Abstract
The anniversary of the journal “Cellulose” is an opportunity to review innovations that were introduced during the past 25 years. Of these, from our perspective, the development of solvents that dissolve cellulose physically, i.e., without formation of covalent bonds is most relevant. The reasons are that cellulose can be regenerated from these media in different shapes and transformed into many important derivatives. Twenty-five years is a long time-span! As the volume of information on the applications of the above-mentioned solvents in cellulose chemistry is extensive, we made choices to reach a balance between the amount of material covered and the length of the review. Consequently, we focus on cellulose derivatization under homogeneous reaction conditions to produce selected derivatives. We dwell on the latter because a comprehensive discussion was recently published on derivatization under heterogeneous and homogeneous conditions (Heinze et al. in Cellulose derivatives, Springer, Cham, pp 259–292, 2018a). The derivatives selected are esters of organic acids, ionic and nonionic ethers because of their tremendous commercial and scientific importance. Cellulose derivatization in homogeneous media is advantageous because of much better control of product properties relative to those obtained under the heterogeneous counterparts. These properties include degree of substitution in the anhydroglucose unit and along the biopolymer back-bone, and regioselectivity. Thus, novel cellulose derivatives were prepared that are not accessible under heterogeneous conditions. The requirement to dissolve cellulose physically is to disrupt hydrogen bonding and hydrophobic interactions. Thus, the solvents employed to dissolve cellulose are usually composed of strong electrolytes whose cations and anions interact preferentially with cellulose. These electrolytes are used pure or as solutions in water or dipolar aprotic solvents. Salient examples include LiCl/N,N-dimethylacetamide, tetra(n-butyl)ammonium fluoride·3H2O/dimethyl sulfoxide, ionic liquids, salts of quaternary amines and super-bases. We discuss briefly the essentials of each solvent in terms of its mechanism of cellulose dissolution and show the most relevant results regarding its application for obtaining esters and ethers and back the discussion with relevant references. This information is summarized at the end of the review. We hope that this historical perspective shows the innovations made since the first publication of “Cellulose” and points out to future possibilities—with potential industrial application—of this renewable raw material and its biocompatible and biodegradable derivatives.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Requirements for cellulose dissolution
The dissolution of cellulose occurs either physically or chemically. The latter strategy leads to formation of covalent bonds, i.e., the formation of cellulose derivatives that are usually soluble in the medium. Chemical dissolution is used in commercial processing of cellulose, in particular the viscose process (Heinze et al. 2018b), and will not be dealt with in this review.
At the outset, we use the term “solvent” to denote both single- and multi-component systems. Example of the former are ionic liquids (ILs), whereas the latter is usually composed of a strong electrolyte in a dipolar aprotic solvent (DAS), LiCl/N,N-dimethylacetamide (DMAc), and tetra(n-butyl)ammonium fluoride trihydrate ([TBA]F·3H2O)/dimethyl sulfoxide (DMSO).
Dissolution of cellulose occurs if the dissolved state is associated with lower free energy than the solid state. Consequently, we should consider the contributions of the enthalpy and entropy terms of Gibbs free-energy equation (Burchard 2003). In view of the strong intra- and intermolecular hydrogen bonding in cellulose (Fig. 1), much emphasis was placed on the ability of the solvent to break these hydrogen bonds (i.e., dissolution enthalpy) as the essential criterion for cellulose dissolution.

Reprinted with permission from (Pinkert et al. 2010), copyright (2010) American Chemical Society
Intra- and intermolecular hydrogen bonding in cellulose.
However, cellulose is not soluble in water, although the energies of hydrogen bonding between cellulose molecules, water molecules and cellulose/water are not very different. Hence, the energy required to break hydrogen bonding is only a fraction of the total free energy necessary to dissolve cellulose. The remaining part is needed to break the hydrophobic interactions, because cellulose has amphiphilic character, with polar (OH) and nonpolar (CH) patches. A consequence of this amphiphilicity is that cellulose chains can stack via hydrophobic interactions to form sheet-like structures that should be disrupted for dissolution to occur (the entropy term) (Lindman et al. 2017). A schematic representation of the cooperative effects of these two types of interactions is shown in Fig. 2. The latter was suggested (based on molecular dynamic, MD, simulations) for the formation of regenerated cellulose from aqueous cellulose solutions (Miyamoto et al. 2009). To follow the argument more easily, however, we inverted the steps, i.e., fibrous cellulose → dissolved biopolymer. We start from cellulose with crystalline and amorphous regions. On contact with the solvent that dissolves the biopolymer physically, the tightly packed aggregates, made of stacked chains, start to separate into smaller sheets with some degree of order, i.e., still containing crystalline and defective regions. As dissolution proceeds, this “peeling” process continues. Finally, the sheets disintegrate into solvated chains or, most probably, smaller solvated cellulose aggregates. The evidence for dissolved cellulose aggregates is based on light scattering data demonstrating that dissolved cellulose is not monomeric in efficient solvents, including LiCl/DMAc (Röder et al. 2001), and ILs (Trulove et al. 2009; Kuzmina et al. 2010).

