant dehydration of anhydroglucose unit, which leads to the formation of carbonyl derivatives through the rearrangement of a resulting enol (Kato and Takahashi 1967; Shafizadeh and Bradbury 1979). Some of dehydration products include levoglucosenone, furan derivatives, and pyran derivatives (Pouwels et al. 1989). The formation of hydroxyacetaldehyde is also facilitated in the presence of K and Ca (Julien et al. 1991). Figure 6b shows the 3D map of carbonyl group on the raw fabric (4 × 4 mm).
The aliphatic and aromatic carbon peaks of char for raw cotton are observable at a temperature (320 °C) lower than that for scoured cotton (350 °C). As the temperature increases, the aromatic and aliphatic carbon peaks are intensified, whereas the peaks of glycosidic carbons gradually diminish resulting from decomposition and depolymerization of cellulose. Like scoured cotton, the glycosidic carbon peaks of raw cotton completely disappear at 380 °C. The aromatic carbon peaks for raw cotton show a slight change in chemical shift upfield with increasing the temperature, suggesting that char continues to lose oxygen from aromatic C–O bonds. This may occur as well for scoured cotton char but is not evident from the acquired spectral results. It is noticeable that, unlike scoured cotton, the aliphatic carbon peak remains at 400 °C for raw cotton. The different composition of char observed for raw cotton cellulose containing abundant K is in agreement with another study (Soares et al. 1998). They showed that the presence of K ions, added as carboxylate salts onto pure cellulose, promoted the formation of aliphatic char. The intensity of the aliphatic carbon peak decreases with increasing the temperature from 380 to 400 °C because of the pyrolysis of aliphatic chars into condensed polycyclic aromatic char. The presence of inorganic components not only affected the kinetics of the cellulose decomposition, but also changed the composition of the intermediate chars.
Discussion
It has been proposed that during the thermal decomposition, cellulose undergoes two competitive pathways, dehydration and depolymerization (Antal et al. 1980; Bradbury et al. 1979; Radlein et al. 1991). TG/DTG experiments on raw cotton fabrics before and after scouring showed that inorganic impurities, most of which were removed during the scouring procedure (Table 2), influence this cellulose thermal decomposition process [there are other noncellulosic components, but these low-molecular-weight organic substances decompose at lower temperatures than cellulose (Abidi et al. 2007)]. Since very small concentrations of the metals (≤4,300 μg/g) exerted such a dramatic effect, their function is considered to be catalytic. It is likely that inorganic salts are involved in the thermal reactions of cellulose occurring at low temperatures, as indicated by the shift of the initial decomposition temperature of cellulose to a lower temperature (Figs. 1, 5) and the reduced activation energy for cellulose thermal decomposition particularly at low conversion levels (Fig. 3). Depolymerization mostly contributes to cellulose decomposition at high temperatures due to its higher activation energy (Basch and Lewin 1973), whereas the dehydration of cellulose occurs at low temperatures and contributes to the charring of cellulose. 13C CP/MAS NMR and ATR-FTIR analyses suggested dominant dehydration of anhydroglucose of raw cotton at low temperatures as compared with scoured cotton. Consistently, raw cotton produced a char amount five times greater than scoured cotton. We also observed that the production of aliphatic intermediate char was promoted in the presence of inorganic salts. It has been reported that various individual metals, when artificially introduced to demineralized cellulosic materials, exhibited different effects on cellulose thermal decomposition (Mayer et al. 2012; Richards and Zheng 1991; Soares et al. 1998). The effects of K, Ca, and Mg, which are abundant in cotton, on char yield and the chemical composition of char are in agreement with our results. There have been attempts to explain the roles of metal ions or metal salts in the pyrolysis of cellulose, but the proposed mechanisms are controversial (Piskorz et al. 1989; Richards 1987; Shafizadeh 1968). The results of this study support the mechanism that inorganic salts (K, Ca, and Mg) catalyze the dehydration pathway at low temperatures and allow for moderately slow-rate thermal decomposition (see DTG thermogram shapes in Fig. 1). As a result, the thermal stability of raw cotton was found to be greater than that of scoured cotton as shown by MCC data (Table 4). Thus, unscoured raw cotton carries natural inorganic components which may prove beneficial, if not entirely enabling, in applications requiring heat and fire resistant characteristics.
