Abstract
Predictive models for simulation of drug release from tablets containing lignin as excipient were developed in this work. Two predictive models including Artificial Neural Network (ANN) and hybrid ANN-Kriging were developed to simulate the tablet dissolution. Measured data was collected on the release rate of aspirin tablets prepared by dry granulation via roll compaction followed by milling and tableting. Two formulations were considered, one with lignin and one without. The main aim is to show the effect of lignin as a bio-based natural polymer in tablet manufacturing to control drug dissolution. For the ANN model development, process and formulation parameters including roll pressure and lignin content were considered as the input, while API dissolution was considered as response. The predictions were compared with measured data to calibrate and validate the model. To improve the predictability of the model, Kriging interpolation was used to enhance the number of training points for the ANN. The interpolated data was trained and validated. The final concentration and the dissolution rate were predicted by ANN as well as ANN-Kriging models, and the R2 of greater than 0.99 for most cases was obtained. The validated model was used to evaluate the effect of process parameters on the release rate and it was indicated that the tablets containing lignin have higher release rate compared to tablets without. Also, it was revealed that process parameters do not have significant effect on the tablet release rate, and the tablet release rate is mainly affected by the lignin content. The results indicated that ANN-based model is a powerful tool to predict the API release rate for tablets containing various formulations, and can be used as a predictive tool for design of controlled release systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most drugs are administered in solid phase, such as crystalline particles which are processed into tablets or capsules by adding excipients (Siepmann and Siepmann 2013). In pharmaceutical manufacturing of solid-dosage oral formulations, there are different unit operations such as blending, granulation, drying, tableting, and coating among which granulation is the key processing step as the tablet properties depend on the granules attributes such as size, API content, porosity, etc. (Hansuld and Briens 2014; Shirazian et al. 2018; Suresh et al. 2017; Vervaet and Remon 2005). In granulation processes, pharmaceutical granules are formed from fine powder (e.g. excipient and API) using various methods such as roller compaction, high-shear wet granulation, twin-screw granulation, fluidised bed granulation, and hot melt extrusion (Asada et al. 2018; Hansuld and Briens 2014; Ko et al. 2018; Passerini et al. 2010; Walker et al. 2007). The main reason for granulation is to improve the powder flowability as well as tablet properties. Among various granulation processes developed so far, dry granulation is a suitable process for moisture and heat sensitive formulations, as no binder is used in the process. Dry granulation is usually carried out using roller compaction process in which the formulation is first compacted to produce ribbons, and then granules are produced by milling the ribbons. The critical process parameters in roller compaction process include roll pressure, roll speed, roll gap, and screw feeder speed (Al-Asady et al. 2015; Omar et al. 2016).
Understanding the relationship between process parameters of the roll compaction process and tablet properties is of great importance for development of pharmaceutical manufacturing and implementing Quality-by-Design (QbD) approach. The effect of process parameters and material properties on critical quality attributes of ribbons and granules have been reported in literature. Both experimental (Khorasani et al. 2015, 2016) and theoretical studies (Loreti et al. 2017; Pérez Gago et al. 2016; Reynolds et al. 2010; Souihi et al. 2015) have been conducted to understand the roller compaction process. In terms of modelling studies, Johanson has proven to be a robust and rigorous mechanistic model for better understanding of roller compaction process (Reynolds et al. 2010). Although, previous studies reveal the correlation between critical process parameters and granules/ribbons properties in roller compactor processes (Pishnamazi et al. 2019a), understanding the relationship between process parameters and tablet dissolution still remains a challenge and opportunities arise in helping to improve tablet dissolution rate by tuning formulation and process parameters.