Adapted from (Miyamoto et al. 2009), copyright (2009), with permission from Elsevier
Schematic representation of the structures formed during cellulose dissolution in aqueous environment: a semi-crystalline cellulose; b breakdown into smaller sheets held by hydrogen bonding and hydrophobic interactions; c formation of the molecular sheets held by van der Waals force; d dissolved cellulose.
The above remarks show the relevance of understanding cellulose dissolution to its derivatization. We develop our discussion, therefore, in terms of the solvent employed, evidence for its mechanism of action, followed by its application in the synthesis of esters and ethers. Our aim is to demonstrate how the introduction of novel solvents contributed to cellulose chemistry during the last 25 years, e.g., by making reactions selective, efficient with satisfactory atom economy and regioselectivity. The selected solvent classes shown below were the most relevant ones developed during this time span:
-
1.
Strong inorganic electrolyte in DAS, LiCl/DMAc;
-
2.
Quaternary ammonium electrolytes with inorganic counter-ions in DAS, e.g. [TBA]F·3H2O/DMSO;
-
3.
Aqueous alkali solutions without and with hydrotropes (urea, thiourea); aqueous quaternary ammonium hydroxides, e.g. [NR4]OH;
-
4.
Quaternary ammonium electrolytes (QAEs) with organic counter-ions in DASs, e.g. [NR4]AcO;
-
5.
Imidazole-based ILs alone and as solutions in DASs.
Cellulose pretreatments
Depending on the solvent employed it is necessary, or convenient to submit cellulose to a pretreatment before dissolution. For efficient dissolution in LiCl/DMAc the cellulose sample should be “activated”, a pretreatment introduced to remove adsorbed water from cellulose and enhance its solubility in the medium (Ishii et al. 2008). The following strategies were employed: substitution of water by organic solvents ending with DMAc, e.g. (water → methanol → DMAc); distillation of a fraction (25 vol%) of DMAc; heating of a mixture of cellulose and LiCl under reduced pressure (El Seoud et al. 2013). The first strategy is laborious (requires ca. one day), expensive (144 mL of methanol plus DMAc/g cellulose) and is recommended where cellulose dissolution without degradation in essential (Dupont 2003). The second strategy does not eliminate water completely leading, e.g., to consumption of a part of the acylating agent (Marson and Seoud 1999). More importantly, however, this activation is associated with biopolymer degradation due to its reaction with the strongly electrophilic species N,N-dimethylketeniminium ion [CH2=C=N+(CH3)2] formed by dehydration of the enol tautomer of DMAc at the b.p. of the solvent (Potthast et al. 2003; Rosenau et al. 2006). Thermal activation of a mixture of cellulose and LiCl under reduced pressure does not cause biopolymer degradation, but DMAc should be introduced before reestablishing atmospheric pressure to avoid cellulose hornification (Regiani et al. 1999). This activation strategy is probably most convenient because the biopolymer and LiCl are dried in situ, simultaneously. Cellulose dehydration is not required for its dissolution/derivatization in [TBA]F·3H2O/DMSO; ILs and their solutions in DAS (Wu et al. 2004; Kostag et al. 2013). Thus, sample of cellulose acetate with the same DS were obtained by acetylation of microcrystalline cellulose (MCC) by acetic anhydride in 1-allyl-3-methylimidazolium chloride [AlMeIm]Cl without and with prior activation (Fidale et al. 2009). Examples are known where the removal of water is not even essential for the success of the reaction of water-sensitive reagents. The reason is that the water activity in IL is greatly reduced (Amigues et al. 2006). A note on the solvent [TBA]F·3H2O/DMSO is worthwhile. Under comparable conditions, this solvent absorbs water even faster than LiCl/DMAc (Fidale et al. 2006). Consequently, water uptake by the precursor electrolyte ([TBA]F·3H2O) and the solvent should be controlled because of the demonstrated deleterious effect of water on [TBA]F/cellulose interactions (Östlund et al. 2009).
Another pretreatment that is used especially with fibrous cellulose is mercerization, i.e., treatment with a base, usually aqueous NaOH followed by base washing and sample drying. There is a massive evidence that this treatment produces cellulose that shows increase in swelling and dissolution, relative to untreated cellulose. The reasons for the beneficial effects of this pretreatment include increase in cellulose accessibility and surface area, the change of cellulose I → cellulose II, and partial removal of lignin and hemi-cellulose (Heinze et al. 2018c). This pretreatment leads to oxidative degradation, a side reaction that can be suppressed by carrying the treatment under reducing conditions (El Seoud et al. 2008).
Cellulose dissolution and derivatization in LiCl/DMAc
Dissolution
The cellulose dissolving efficiency of Lithium halides/DAS depends on the electrolyte and DAS. For the same organic solvent, LiCl is more efficient than LiBr or LiI; for LiCl, DMAc is a better solvent than DMF or DMSO (Furuhata et al. 1992; Morgenstern and Berger 1993; Wang et al. 2009). Therefore, we concentrate on LiCl/DMAc, introduced to dissolve, inter alia, chitin (Austin 1977), cellulose (McCormick et al. 1985), and aromatic polyamides (Kwolek et al. 1977; Morgan 1977).
A brief note about the state of cellulose aggregation in these solutions is in order because this aggregation affects the biopolymer reactivity, e.g., toward derivatization. Obtaining clear cellulose solutions in LiCl/DMAc and, in fact, in any solvent does not necessarily mean that the biomacromolecule is molecularly dispersed. Most certainly these solutions contain aggregates of still ordered cellulose molecules (Morgenstern and Kammer 1999; Burchard 2003). In fact, aggregate-free solutions of polysaccharides are hard to prepare (Rinaudo 1993; Potthast et al. 2002). The state of these aggregates, in particular the aggregation number, depends on cellulose and electrolyte concentrations, and the method of solution preparation (Sjöholm et al. 1997; Ciacco et al. 2010). Dynamic light scattering (DLS) data indicated that the average lengths of dissolved MCC chains are practically equal to their persistent lengths, i.e., there is no biopolymer chain-coiling. The flexibility of long chain celluloses, e.g., cotton linters leads to coiling hence formation of strong intermolecular hydrogen bonding and van der Waals interactions. Consequently, the properties of cellulose, e.g., its average degree of polymerization (DP) and index of crystallinity (Ic), the concentrations of cellulose and LiCl affect the state of biopolymer aggregation, hence the ease of its dissolution and efficiency of derivatization (Strlič and Kolar 2003; Aono et al. 2006), as evidenced by the DS of product obtained (Ramos et al. 2011).
A major advantage of LiCl/DMAc as solvent is its ability to dissolve celluloses of different molar masses (MMs) and Ic, including cotton linters and bacterial cellulose (BC). Therefore, it is frequently employed in analytical applications, e.g., determination of the average MM by viscosity from the Mark–Houwink–Sakurada equation and by SEC with multiangle light scattering (MALS) detection (Striegel and Timpa 1996; Schult et al. 2002; Potthast et al. 2015). It is also a solvent of choice when new sample of cellulose is tested, or new derivatization protocol is introduced.
The mechanism of cellulose dissolution by this solvent was deduced from conductance, FTIR and NMR spectroscopy. It is instructive to analyze this problem in terms of the following: interactions of LiCl with DMAc; effects of dissolving cellulose, or cellobiose (model for cellulose) on the LiCl/DMAc solvent. Conductivity measurements showed that LiCl and several other strong electrolytes are weakly associated in DMAc; the Li+ is strongly solvated by the solvent whereas the Cl− is weakly solvated (Das et al. 2002). The same conclusion was corroborated by FTIR (νC=O of DMAc), 13C NMR (δ CH3CON(CH3)2), and 7Li NMR (δ and peak width). These techniques also indicated the association of Li+ with the C=O group of several molecules of DMAc, as seen in Fig. 3 (Morgenstern et al. 1992; Striegel 2003; Zhang et al. 2014).

Reprinted (adapted) with permission from (Zhang et al. 2014), copyright (2014) American Chemical Society
Scheme for the solvation of LiCl by DMAc.
1H NMR studies on solutions of cellobiose (Gagnaire et al. 1983) and cellulose (Burchard 2003) dissolved in LiCl/DMAc revealed that all OH groups are complexed with the solvent. The corresponding chemical shifts increase with LiCl concentration and decrease with temperature. The dependence on cellobiose concentration of δ and peak width of 35Cl NMR confirmed that this hydrogen bonding is largely between the hydroxyl groups and the chloride ion (Zhang et al. 2014). 7Li NMR chemical shift and peak width in absence and presence of cellulose indicated that the molecular environment of Li+ changes progressively as cellulose is dissolved. This interaction presumably involves weakening of Li+/DMAc interactions or, in limiting cases, an exchange between one DMAc molecule in the inner coordination shell of Li+ with a cellulosic hydroxyl group (Morgenstern et al. 1992).
From the above-mentioned results, we summarize cellulose dissolution in LiCl/DMAc as follows: cellulose dissolution results in the formation of strong hydrogen bonds between the hydroxyl groups of the AGU and the weakly solvated Cl− as well as Coulombic interaction with the solvated Li+. The former hydrogen bonding is at the expense of [Li(DMAC)n]+···Cl− electrostatic interactions. This loss is attenuated by increasing the solvation number of Li+ by DMAc. The following dissolution scheme (Fig. 4) is based on the work of Zhang et al. (2014).