References
Abidi N, Hequet E, Ethridge D (2007) Thermogravimetric analysis of cotton fibers: relationships with maturity and fineness. J Appl Polym Sci 103:3476–3482
Alongi J, Camino G, Malucelli G (2013) Heating rate effect on char yield from cotton, poly(ethylene terephthalate) and blend fabrics. Carbohydr Polym 92(2):1327–1332
Antal MJ, Friedman HL, Rogers FE (1980) Kinetics of cellulose pyrolysis in nitrogen and steam. Combust Sci Technol 21(3–4):141–152
Atalla RH, Vanderhart DL (1984) Native cellulose: a composite of two distinct crystalline forms. Science 223:283–285
Atalla RH, Vanderhart DL (1999) The role of solid state 13C NMR spectroscopy in studies of the nature of native celluloses. Solid State Nucl Magn Reson 15(1):1–19
Basch A, Lewin M (1973) The influence of fine structure on the pyrolysis of cellulose. I. Vacuum pyrolysis. J Polym Sci 11(12):3071–3093
Bradbury AGW, Sakai Y, Shafizadeh F (1979) Kinetic-model for pyrolysis of cellulose. J Appl Polym Sci 23(11):3271–3280
Cabrales L, Abidi N (2010) On the thermal degradation of cellulose in cotton fibers. J Therm Anal Calorim 102(2):485–491
David K, Pu Y, Foston M, Muzzy J, Ragauskas A (2009) Cross-polarization/magic angle spinning (CP/MAS) 13C nuclear magnetic resonance (NMR) analysis of chars from alkaline-treated pyrolyzed softwood. Energy Fuels 23(1):498–501
DeGroot WF, Shafizadeh F (1984) The influence of exchangeable cations on the carbonization of biomass. J Anal Appl Pyrolysis 6(3):217–232
Doyle CD (1961) Kinetic analysis of thermogravimetric data. J Appl Polym Sci 5(15):285–292
Flynn JH, Wall LA (1966) General treatment of thermogravimetry of polymers. J Res Natl Bur Stand 70A(6):487–523
Halpern Y, Patai S (1969) Pyrolytic reactions of carbohydrates. Part V. Isothermal decomposition of cellulose in vacuo. Isr J Chem 7:673–683
Horrocks AR (2011) Flame retardant challenges for textiles and fibers: new chemistry versus innovatory solutions. Polym Degrad Stab 96(3):377–392
Hu S, Hu Y, Song L, Lu H (2011) Effect of modified organic–inorganic hybrid materials on thermal properties of cotton fabrics. J Therm Anal Calorim 103(2):423–427
Julien S, Chornet E, Tiwari PK, Overend RP (1991) Vacuum pyrolysis of cellulose: Fourier transform infrared characterization of solid residues, product distribution and correlations. J Anal Appl Pyrolysis 19:81–104
Kato K, Takahashi N (1967) Pyrolysis of cellulose part II. Thermogravimetric analyses and determination of carbonyl and carboxyl groups in pyrocellulose. Agric Biol Chem 31:519–524
Kelly J, Mackey M, Helleur RJ (1991) Quantitative analysis of saccharides in wood pulps by quartz-tube pulse pyrolysis—polar phase gas chromatography. J Anal Appl Pyrolysis 19:105–117
Kilzer FJ, Broido A (1965) Speculation on the nature of cellulose pyrolysis. Pyrodynamics 2:151–163
Kissinger HE (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand 57(4):217–221
Lyon RE, Walters RN, Stoliarov SI (2007) Screening flame retardants for plastics using microscale combustion calorimetry. Polym Eng Sci 47(10):1501–1510
Mayer ZA, Apfelbacher A, Hornung A (2012) A comparative study on the pyrolysis of metal- and ash-enriched wood and the combustion properties of the gained char. J Anal Appl Pyrolysis 96:196–202
McCall ER, Jurgens JF (1951) Chemical composition of cotton. Text Res J 21:19–21
Nassar MM (1999) Thermal analysis kinetics of bagasse and rice straw. Energy Sources 21:131–137
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38(11):1881–1886