Drug dissolution is an important quality attribute of pharmaceutical tablets in which the kinetics and equilibrium concentration (solubility) of Active Pharmaceutical Ingredient (API) release from the tablets is of utmost importance (Siepmann and Siepmann 2013). The main focus of controlled release systems is to manipulate the release rate of APIs through different techniques such as incorporating into polymeric matrix (Castro-Dominguez et al. 2017), and loading drug in stimuli-responsive nanocarriers (Fleige et al. 2012), and therefore enhancing bioavailability for poorly water soluble APIs. Nowadays, a big challenge facing the pharma industry is poor solubility of newly produced drugs in the body, i.e. bioavailability. According to Biopharmaceutics Classification System, BCS Class II and BCS Class IV many drugs are of poor solubility and bioavailability (Daousani and Macheras 2016). It has been recognised that seven out of ten of drugs never reach the patients. Development of solid dispersions have been reported to be effective in improving the solubility of BCS Class II drugs (Van den Mooter 2012). Amorphous solid dispersions provide high dissolution rates because of their disordered structure and higher Gibbs free energy compared to crystalline APIs (Ziaee et al. 2017).
Recently, lignin has attracted much attention as it may be used to improve the release of bio-active compounds (Chowdhury 2014; Collins et al. 2019). Lignin is a cross-linked natural polymer, cheap, and available (Culebras et al. 2018). Due to amorphous nature of lignin which has higher free energy, it can be used as modifier to enhance the bioavailability of poorly soluble APIs. However, understanding the effect of lignin on dry granulation using roller compactor, and finding a correlation between lignin content and tablet dissolution rate remains a big challenge. Developing a robust predictive model for designing controlled release systems based on natural polymers is of great importance for pharmaceutical industry.
Therefore, there is a definite need for a comprehensive study to correlate the critical process parameters of roller compactor as well as formulation with tablet dissolution as the key critical quality attribute. A powerful tool is the development of a process model where inputs and outputs can be correlated. Different models have been used for pharmaceutical manufacture such as mechanistic models (Sajjia et al. 2017; Shirazian et al. 2018) and soft computing approaches (Mustafa et al. 2017; Shirazian et al. 2017). Artificial Neural Network (ANN) is a soft computing method which is capable of predicting the process and making correlation between process inputs and outputs (Ismail et al., 2019a, b). Applicability, robustness, and reliability of ANN in pharmaceuticals have been verified in the literature (Das and Chakraborty 2016; Mustafa et al. 2017; Shirazian et al. 2017).
The main objective of the current study is the development of a comprehensive ANN-based model for prediction of dissolution rate of tablets prepared by roller compaction followed by milling and tableting. In order to enhance the bioavailability of API, lignin is used as excipient and the tablet dissolution rate is measured for tablets containing lignin and without lignin. Aspirin is used as model API in this study.
Experiments
Materials and methods
Two different formulations containing API and excipients were considered in this study for preparation of tablets. The excipients used include microcrystalline cellulose (MCC 102, SANAQ®), lactose monohydrate (Lennox USP, NF, BP, Ph, pure pharma grade), and Alcell lignin (Tecnaro, Germany). More details on the lignin used in this study can be found elsewhere (Culebras et al. 2018). Acetylsalicylic acid (Alfa Aesar, 99% C9H8O4) was utilised as API in both formulations. Magnesium stearate (Sigma-Aldrich, Ph. Eur., BP, ≥ 90%) was used as lubricant for the compaction experiments, and Croscarmellose sodium (CCS) (IMCD NF, PH.Eur., JP) was used as disintegrant in both formulations. The percentage of the materials used in two different formulations are listed in Table 1. To prepare the mixture, all the materials were blended using a Morphy Richards Stand Mixer. HCl acid (ACS, ISO, Reag. Ph Eur, Hydrochloric acid fuming 37%) was used for preparation of buffer solutions for dissolution tests. For preparation of the mobile phase for analysing the API concentration with HPLC, Ortho-phosphoric acid (analytical reagent grade, Fisher Scientific UK) and acetonitrile, HPLC grade, 99.7 + % min Liquid (Alfa Aesar) were mixed together.