Reprinted (adapted) with permission from (Zhang et al. 2014), copyright (2014) American Chemical Society
Schematic representation of interaction of cellulose and LiCl/DMAc during dissolution.
Few additional remarks on cellulose dissolution in LiCl/DMAc are worth mentioning:
-
1.
Although the above-mentioned spectroscopic results attribute cellulose dissolution to hydrogen bonding, contribution from the hydrophobic interactions between cellulose and the methyl groups of DMAc cannot be ruled out as shown, e.g., for the solvation of glucose in DAS (Vasudevan and Mushrif 2015), and by the fact that cellulose is more soluble in LiCl/DMAc than LiCl/DMF. Note that DMAc is more hydrophobic, although less polar than DMF (values of log P (partition coefficient between n-octanol and water = − 0.25 and − 1.01; values of ET(30) = 42.9 and 43.2 kcal mol−1, for DMAc and DMF, respectively);
-
2.
Whereas the order of the dissociation constants in DMAc is LiI > LiBr > LiCl (Das et al. 2002) the order of efficiency (as cellulose solvent) in the same DAS is LiCl > LiBr > LiI. Consequently, charge density on the anion and not the concentrations of free ions controls the observed electrolyte efficiency;
-
3.
The presence of adventitious water leads to several deleterious effects: it affects the solubility of LiCl in DMAc; decreases the solubility of cellulose in the solvent; increases the aggregation of dissolved cellulose (Rosenau et al. 2001; Potthast et al. 2002) and consumes reactive derivatizing agents, e.g., acid anhydrides and acyl chlorides. Because the water present is tightly bound to the electrolyte, Karl–Fischer titration may give false results. The concentration of water can be quickly and conveniently determined using solvatochromic probes (Potthast et al. 2002; Fidale et al. 2006). In this regard, the above-mentioned cellulose thermal activation under reduced pressure is probably most convenient because the biopolymer and LiCl are dried in situ, simultaneously.
Esterification of cellulose in LiCl/DMAc
Esters of carboxylic acids
As solvent, LiCl/DMAc was successfully employed for the synthesis of esters and mixed esters of celluloses with different DP, some of which cannot be obtained under heterogeneous conditions, e.g., long-chain fatty esters. In the account that follows, we concentrate on the strategies employed for derivatization and list selected results.
At the outset, the properties of LiCl/DMAc, both macroscopic and microscopic, ensure obtaining good yields and controlled DS for reactions where reagent diffusion is important (as in polymer reactions) and the transition state is more polar than the reactant state, e.g., esterification. Regarding macroscopic properties consider solution viscosity. According to the Stokes–Einstein diffusion equation, lower solution viscosity leads to higher diffusion rates of the species present in solution, which corresponds to an increase in mass transfer, hence increase in reaction rate/yield. This expectation was demonstrated experimentally, e.g., for Diels–Alder reactions in pure ILs (Baba et al. 2006; Tiwari and Kumar 2012), and their mixtures with molecular solvents (Khupse and Kumar 2011). The viscosity of cellulose solutions (1 wt%) in LiCl/DMAc are not elevated even for biopolymer samples with high DP. For example, viscosities of 0.31 and 4.78 Pa s at 50 °C were reported for cellulose samples with DP = 280 and 643, respectively (Wei and Cheng 2007). Under comparable conditions, the viscosity of MCC in LiCl/DMAc is 15% that in the IL 1-allyl-3-butylimidazolium chloride (Possidonio et al. 2010).
Solvatochromic parameters are used as indication for microscopic properties of this, and other cellulose solvents. Values of ET(30) of LiCl/DMAc, are relatively high and increase in the sequence (42.8 → 47.3 → 51.0 kcal mol−1) on going from pure DMAc to LiCl/DMAc solutions containing 0.5, 5.0 wt% electrolyte, respectively (Spange et al. 1998). The last ET(30) value is in the same polarity range of 2-ethoxyethanol (51.0 kcal/mol) and 1-propanol (50.7 kcal/mol) (Reichardt and Welton 2010), i.e., the solvent is quite polar. As argued elsewhere, the efficiency of a solvent in dissolving cellulose is related to its “net or effective basicity”, taken as the difference between its Lewis basicity (SB) and Lewis acidity (SA), solvents with high net basicity (SB − SA ≥ 0.5) are efficient (Parviainen et al. 2013). The values of net basicity are 0.78, 1.62, and 1.49 for pure DMAc, 0.5 and 5 wt% LiCl in DMAc, respectively (Spange et al. 1998). In summary, the solvent LiCl/DMAc possess favorable characteristics for cellulose dissolution and derivatization, both macroscopic (low viscosity) and microscopic (high empirical polarity and net basicity).
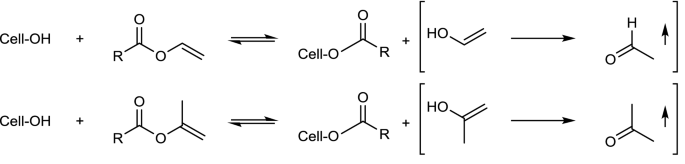
Cellulose esterification with carboxylic acids is inefficient and requires drastic reaction conditions (Thomas 1970). Therefore, esterification is usually carried out with activated carboxylic acids; with reactive derivatives of carboxylic acids (anhydrides and acyl chlorides), or by transesterification with vinyl and isopropenyl esters.
Carboxylic acids can be activated by conversion, in situ, into anhydrides, mixed (i.e., asymmetric) anhydrides, and N-acyl-diazole or N-acyl-benzotriazole. Thus, the reaction of carboxylic acid with tosyl chloride (TsCl) leads to the formation first of carboxylic–sulfonic mixed anhydride that further reacts to yield acyl chloride and carboxylic acid anhydride, both efficient acylating agents (Sealey et al. 1996). This mechanism of activation was demonstrated with 1H NMR spectroscopy, see Fig. 5.

Reprinted by permission from (Heinze et al. 2006), Springer Nature, copyright (2006)
Evolution as a function of time of the 1H NMR spectra of acetic acid/TsCl mixture, showing the formation of a mixture of acetic acid anhydride and acetyl chloride.
Carboxylic acid activation with carbonyldiimidazole (CDI), benzotriazole + SOCl2, and N,N′-dicylohexylcarbodiimide (DCC) converts the carboxylic acid into N-acylimidazole, N-acylbenzotriazole, and acid anhydride respectively.
Carboxylic acid anhydrides are used alone, or in the presence of tertiary bases, e.g., pyridine, 4-N,N-dimethylaminopyridine, or imidazole. The effective acylating agent depends on the relative concentration (tertiary base/anhydride). At low ratios (< 2) the reaction proceeds via anhydride plus N-acylated pyridine or N-acylated imidazole. At higher ratios (≥ 2) the anhydride is quantitatively converted into, e.g., N-acylimidazole, whose intermediate formation in LiCl/DMAc was demonstrated by 1H NMR, see Fig. 6. Mixed fatty carboxylic–acetic anhydride can be obtained, in situ, from the (catalyzed) reaction of fatty acid and acetic anhydride (Peydecastaing et al. 2008b, 2009), and used to esterify cellulose. Under these conditions, the product is a mixed cellulose ester with predominance of the acetate group, presumably due to steric effects (Vaca-Garcia et al. 1998; Vaca-Garcia and Borredon 1999). Use of trifluoroacetic anhydride instead of acetic anhydride and elimination of the (labile) trifluoroacetate yields pure fatty-ester of cellulose (Huang 2012). Cellulose mixed esters were also prepared by the simultaneous reaction of dissolved cellulose with mixtures of acid anhydrides (Liebert and Heinze 2005; Possidonio et al. 2010).