Piskorz J, Radlein D, Scott DS, Czernik S (1989) Pretreatment of wood and cellulose for production of sugars by fast pyrolysis. J Anal Appl Pyrolysis 16(2):127–142
Pouwels AD, Eijkel GB, Boon JJ (1989) Curie-point pyrolysis-capillary gas chromatography-high-resolution mass spectrometry of microcrystalline cellulose. J Anal Appl Pyrolysis 14:237–280
Radlein D, Piskorz J, Scott DS (1991) Fast pyrolysis of natural polysaccharides as a potential industrial process. J Anal Appl Pyrolysis 19:41–63
Richards GN (1987) Glycolaldehyde from pyrolysis of cellulose. J Anal Appl Pyrolysis 10(3):251–255
Richards GN, Zheng G (1991) Influence of metal ions and of salts on products from pyrolysis of wood: applications to thermochemcial processing of newsprint and biomass. J Anal Appl Pyrolysis 21(1–2):133–146
Shafizadeh F (1968) Pyrolysis and combustion of cellulosic materials. Adv Carbohydr Chem 23:419–474
Shafizadeh F (1982) Introduction to pyrolysis of biomass. J Anal Appl Pyrolysis 3(4):283–305
Shafizadeh F (1985) Pyrolytic reactions and products of biomass. In: Overend RP, Milne TA, Mudge LK (eds) Fundamentals of thermochemical biomass conversion. Elsevier, Amsterdam, pp 183–217
Shafizadeh F, Bradbury AGW (1979) Thermal degradation of cellulose in air and nitrogen at low temperatures. J Appl Polym Sci 23:1431–1442
Shafizadeh F, Fu YL (1973) Pyrolysis of cellulose. Carbohydr Res 29(1):113–122
Shafizadeh F, Furneaux RH, Cochran TG, Scholl JP, Sakai Y (1979) Production of levoglucosan and glucose from pyrolysis of cellulosic materials. J Appl Polym Sci 23(12):3525–3539
Soares S, Camino G, Levchik S (1998) Effect of metal carboxylates on the thermal decomposition of cellulose. Polym Degrad Stab 62(1):25–31
Tang WK, Neil WE (1964) Effects of flame retardants on pyrolysis and combustion of α-cellulose. J Polym Sci C Polym Symp 6(1):65–81
Teng H, Wei Y-C (1998) Thermogravimetric studies on the kinetics of rice hull pyrolysis and the influence of water treatment. Ind Eng Chem Res 37(10):3806–3811
Vyazovkin S, Vincent L, Sbirrazzuoli N (2007) Thermal denaturation of collagen analyzed by isoconversional method. Macromol Biosci 7(11):1181–1186
Wakelyn PJ, Bertoniere NR, French AD, Thibodeaux DP, Triplett BA, Rousselle M, Goynes WR, Edwards JV, Hunter L, McAlister DD, Gamble GR (2006) Cotton fiber chemistry and technology. CRC Press, Boca Raton
Walters RN, Lyon RE (2003) Molar group contributions to polymer flammability. J Appl Polym Sci 87(3):548–563
White JE, Catallo WJ, Legendre BL (2011) Biomass pyrolysis kinetics: a comparative critical review with relevant agriculture residue case studies. J Anal Appl Pyrolysis 91(1):1–33
Williams PT, Horne PA (1994) The role of metal salts in the pyrolysis of biomass. Renewable Energy 4(1):1–13
Yang CQ, He QL (2012) Textile heat release properties measured by microscale combustion calorimetry: experimental repeatability. Fire Mater 36(2):127–137
Yang CQ, He Q, Lyon RE, Hu Y (2009) Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym Degrad Stab 95(2):108–115
Acknowledgments
We thank Dr. Robert H. White for his review and also thank Qingliang He and Dr. Qi Zhao for their valuable discussions. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nam, S., Condon, B.D., Foston, M.B. et al. Enhanced thermal and combustion resistance of cotton linked to natural inorganic salt components. Cellulose 21, 791–802 (2014). https://doi.org/10.1007/s10570-013-0133-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0133-y