Equipment and instruments
Dry granulation method was utilised to produce the tablets for two different formulations. In order to do dry granulation process, roller compaction was carried out. The roller compactor, (“Freund TF-MINI”), with roll diameter of 100 mm and width of 25 mm integrated with a vertical screw feeder was used to produce ribbons. The roll pressure was changed between 30 and 50 bar in the experiments. A conical mill (Laboratory Comil 193 AS) with a screen (mesh size of 813 µm) and impeller speed of 3000 rpm was used to produce the granules from the ribbons. The considered process parameters included screw speed (SS) and roll pressure (RP), while roll speed was kept constant at 4 rpm. The screw speed was changed between 10 and 14 rpm, and roll pressure was changed between 30 and 50 bars in the ribbon production experiments.
For tableting process, a single punch tablet press (Gamlen Tableting GTD-1 D series) was applied. For preparation of each tablet, 100 mg of granules of each formulations were measured and compacted to produce tablets in a 6 mm die. The tablet compression was carried out at 180 mm/min speed under fixed load of 400 kg.
Dissolution procedure
For the dissolution analysis, buffer solution at pH = 1.2, including 0.1 N HCl (ACS, ISO, Reag. Ph Eur, Hydrochloric acid fuming 37% wt.) was prepared as dissolution medium. 500 ml of the dissolution test chamber was filled with the buffer solution, with the constant temperature at 37 ± 0.5 °C and 75 rpm stirrer speed. The experiment was run for 120 min, and the samples (Three mL) were withdrawn from the dissolution chamber at 5, 10, 20, 30, 40, 50, 60, 120 min. Afterward, the samples were filtered and the concentration of API was measured using HPLC at wavelength of 200 nm. The dissolution tests were carried out in triplicate, and the average values were used for modeling.
High-Performance Liquid Chromatography (HPLC) was used to measure the API concentration in each samples via an Agilent (Agilent Technologies, Waldbronn, Germany) 1260 Infinity II HPLC system. The HPLC setup consisted of a quaternary pump G1311B, a diode array detector G1315D set at wavelengths of 200 nm for acetylsalicylic acid, auto-sampler G1329 B and a thermostated column compartment G1316A set at temperature of 25 °C. The system operated under isocratic flow at 0.75 mL/min using mobile phases consisting of A) 0.1% Ortho-phosphoric acid; B) acetonitrile; A/B = 50/50, v/v. The injection volume was 10 mL. The total run time was 10 min, and the type of column used was Kromasil 5C18 (250 × 4.6 mm) (Pishnamazi et al. 2019b).
Model development
ANN structure
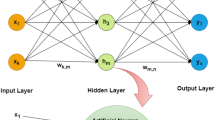
In order to develop a predictive model, artificial neural network (ANN) approach was used in which the process parameters and material properties are correlated with critical quality attributes. Roll pressure, screw speed, and lignin content in the formulation were considered as the inputs, whereas the kinetics and final API dissolution were considered as outputs for developing ANN. The preliminary results indicated that screw speed has negligible effects on the quality attributes of ribbons, therefore, screw speed was omitted from the process parameters. An ANN model consisting of two hidden layers was developed as shown in Fig. 1. JMP Pro 14 software was used for developing the ANN model and analysing the results.
To find the optimum ANN structure, different transfer functions were tested, and the best results were obtained for the structure consisting of 6 non-liner (hyperbolic tangent), 2 liners, and 2 Gaussian functions in the first layer; with 6 non-linear nodes for the second layer (see Fig. 1). In ANN modelling using JMP, the linear combination of input variables (roll pressure and lignin content) are not transformed when using the linear activation function, while for the non-linear function, the hyperbolic tangent term is used as follows (SAS_Institute 2016; Shirazian et al. 2017):
where z is a linear combination of input variables.