Reprinted from (Nawaz et al. 2013), copyright (2013), with permission from Elsevier
Mechanism of imidazole-catalyzed acylation of cellulose in LiCl/N,N-dimethylacetamide.
Acyl chlorides are usually employed in the presence of a tertiary base to scavenge the produced HCl and avoid cellulose degradation. Again, depending on the ratio base/RCOCl, the acylating agent may be partially, or solely the N-acylated base. This esterification was carried out by conventional, i.e., thermal heating and under microwave irradiation (Joly et al. 2005; Ratanakamnuan et al. 2012; El Seoud et al. 2013).
Although the reaction of RCOCl/tertiary base with cellulose represents a direct route to obtain esters of long-chain carboxylic acids, the use of the vinyl and isopropenyl esters of these acids represents an attractive alternative to the use of corrosive RCOCl. This (catalyzed or uncatalyzed) transesterification reaction is given by Fig. 7 for vinyl esters.
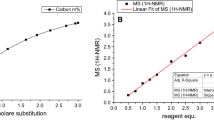
Thus, these reactions whose equilibrium constants are not far from unity are driven to the right-hand side by elimination of the volatile products, acetaldehyde and acetone (Otera 1993). Cellulose acetoacetates with DS up to 1.84 was obtained without catalysis using 2,2,6-trimethyl-4H-1,3-dioxin-4-one as reagent. Contrary to other procedures, a simple to handle, commercially available reagent was employed. Moreover, the synthesis requires a short reaction time to obtain pure products that are promising starting materials for the design of advanced cellulose-based materials. The hydrophobic cellulose acetoacetates can be transferred into reactive nanoparticles with particle sizes ranging from 120 to 300 nm (Würfel et al. 2018).
After reaction mixture workup, it is necessary to determine the yield (from product mass) and structure of the obtained esters. Among these parameters are the (average) DS and, where DS < 3, the distribution of the acyl group among O-2, O-3, and O-6 of the AGU. Average DS can be determined by saponification with a base, followed by back titration of the excess base (ASTM D871 - 96 2004). Although this (standard) method is simple, its use, even with single esters of cellulose, presents the following limitations:
-
1.
The method requires a large amount of sample (1.9 g ester/run) (ASTM D871 - 96 2004);
-
2.
For cellulose esters of fatty acids, the titration end-point is subject to the so-called “colloid error” due to the stable emulsion formed (sodium soap) (Edgar et al. 2001; Freire et al. 2005);
-
3.
The method is not suitable for mixed esters of two very different acids, e.g., carboxylate–tosylate (Casarano et al. 2011).
These problems were solved by use of several techniques: Quantitative determination of FTIR peak area or νC=O and comparison with (pre-established) calibration curve (plots between peak area or νC=O vs. DS); determination of ET(solvatochromic probe) of the solid sample followed by determination of the corresponding DS from a (pre-established) calibration curve (ET(probe) vs. DS); transformation of the cellulose ester into a volatile derivative (e.g., by transesterification) followed by GC analysis of the volatile products (Peydecastaing et al. 2008a; Fidale et al. 2013; Ferreira et al. 2016). The distribution of the acyl group between positions O-2, O-3 and O-6 of the AGU is readily done by 13C NMR peak integration using the inverse gated-decoupling experiment (Berger and Braun 2004), because δC=O of these 3 acyl groups are well separated (167–170 ppm) (Kamide and Okajima 1981; Kamide et al. 1981; Iwata et al. 1992; Regiani et al. 1999; Marson and Seoud 1999; El Seoud et al. 2000). Additional details on the effects of cellulose DP, acylating agents, reaction conditions on the yields and DS values (partial and total) of cellulose esters synthesized in LiCl/DMAc can be found in a recent publication (Heinze et al. 2018b). Representative examples of cellulose esterification and etherification are listed in Table 5 at the end of this review.
Esters of sulfonic acids
Several cellulose sulfonates are known, including aromatic, e.g., 4-X-benzene sulfonates (X=H, CH3, Br, and NO2) and aliphatic, e.g., methane- and trifluoromethane esters. Of these, we dwell on cellulose 4-methylbenzene sulfonates (cellulose tosylates, CTs) because they were most extensively employed in cellulose chemistry. Tosylation by TsCl is a regioselective reaction, i.e., substitution is mostly at O-6 if the DSTs is ≤ 1. This behavior is exploited in making interesting cellulose derivatives, in particular, the 6-deoxy derivatives by nucleophilic substitution (SN) of the (good) leaving tosylate group, as shown by the example below (Fig. 8).

Redrawn from (Liu and Baumann 2002)
Regioselective synthesis of 6-amino-6-deoxy cellulose and its N-sulfonated and N-carboxymethylated derivatives.
Introduction of the azido group is interesting because it can be reduced to the amino group, that can be further functionalized, e.g., quaternized to give a cellulose polyelectrolyte, or used in the click chemistry approach, a term introduced to describe a group of atom efficient reactions (Lewis et al. 2002). Figure 9 shows a typical example for the synthesis of regioselectively substituted CTs followed by conversion into cellulose-based polyelectrolytes via a click chemistry (Huisgen) reaction (Furuhata et al. 1992; Fox and Edgar 2012).
CTs were synthesized in LiCl/DMAc, e.g., with DSTs of up to 2.4. This ester was transformed almost completely into chlorodeoxy cellulose (DS = 2.3) by further heating in the reaction mixture (Cl− of LiCl acting as the nucleophile) (McCormick and Callais 1987; Rahn et al. 1996). Usually, the formation of chlorodeoxy cellulose is a side reaction in the synthesis of CTs whose extent is calculated from elemental analysis (Gericke et al. 2012b; Ferreira et al. 2016). This formation, however, can be suppressed by using tertiary base catalysts, e.g., triethylamine or 4-N,N-dimethyaminopyridine and low reaction temperature (10 °C) (McCormick et al. 1990). For the reaction in LiCl/DMAc, catalyzed by triethylamine, it was demonstrated that the values of DSTs is a function of the molar ratio of TsCl to AGU. The solubility of the CTs depends on DSTs. For example, up to DSTs of 0.46, CTs are soluble in DMAc and DMSO, 1.43 in dioxane or acetone and 2.02 in THF (Rahn et al. 1996). CTs can be transformed into other products without removing the tosylate group, in particular the mixed esters such as tosylate/carboxylates (Heinze et al. 1996; Ferreira et al. 2016; Bioni et al. 2018), tosylate/urethanes (Tiller et al. 2000), and tosylate/sulfates (Heinze and Rahn 1997). A recent work on the thermal behavior of cellulose tosylate/carboxylate with fixed DSTs and variable DSCarboxylate (acetate, butyrate and hexanoate) showed that that the first reactions that occurs during thermal decomposition of the mixed esters is their deacylation. Additionally, the temperatures of the first decomposition (splitting of the acyl group) correlate linearly with DSCarboxylate (Ferreira et al. 2016).
In summary, CTs are usually synthesized in LiCl/DMAc by reaction of TsCl/tertiary base, the reaction is regioselective, and the products can be used directly or further modified into cellulose deoxy derivatives by SN reaction, or cellulose tosylate/second substituent by reaction of the remaining hydroxyl groups of the AGU.
Etherification of cellulose in LiCl/DMAc
Homogeneous alkylation of cellulose with iodomethane, iodoethane, bromoethane, 1-bromopropane, or 1-iodobutane in LiCl/DMSO solvent in the presence of dimsyl sodium was carried out successfully (Petruš et al. 1995). Methyl cellulose was also obtained in LiCl/DMAc with DS-values of 0.9–2.2 and compared to heterogeneously prepared samples (Hirrien et al. 1996). A deviation in the substitution pattern for samples with similar DS was found indicating a more uniform substituent distribution along the polymer backbone for homogeneously prepared methyl cellulose.
Bulky silyl and aryl containing substituents are interesting as regioselective protecting groups to attain a better understanding of structure–property relationships. This knowledge leads to better properties and ultimately improves the performance of new materials. The most commonly used protecting groups are thexyldimethylsilyl and trityl (triphenylmethyl) and its derivatives. The chloride of the latter one was reacted with cellulose in LiCl/DMAc to obtain 6-O-tritylcellulose with acceptable regioselectivity (Takahashi et al. 1986; Kondo and Gray 1991). Using methoxy-substituted trityl chlorides increased reaction rate significantly (Heinze et al. 1994b; Gomez et al. 1996). Additionally, the acid-catalyzed splitting of the methoxytrityl substituent is accelerated compared to the trityl group.
Even higher regioselectivity was achieved using thexyldimethylsilyl chloride; cellulose 2,6-O- or 6-O-substitution products are obtained. Whereas the latter ether can be obtained by the heterogeneous reaction in DMF/ammonia at − 15 °C (Klemm and Stein 1995), 2,6-O-thexyldimethylsilyl cellulose was prepared in LiCl/DMAc with pyridine (Koschella and Klemm 1997) or imidazole (Koschella et al. 2001) as base Fig. 10. A mild removal of the silyl groups with [TBA]F avoids undesired cellulose degradation. More detailed information on this topic can to found in a comprehensive review (Fox et al. 2011).

Reprinted with permission from (Fox et al. 2011), copyright (2011) American Chemical Society
Heterogeneous and homogeneous thexyldimethylsilylation of cellulose.
Cellulose derivatization in [TBA]F·3H2O/DMSO
Cellulose dissolution in [TBA]F·3H2O/DMSO
Dimethyl sulfoxide containing [TBA]F·3H2O dissolves easily cellulose with a DP as high as 650 within 15 min at room temperature without pre-treatment. This solvent is a non-derivatizing one, as concluded from 13C NMR spectroscopic measurements (Heinze et al. 2000). In the 13C NMR spectrum, six signals appear that were unambiguously assigned to the different C-atoms of the AGU (Fig. 11).

Reprinted from (Heinze and Liebert 2012), copyright (2012), with permission from Elsevier
13C NMR spectrum of cellulose dissolved in DMSO-d6/[TBA]F·3H2O recorded at 50 °C.
19F and 1H NMR spectroscopic measurements of solutions of cellulose in ([TBA]F·xH2O/DMSO) containing varying amounts of water indicated that the disruption of hydrogen bonds is the result of the formation of strong Cel-OH···F− bonds and the subsequent electrostatic repulsion between the negatively charged Cel-OH···F− chains (Östlund et al. 2009). A sheath of the [TBA]+ cations most likely surrounds the negative chains, which leads to a synergism of electrostatic repulsion (Cel-OH···F−) and steric repulsion (Cel-OH···anion/cation complex) preventing chain re-association. Due to strong F–water interactions, this solvent tolerates a certain amount of water only. Water may remove the fluoride ions from the cellulose backbone allowing formation of hydrogen bond networks that that yield solutions of increasing viscosity or even gels and finally precipitation of the biopolymer (Fig. 12).