In order to build ANN model, 60% of the measured data was used to train the network, while 40% was used for model validation and testing the developed model in prediction of the API release rate. The network was trained to predict the concentration of API at different sampling time intervals, as function of lignin content and roll pressure of roller compactor. The developed ANN model was used for prediction of API release rate, in which the predictive behaviour and accuracy of the model is assessed by comparing the predicted values and the measured values. The coefficient of determination (R2) which indicates the goodness of fitting is calculated as (Barrasso et al. 2015; Shirazian et al. 2017):
where f refers to the predicted points, and y refers to the observed values. i denotes the set of experimental run. Also, root-mean-squared error (RMSE) is calculated as:
where n denotes the number of measurements.
ANN-Kriging hybrid model
In order to improve the predictability of the developed ANN model for API dissolution, Kriging interpolation method was used to enhance the number of training and validating points for the ANN. Ordinary Kriging interpolation was developed based on two inputs and eight outputs which are the dissolution percentage at various times. Kriging method predicts a response yk at an interpolated point xk as a weighted sum of the observed responses (y1, y2…yn) where xk falls in the neighbourhood of their corresponding sampling points (x1, x2, x3…xn) (Boukouvala et al. 2011):
\(w_{i}\) is the weighted sum (kriging weights) which depends on the Euclidian distance \(h\):
In Kriging algorithm, the main objective is to calculate the set of Kriging weights assigned to each group of n clustered points in the neighbourhood of \(x_{k}\) where the derived variogram model leads the sum of the weights to unity. In calculating the interpolated prediction \(f\left( {x_{k} } \right)\) at \(x_{k}\), the observed responses (y1, y2…yn) for sampled points (x1, x2, x3…xn) that are in the neighbourhood and nearer to \(x_{k}\) will have more influence on predicting \(f\left( {x_{k} } \right)\). Indeed, the higher number of neighbouring points and the nearer these neighbouring points are to \(x_{k}\), \(f\left( {x_{k} } \right)\) will be calculated with more confidence (Ismail et al., 2019a, b).
In ordinary kriging interpolation, the experimental variogram is calculated from the experimental data points to statistically quantify the dataset in a form that fits statistical equations (exponential, Gaussian, cubic…etc.). After fitting the experimental and theoretical variograms, kriging weights are then calculated to determine the interpolated response.
A two-dimensional kriging interpolation was conducted on the experimental data obtained from the dissolution experiments to predict the dissolution of API at new data points. The reliability and validity of the hybrid ANN-kriging has been proved in our previous works (Ismail et al., 2019a, b). Ordinary Kriging interpolation was performed in Matlab where the lignin content (%) and roller pressure (bar) were taken as input parameters and API dissolution at 5, 10, 20, 30, 40, 50, 60 and 120 min as output parameters. Interpolation was conducted at 10 new points for each dimension in which 121 points were obtained after applying Kriging. The interpolated kriging data was used to improve the empirical ANN model prediction compared to using just experimental data.
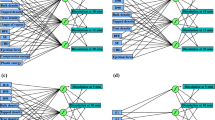
The structure of the developed ANN-Kriging is shown in Fig. 2. As seen, the hybrid model contains two hidden layers, each layer constitutes of three nodes which makes the model simpler and faster to solve. Also, a combination of linear and nonlinear transfer functions has been used for the hybrid model to train the network for prediction of data.
Results and discussion
ANN model
The developed ANN model was first trained using the experimental data collected on the release rate of tablets containing aspirin. The trained network was then used to validate the model. The results of training and validation are listed in Table 2 for the concentration of API at different times, and the final concentration of API at the time of 120 min (final). As seen, the model is very well trained with the experimental data, and the R2 of 1 is obtained for all points, while for the validation R2 of 0.99 is obtained for most cases except for predicting the concentration of API after 5 min which could be attributed to high release rate at the beginning of the dissolution test. It is clearly observed that the model can predict the release rate of API with high accuracy, and can be used as a powerful predictive tool for the design of release systems based on lignin. However, it should be pointed out that there might be a risk of over-prediction in this system as small number of experimental points are used. Therefore, Kriging approach was used to train the network for more data points and prevent the risk of over-prediction.