Reprinted with permission from Östlund et al. (2009), copyright (2009) American Chemical Society
Photograph and plot of samples at varied cellulose and water content: (a) samples at isotropic solutions; (b) transparent gels; (c) opaque gels. Inspection of the parallel lines in the background of the photograph shows the difference in transparency between the samples. Vial (a) 1% cellulose (data point a in plot); vial (b) 2.3% H2O and 1% cellulose (data point b in plot); vial (c) 10% H2O and 1% cellulose (data point c in plot) all in [TBA]F/DMSO.
Consequently, water-free [TBA]F is of interest to dissolve the cellulose. Direct dehydration of [TBA]F·3H2O is not feasible because anhydrous [TBA]F is unstable, undergoing a rapid E2-elimination (Hofmann degradation), resulting in the formation of hydrogen difluoride anions (Sharma and Fry 1983). However, anhydrous [TBA]F is accessible in situ by reacting tetra(n-butyl)ammonium cyanide with hexafluorobenzene in dry DMSO (Sun and DiMagno 2005). The freshly prepared mixture of DMSO and water-free [TBA]F solution dissolves cellulose very easily, even in the presence of the byproduct C6CN6 (Köhler and Heinze 2007). Surprisingly, the dissolution of bleached cotton fibers (DP = 3743) occurs within 1 min. On the contrary to the findings of Östlund et al., no difference in the viscosity of a solution of the same cellulose in [TBA]F·3H2O/DMSO or in the water-free system (Östlund et al. 2009).
Other quaternary ammonium fluorides/DAS were investigated as cellulose solvents. Thus, benzyltrimethylammonium fluoride monohydrate ([BTMA]F·H2O)/DMSO dissolves cellulose, whereas tetramethylammonium fluoride (TMAF)/DMSO does not. A simple explanation for this difference is the solubility of the parent electrolyte in DMSO. At room temperature, the solubilities in DMSO are 0.94, 0.025 mol/L for [TBA]F·3H2O, and BTMAF·H2O, respectively; TMAF is practically insoluble in this DAS. It was argued that a certain amount of fluoride ions (at least 2.2 mol fluoride per mol AGU) are needed to dissolve cellulose (Köhler and Heinze 2007).
For [TBA]+ with different halide ions (X−), IR spectroscopy showed that the interactions X−···HO-polyol (2,2-bis(hydroxymethyl)-1,3-propanediol (a model for cellulose) increased as a function of increasing the charge density and decreasing the volume of the anion (Papanyan et al. 2013). The same was found for solutions of the 1-ethyl-3-methylimidazolium halides ([EtMeIm]X, X = halide). Because the fluoride anion is the hardest base according to the Hofmeister series, the high cellulose dissolution capacity of QA fluorides/DMSO is expected; other QA halides/DMSO are not efficient (Papanyan et al. 2013).
As shown above for LiCl/DMAc, [TBA]F·3H2O/DMSO was also used as solvent for determination of MM of cellulose samples by SEC analysis (Lu and Ralph 2003; Yusup et al. 2015). For cellulose samples with low molar mass, e.g., MCC pullulan was used for calibration. The high concentration of [TBA]F·3H2O needed for dissolution of cellulose samples with high MM, however, prevented use of this solvent, due to column saturation with the electrolyte (Rebière et al. 2017).
Esterification of cellulose in [TBA]F·3H2O/DMSO
[TBA]F·3H2O/DMSO was applied as reaction medium for a variety of homogeneous derivatization reactions. The acetylation of MCC (2.9 wt%) dissolved in 16 wt% DMSO/[TBA]F·3H2O with 2.3 mol acetic anhydride/mol AGU at 40 °C for 70 h gave a cellulose acetate soluble in DMSO and DMF with a DSAc of 0.83 (Heinze et al. 2000). This DS was reached although the reaction mixture contains water (from [TBA]F·3H2O), which is the 4.4-fold molar amount of acetic anhydride employed. Partial DSAc values were calculated by 1H NMR spectroscopy; the order is O-6 ≥ O-2 > O-3, akin to the result in LiCl/DMAc (Ass et al. 2004).
The transesterification of cellulose dissolved in [TBA]F·3H2O/DMSO with vinyl acetate under comparable conditions led to a cellulose acetate with DS=1.04, presumably because vinyl acetate reacts with water slower than acetic anhydride. Most of the hydroxyl groups can be acetylated applying a molar ratio of 10 mol vinyl acetate/AGU yielding cellulose acetate with a DS = 2.72 (Heinze et al. 2000). Table 1 shows a comparison of the acylation results reactions in 3 solvents.
The conversion of cellulose in [TBA]F·3H2O/DMSO with tosyl chloride/triethylamine yields organo-soluble CTs (DSTs = 1.15) with insignificant chlorodeoxy formation (DSChlorodeoxy = 0.024%). Figure 13 shows the use of in situ activation of carboxylic acids to synthesize bearing unsaturated, chiral, crown ether, and cyclodextrin moieties (Ciacco et al. 2003; Hussain et al. 2004; Liebert and Heinze 2005; Heinze et al. 2006).
Functionalization of cellulose with furan-2-carboxylic acid, activated with CDI yields products of DS of up to 2.4, whereas the homogeneous preparation of cellulose furoate in LiCl/DMAc gave a DS of 0.6 under comparable conditions. Thus, quaternary ammonium fluorides/DMSO are more efficient reaction media for this specific synthesis. Moreover, the cellulose esters formed possess comparable properties (e.g., solubility in organic solvents) with those obtained in LiCl/DMAc (Köhler and Heinze 2007).
On the contrary, the reaction of cellulose with 3-isopropenyl-α,α-dimethylbenzyl isocyanate, is more efficient applying LiCl/DMAc as medium; highest DS of 1.8 was obtained (Köhler and Heinze 2007). The cellulose-3-isopropenyl-α,α-dimethylbenzyl carbamate is soluble in various solvents such as DMSO, which can be used to study the structure by NMR spectroscopy (Fig. 14), on one hand. On the other, it may undergo crosslinking in the presence of light. Nevertheless, there is no general conclusion possible which solvent give the best results regarding DS for the reaction in question. However, it should be pointed out again that the dissolution of cellulose in the mixture of quaternary ammonium fluoride/DMSO is very simple and fast compared to the laborious dissolution of the biopolymer in LiCl/DMAc.