ANN-Kriging model
The predicted release rate versus the measured values for both training and validation for the hybrid ANN-Kriging model are depicted in Fig. 3. Moreover, the statistical data of the calibration and validation for the hybrid ANN-Kriging is listed in Table 3. After Kriging, the number of data points are increased to 121 points to make a more robust predictive model. 1/3 of the data points were used for validation, while 2/3 were used for training the network. It is clearly seen that the model is well trained for all the training points and the cross validation can confirm the model can be used for prediction of the dissolution. Also, similar to the ANN model, some deviations are observed for the dissolution data after 5 min, however R2 has been significantly improved for 5 min data from 0.52 to 0.67 for the validation stage.
Simulation of the release rate using ANN model
The validated model was used to simulate the release rate of API for the tablets prepared with two formulations. The experimental and predicted release rates for the tablet without lignin, and the tablets containing lignin are represented in Figs. 4 and 5, respectively. The graphs of release rate indicate that the release rate is very high at the beginning of dissolution test, and more than 60% of the API is released after 5 min. After 20 min, the drug concentration in the solution reaches the highest values, and then decreases, and finally become plateau which is considered as the equilibrium point. The reason for reduction in the concentration of API after 20 min could be attributed to the dissociation of aspirin which undergoes hydrolysis during the dissolution test. Aspirin is partially hydrolysed to salicylic acid and acetyl salicylic acid upon exposure to aqueous solutions (Pishnamazi et al. 2019b).
It is also observed in Figs. 4 and 5 that the release rate of aspirin is higher in the tablets containing lignin such that higher dissolution rate is observed in the dissolution test of tablets containing lignin. This could be due to amorphous nature of lignin which enhances the dissolution of aspirin. It is observed that the model is robust and can predict the release rate and the final concentration as well.
Design space for the API release
The developed model was used to understand the effect of process parameters of roll compaction as well as formulation on the release rate of API. In roller compaction process, roll pressure is the most important parameter compared to other parameters such as screw speed and roll speed (Pishnamazi et al. 2019a). The effect of roll pressure and lignin content on the equilibrium concentration of API in the buffer solution is shown in Fig. 6. The roll pressure was considered between 30 and 50 bar, and the lignin content between 0 and 20 wt%. It is indicated that by increasing the lignin content in the formulation, the dissolution of API increases significantly which consequently can enhance the bioavailability of API. Also, it is seen that by increasing the pressure, the dissolution decreases, however the effect of roll pressure on the dissolution is not significant compared to the effect of lignin content because the dissolution of API is highly dependent on the chemistry of formulation and the dissolution medium. It is observed that increasing roll pressure decreases the equilibrium concentration of API which is attributed to the size of granules. As the roll pressure increases, denser ribbons are produced, which results in formation of larger granules in the milling step. Larger granules in the prepared tablets results in lower dissolution as the surface area of the granules decreases and reduce the surface energy and dissolution.
Conclusions
A new formulation containing lignin was designed in this work to enhance the bioavailability of drugs. The tablets were prepared using dry granulation method followed by milling and tableting. In order to design and predict the release rate of API, an artificial neural network (ANN) model was developed considering two hidden layers and combining various activation functions, i.e. linear, hyperbolic tangent, and Gaussian. The ANN model as well as hybrid ANN-Kriging were developed to predict the dissolution of the drug. Two formulations, one containing lignin, and the other one without lignin were considered to investigate the effect of lignin on the API release rate. The results of release rate indicated that the tablets containing lignin have higher release rates of API. The results of simulation revealed that the developed model can predict the release rate with high accuracy and R2= 0.99 was obtained for most cases. The model was used to predict the kinetics and equilibrium of the release rate and great agreement was obtained between the predicted and measured data. The validated model was then used to understand the effect of process parameters on the release rate, and it was revealed that increasing roll pressure decreases the release rate, because larger granules are produced which in turn results in lower release rate.