Reprinted with permission from Köhler and Heinze (2007), copyright (2007), with permission from Elsevier
13C NMR spectrum of cellulose-3-isopropenyl-α,α-dimethylbenzyl carbamate with DS = 1.79 acquired in DMSO-d6.
The graft polymerization of cyclic compounds such as lactones and N-carboxy-α-amino acid anhydrides on cellulose dissolved in [TBA]F·3H2O/DMSO could be carried out as well (Ikeda et al. 2003).
An approach for the synthesis of regioselectively functionalized cellulose esters with bulky substituents in [TBA]F·3H2O/DMSO, LiCl/DMAc and [AlMeIm]Cl was attempted by Xu et al. (2011). The esterifications were quasi-selective for the primary O-6, but still acylation on the secondary O-2/3 was observed. Thus, they couldn’t identify conditions for truly regiospecific acylation (at O-6). They conclude regioselective esterification is likely to be successful for large dendritic acyl moieties. A comprehensive review about regioselectivity in cellulose esterification and etherification is available (Fox et al. 2011). An interesting result is the observation of regioselective deacetylation of silylated cellulose acetate during deprotection with [TBA]F·3H2O/THF at the positions O-2 and O-3 of the AGU. The deacetylation takes place via ketene intermediate formation (Xu and Edgar 2012; Zheng et al. 2013a, b). The succinylation of cellulose was compared in the solvent systems [N2222]Cl/DMSO and [TBA]F·3H2O/DMSO. The reaction is slightly more efficient in the latter solvent and depends strongly on the QAE+ concentration. Polyelectrolyte-like complexes of cellulose and QAE could promote the succinylation of cellulose (Chen et al. 2014).
Etherification of cellulose in [TBA]F·3H2O/DMSO
Unlike typical cellulose carboxylic esters that are soluble in the solvents employed for cellulose dissolution, the hydrophobic and less polar cellulose ethers are not soluble in the reaction medium. Thus, the reaction starts homogeneously but becomes heterogeneous. This (phase separation) problem also applies to ionic cellulose ethers like sodium carboxymethyl cellulose. There are some exceptions, however: SO2/diethylamine/DMSO dissolves cellulose and homogeneous carboxymethylation was carried out (Kamida et al. 1984; Isogai et al. 1984a).
Commercially, etherification is carried out exclusively under heterogeneous reaction conditions applying aqueous NaOH to activate cellulose, i.e., to enhance the reactivity by swelling and increase the nucleophilicity of the hydroxyl groups by forming the alkali cellulose (Heinze et al. 2018c). The addition of aqueous NaOH to dissolved cellulose usually leads to precipitation, i.e., the homogeneous system becomes heterogeneous, containing unreactive cellulose. On the contrary, cellulose samples dissolved in [TBA]F·3H2O/DMSO were treated with solid NaOH suspended in DMSO or with an aqueous base solution yielding a highly swollen material. The reaction with benzyl chloride yielded benzyl celluloses with DS values up to 2.8. Interestingly, the reactivity of the cellulose depends not only on the molar ratio of benzyl chloride/AGU, but also on the amount of [TBA]F·3H2O used for dissolving the biopolymer (Ramos et al. 2005b). SEC measurements revealed polymer aggregation in samples of low DS synthesized in a solvent containing 9% [TBA]F·3H2O while at higher concentration of the salt, the benzyl cellulose samples obtained do not form aggregates. Fully substituted organo-soluble allylcellulose was prepared by a similar procedure (Heinze et al. 2008).
Carboxymethylation of cellulose in [TBA]F·3H2O/DMSO was successfully carried out with DS-values up to 1.87 (Heinze et al. 2000). 1H NMR spectroscopy of the CMC showed partial DS of carboxymethyl groups in the order O-6 > O-3 ≥ O-2 (6 = 0.734, 2 = 0.558, 3 = 0.574), which is comparable to the same product synthesized in aqueous Ni[tris(2-aminoethyl)amine](OH)2 system. However, the substituents were distributed in a non-statistical manner because of the high amount of fully carboxymethylated AGUs. Later, a more detailed study of this reaction was carried out (Ramos et al. 2005a). Using solid NaOH, products with DS as high as 1.6 can be obtained independent of the DP of the cellulose used. With aqueous NaOH a maximum DS of 2.17 was reached in a one-step conversion. On the contrary, a two-step procedure is necessary to get such a high DS value by the conventional heterogeneous procedure. The derivatives synthesized in [TBA]F·3H2O/DMSO exhibit a deviation from the statistical substituent distribution, observed for products obtained in the conventional heterogeneous process (Heinze et al. 1994a; Heinze 1998). The carboxymethylation is also much more efficient compared to the reaction carried out in LiCl/DMAc, because a rather high excess of etherifying agent (up to 5 mol/AGU) must be employed. Synthesis of CMC from an empty fruit bunch could be accomplished, which seems to be useful for diverse applications such as paper coating and food packaging (Eliza et al. 2015).
Cellulose derivatization in aqueous media
Cellulose dissolution in aqueous media
Regarding the dissolution of cellulose in aqueous environment, theoretical calculations revealed that the entropy loss due to water–cellulose interactions is not compensated by the concomitant entropy gain due to the increased chain conformations upon dissolution, consequently, cellulose dissolution in energetically unfavorable (Bergenstråhle et al. 2010; Parthasarathi et al. 2011; Bao et al. 2015). This energy balance, however, changes if the polymer is ionized (by an added base), i.e., becoming a polyelectrolyte, because the counter ions and Coulombic interactions contribute largely to the entropy gain (Schneider and Linse 2002, 2003). As a result, polymers that ionize are generally soluble in water, even if they are not very polar (Lindman et al. 2017). Cellulose ionization was inferred from NMR data by considering that the OH-groups don’t need be completely dissociated, but form dissociated structures of relatively short duration (Isogai 1997). Later, this assumption was substantiated with electrophoretic NMR on cellobiose. The results revealed base-independent (NaOH and KOH) dissociation steps at pH 12 and 13.5 (Bialik et al. 2016). A comprehensive review of cellulose in NaOH aqueous solutions with and without additives was provided by Budtova and Navard (2015). Cellulose dissolution in systems based on aqueous metal complex (Burchard et al. 1994; Klüfers and Schuhmacher 1994; Saalwächter et al. 2000) or zinc chloride (Letters 1932; Xu and Chen 1999) needs deprotonation of OH-groups (O-2 and O-3) before the complex can be established at those positions. This emphasizes the argumentation of the (transient) deprotonation of cellulose in basic media. In this context, the decrease in cellulose solubility in basic solution upon addition of additives, ionic or non-ionic can be rationalized because of the concomitant change (decrease) of entropy (Alves et al. 2016a, b; Medronho et al. 2016). On the other hand, urea, thiourea, guanidine and their derivatives decrease hydrophobic interactions. In aqueous solutions, these additives cause, inter alia, protein denaturation, demicellization of surfactant aggregates, and cellulose dissolution (in presence of base) (Lilienfeld 1924; Zhou and Zhang 2000; Cai and Zhang 2005, 2006; Cai et al. 2006, 2007; Ruan et al. 2008; Egal et al. 2008; Qi et al. 2008; Cai et al. 2008; Liu and Zhang 2009; Lindman et al. 2017). Note that thiourea is the more efficient additive than urea, although the dissolution mechanism of both additives is similar, namely by channel inclusion complexes [Fig. 15 (Cai et al. 2007; Luo and Zhang 2013)].

Reprinted from (Luo and Zhang 2013), copyright (2013), with permission from Elsevier
TEM image (left) and proposed mechanism of the channel inclusion complex (right) of cellulose in LiOH/urea aqueous solution.
Aqueous tetraalkylammonium hydroxide ([NR4]OH), in particular tetra(n-butyl)ammonium hydroxide ([TBA]OH), solutions dissolve cellulose well. The conclusion may raise that solutions of organic acids or bases are much better solvents than those of inorganic ones (Gubitosi et al. 2016; Lindman et al. 2017). It was found, that 1.2 [TBA]+ ions bind per AGU, which suggests electrostatic interactions of the cation with the deprotonated hydroxyl groups of cellulose, in addition to hydrophobic interactions (Gentile and Olsson 2016). Furthermore, cellulose solubility increases as a function of increasing cation hydrophobicity, as illustrated in Fig. 16 (Wang et al. 2018). NMR analyses (2D NOESY) of [NR4]OH/cellulose or cellobiose indicated that the cations α-methylene group interacts with the electropositive C-1 atom of cellulose via electrostatic and Van der Waals forces, forming a [NR4]+/cellulose “complex” leading to further cellulose structural disruption [Fig. 17 (Zhong et al. 2017)].

Reproduced from Ref. (Wang et al. 2018), with permission from the Centre National de la Recherche Scientifique (CNRS) and The Royal Society of Chemistry
Solubility of cellulose in aqueous quaternary ammonium hydroxides in dependence of the cation volume.

Reprinted with permission from Zhong et al. (2017), copyright (2017), with permission from Elsevier
Proposed interactions of aqueous [NR4]OH with cellulose.
In summary, cellulose is likely to dissolve in aqueous media if it acquires a charge (albeit transient), concomitant with disruption of hydrophobic interactions. For example, at pH > 12 the translational and configurational entropy increases. Organic hydroxides such as [NR4]OH are more efficient than their inorganic counterparts because the cations of the former disrupts hydrophobic interactions.
Etherification of cellulose in aqueous media
Anionic cellulose ethers
Carboxymethyl cellulose (CMC) is one of the most relevant industrial cellulose derivative (Heinze et al. 2018d). Its industrial production is carried out heterogeneously in a slurry containing an alcohol (often isopropanol), aqueous NaOH and chloroacetic acid or the corresponding sodium salt. The first successful homogeneous carboxymethylation of cellulose was carried out in Ni[tris(2-aminoethyl)amine](OH)2 in the presence of alkaline solution (Heinze et al. 1999). DS-values between 0.11 and 0.72 were obtained after 3–4 h reaction time at 80 °C. The samples became water soluble at DS > 0.4, which is comparable with heterogeneously prepared products. Interestingly, structure analysis by means of HPLC and 1H NMR after chain degradation showed comparable results as for CMC obtained by the heterogeneous slurry process, i.e., a statistical distribution of the substituents along the polymer chain. The partial DS-values, however, are different: in the heterogeneous slurry O-2 ≥ O-6 > O-3 was found, whereas it was O-6 ≥ O-2 > O-3 in Ni[tris(2-aminoethyl)amine](OH)2. Thus, cellulose activated by NaOH aqueous solutions as in the slurry process or homogeneously dissolved in Ni[tris(2-aminoethyl)amine](OH)2 exhibit overall the same reactivity but a slightly different distribution of the partial DS. Later, the same group applied NaOH/urea (Qi et al. 2009) and LiOH/urea (Qi et al. 2010) as homogeneous reaction media for the carboxymethylation of cellulose. Water-soluble CMC with DS = 0.18–0.62 were obtained in NaOH/urea and with DS = 0.36–0.65 in LiOH/urea. Here, partial DS-values in the order O-6 > O-2 > O-3 were found, which is in accordance with the nickel-based solvent (Qi et al. 2009). The homogeneous carboxymethylation of cellulose is less effective than the heterogeneous synthesis (maximum DSheterogeneous = 1.24 vs. DShomogeneous = 0.72), most likely because of an increased hydrolysis of sodium monochloroacetate. On the other hand, water-soluble derivatives with DS-values as low as 0.18 could be obtained via the homogeneous route, which must be attributed to the slight differences in the partial DS-values.
The homogeneous Michael addition of cellulose with acrylamide in NaOH/urea and partial hydrolysis of the acylamino group yielded water-soluble (mixed) derivatives in the range of 0.36–0.84 (Song et al. 2008b). Figure 18 shows the reaction scheme.