References
Al-Asady RB, Osborne JD, Hounslow MJ, Salman AD (2015) Roller compactor: the effect of mechanical properties of primary particles. Int J Pharm 496:124–136
Asada T, Nishikawa M, Ochiai Y, Noguchi S, Kimura S-I, Iwao Y, Itai S (2018) Mechanism of the formation of hollow spherical granules using a high shear granulator. Eur J Pharm Sci 117:371–378
Barrasso D, El Hagrasy A, Litster JD, Ramachandran R (2015) Multi-dimensional population balance model development and validation for a twin screw granulation process. Powder Technol 270, Part B, 612–621
Boukouvala F, Muzzio FJ, Ierapetritou MG (2011) Dynamic data-driven modeling of pharmaceutical processes. Ind Eng Chem Res 50:6743–6754
Castro-Dominguez B, Moroney K, Schaller B, O’Connor S, Cloonan A, Vo TTN, Walker G, O’Reilly EJ (2017) Electrospun API-loaded mixed matrix membranes for controlled release. RSC Adv 7:43300–43309
Chowdhury MA (2014) The controlled release of bioactive compounds from lignin and lignin-based biopolymer matrices. Int J Biol Macromol 65:136–147
Collins MN, Nechifor M, Tanasă F, Zănoagă M, McLoughlin A, Stróżyk MA, Culebras M, Teacă C-A (2019) Valorization of lignin in polymer and composite systems for advanced engineering applications—a review. Int J Biol Macromol 131:828–849
Culebras M, Beaucamp A, Wang Y, Clauss MM, Frank E, Collins MN (2018) Biobased structurally compatible polymer blends based on lignin and thermoplastic elastomer polyurethane as carbon fiber precursors. ACS Sustain Chem Eng 6(7):8816–8825
Daousani C, Macheras P (2016) Biopharmaceutic classification of drugs revisited. Eur J Pharm Sci 95:82–87
Das MK, Chakraborty T (2016) Chapter 14 - ANN in pharmaceutical product and process development. In: Puri M, Pathak Y, Sutariya VK, Tipparaju S, Moreno W (eds) Artificial neural network for drug design, delivery and disposition. Academic Press, Boston, pp 277–293
Fleige E, Quadir MA, Haag R (2012) Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Adv Drug Deliv Rev 64:866–884
Hansuld EM, Briens L (2014) A review of monitoring methods for pharmaceutical wet granulation. Int J Pharm 472:192–201
Ismail HY, Shirazian S, Skoretska I, Mynko O, Ghanim B, Leahy JJ, Walker GM, Kwapinski W (2019a) ANN-Kriging hybrid model for predicting carbon and inorganic phosphorus recovery in hydrothermal carbonization. Waste Manag 85:242–252
Ismail HY, Singh M, Darwish S, Kuhs M, Shirazian S, Croker DM, Khraisheh M, Albadarin AB, Walker GM (2019b) Developing ANN-Kriging hybrid model based on process parameters for prediction of mean residence time distribution in twin-screw wet granulation. Powder Technol 343:568–577
Khorasani M, Amigo JM, Sonnergaard J, Olsen P, Bertelsen P, Rantanen J (2015) Visualization and prediction of porosity in roller compacted ribbons with near-infrared chemical imaging (NIR-CI). J Pharm Biomed Anal 109:11–17
Khorasani M, Amigo JM, Bertelsen P, Sun CC, Rantanen J (2016) Process optimization of dry granulation based tableting line: extracting physical material characteristics from granules, ribbons and tablets using near-IR (NIR) spectroscopic measurement. Powder Technol 300:120–125
Ko SJ, Lee J-H, Kang C-Y, Park J-B (2018) Granulation development in batch-to-batch and continuous processes from a quality by design perspective. J Drug Deliv Sci Technol 46:34–45
Loreti S, Wu CY, Reynolds G, Mirtič A, Seville J (2017) DEM–PBM modeling of impact dominated ribbon milling. AIChE J 63:3692–3705
Omar CS, Dhenge RM, Palzer S, Hounslow MJ, Salman AD (2016) Roller compaction: effect of relative humidity of lactose powder. Eur J Pharm Biopharm 106:26–37
Passerini N, Calogerà G, Albertini B, Rodriguez L (2010) Melt granulation of pharmaceutical powders: a comparison of high-shear mixer and fluidised bed processes. Int J Pharm 391:177–186
Pérez Gago A, Reynolds G, Kleinebudde P (2016) Impact of roll compactor scale on ribbon density. Powder Technol 337:92–103
Pishnamazi M, Casilagan S, Clancy C, Shirazian S, Iqbal J, Egan D, Edlin C, Croker DM, Walker GM, Collins MN (2019a) Microcrystalline cellulose, lactose and lignin blends: process mapping of dry granulation via roll compaction. Powder Technol 341:38–50
Pishnamazi M, Iqbal J, Shirazian S, Walker GM, Collins MN (2019b) Effect of lignin on the release rate of acetylsalicylic acid tablets. Int J Biol Macromol 124:354–359
Reynolds G, Ingale R, Roberts R, Kothari S, Gururajan B (2010) Practical application of roller compaction process modeling. Comput Chem Eng 34:1049–1057
Sajjia M, Shirazian S, Kelly CB, Albadarin AB, Walker G (2017a) ANN analysis of a roller compaction process in the pharmaceutical industry. Chem Eng Technol 40:487–492
Sajjia M, Shirazian S, Egan D, Iqbal J, Albadarin AB, Southern M, Walker G (2017b) Mechanistic modelling of industrial-scale roller compactor ‘Freund TF-MINI model’. Comput Chem Eng 104:141–150
SAS_Institute (2016) Using JMP® 12.2.0, Cary, NC
Shirazian S, Kuhs M, Darwish S, Croker D, Walker GM (2017) Artificial neural network modelling of continuous wet granulation using a twin-screw extruder. Int J Pharm 521:102–109
Shirazian S, Darwish S, Kuhs M, Croker DM, Walker GM (2018) Regime-separated approach for population balance modelling of continuous wet granulation of pharmaceutical formulations. Powder Technol 325:420–428
Siepmann J, Siepmann F (2013) Mathematical modeling of drug dissolution. Int J Pharm 453:12–24
Souihi N, Reynolds G, Tajarobi P, Wikström H, Haeffler G, Josefson M, Trygg J (2015) Roll compaction process modeling: transfer between equipment and impact of process parameters. Int J Pharm 484:192–206
Suresh P, Sreedhar I, Vaidhiswaran R, Venugopal A (2017) A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chem Eng J 328:785–815
Van den Mooter G (2012) The use of amorphous solid dispersions: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Technol 9:e79–e85
Vervaet C, Remon JP (2005) Continuous granulation in the pharmaceutical industry. Chem Eng Sci 60:3949–3957
Walker GM, Bell SEJ, Andrews G, Jones D (2007) Co-melt fluidised bed granulation of pharmaceutical powders: improvements in drug bioavailability. Chem Eng Sci 62:451–462
Ziaee A, Albadarin AB, Padrela L, Faucher A, O’Reilly E, Walker G (2017) Spray drying ternary amorphous solid dispersions of ibuprofen – An investigation into critical formulation and processing parameters. Eur J Pharm Biopharm 120:43–51
Acknowledgments
This research was conducted with the financial support of the Synthesis and Solid State Pharmaceutical Centre (SSPC), funded by SFI and is co-funded under the European Regional Development Fund under Grant Number 14/SP/2750.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pishnamazi, M., Ismail, H.Y., Shirazian, S. et al. Application of lignin in controlled release: development of predictive model based on artificial neural network for API release. Cellulose 26, 6165–6178 (2019). https://doi.org/10.1007/s10570-019-02522-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02522-w