Redrawn from (Song et al. 2008b)
Homogenous etherification of cellulose with acrylamide in NaOH/urea aqueous solutions results in a mixture of non-ionic and ionic (carboxyethyl cellulose) cellulose derivative.
Cationic cellulose ethers
Cationic cellulose ethers are mainly prepared in aqueous solutions of NaOH/urea. The reagents used to obtain tetraalkylammonium functionalized cellulose derivatives, so-called “quaternized cellulose” are (3-chloro-2-hydroxypropyl)trimethylammonium chloride (CHPTMA Cl) or (2,3-epoxypropyl)trimethylammonium chloride (EPTMA Cl, Fig. 19). Water-soluble products with DS-value of 0.20–0.63 were obtained with CHPTMA Cl (Song et al. 2008a), and from 0.17 to 0.50 with EPTMA Cl as reagents. The reactivity of the 3 OH-groups was found to be in the same order as for the carboxymethylation: O-6 > O-2 > O-3. Bacterial cellulose was homogeneously modified in the same manner (Zhang et al. 2016). In comparison, under heterogeneous conditions only water-insoluble samples with DS-values < 0.2 were obtained (Heinze et al. 2018d).

Adopted by permission from Heinze et al. (2018d), Springer Nature, copyright (2018)
Reaction scheme and molecular structure of hydroxypropyltrimethylammonium (HPTMA) cellulose synthesized in aqueous media.
Quaternized cellulose was of increased interest for the preparation of cellulose-based hydrogels. These are water-swollen, and cross-linked polymeric networks or polymeric materials that exhibits the ability to swell and retain a significant fraction of water within its structure (Ahmed 2015). They are attractive soft materials receiving increased attention due to the applications in the fields of food, food packaging, pharmaceuticals, agriculture, personal care products, and electronics (Luo and Zhang 2013). Thus, HPTMA cellulose was crosslinked with cellulose (Peng et al. 2016) or CMC (Chang et al. 2011) in the presence of epichlorohydrin. An amphoteric cellulose derivative with terminal quaternary ammonium and sulfonium groups was obtained by simultaneous conversion of cellulose with CHPTMA Cl and (3-chloro-2-hydroxypropane)sulfonic acid (DS = 0.23–0.76) improving mechanical properties of fibers (Song et al. 2013).
Miscellaneous cellulose ethers
We dwell here on classes of non-ionic (e.g. alkyl, hydroxyl alkyl, aryl) cellulose ethers as well as mixed ethers, that may or may not contain ionic groups, synthesized in aqueous media. Aqueous NaOH/urea is the dominantly employed solvent system for these etherification reactions. Hydroxypropyl cellulose (HPC) and methyl cellulose (MC) were the first derivatives prepared in this solvent (Zhou et al. 2004). The DS values were 0.85–1.73 and 1.48–1.69, for HPC and MC, respectively. Interestingly, the partial DS-values of O-2 were in all cases higher than that of O-6, which is in contrast to the examples cited above. Structure property relationships of MC were reported later (Ke et al. 2006; Zhou et al. 2008). The synthesis of O-(2,3-dihydroxypropyl) cellulose with glycidol as reagent was performed as well (Chang et al. 2013). Molar degrees of substitution were determined to be 1.02–4.84, which indicates extensive consecutive reaction at the newly formed OH-groups. The partial DS-values were in the order O-6 > O-2 > O-3 with O-2 being only marginally lower substituted than O-6. Hydroxyethyl cellulose (HEC) exhibited MS-values between 0.54 and 1.44 with similar DS (Zhou et al. 2006). Thus, only minor consecutive reaction is observed under these conditions. Analysis of the partial substitution pattern revealed the preferred substitution of O-2.
Unsaturated substituents could be attached to the cellulose backbone in NaOH/urea. Organo-soluble or swellable (DMSO, DMF, DMAc, pyridine) benzyl cellulose was obtained with a DS as low as 0.4 (Li et al. 2011a). Heterogeneously prepared benzyl cellulose is not soluble in molecular solvents at this DS. However, other homogeneous reaction media such as LiCl/DMAc were more efficient for the benzylation (Isogai et al. 1984b). A rapid benzylation of cellulose in a 47 wt% tetra(n-butyl)phosphonium hydroxide aqueous solution was reported yielding a DS of up 2.5 within 10 min at room temperature. The reaction starts homogeneously but the product precipitates with ongoing conversion. The high efficiency of this solvent system compared to others such as NaOH/urea was ascribed to the good solubility of benzyl bromide in the mixture due to the amphiphilic character of the quaternary phosphonium cation. Figure 20 illustrates the benzylation (Abe et al. 2017).

Reprinted with permission from Abe et al. (2017), copyright (2017) American Chemical Society
Proposed scheme of the benzylation of cellulose in tetra(n-butyl)phosphonium hydroxide aqueous solution: a beginning of benzylation after benzyl bromide addition; b growth of benzylation in a temporarily stabilized micelle; c ending of benzylation accompanied by a precipitation of benzyl cellulose.
Organo-soluble allyl cellulose with DS-values of 0.98–1.65 were synthesized as well (Hu et al. 2015). They were further used for thiol-ene click reactions to obtain cellulose derivatives with various functionalities. Partial DS-values were not determined for the allyl ether substituents.
Hydrogels were prepared from the above-mentioned cationic cellulose ethers. For example, a cellulose/alginate composite was obtained by crosslinking with epichlorohydrine (Chang et al. 2009a). Moreover, a fluorescent hydrogel for fluoro-immunoassays and biological labelling was synthesized by crosslinking of cellulose and incorporation of quantum dots into the cellulose matrix (Chang et al. 2009b). Crosslinking of cellulose (Ciolacu et al. 2016), and CMC (Chang et al. 2010a, b) with N,N′-methylene bisacrylamide (Geng 2018) or 1,4-butanediol diglycidyl (Liu et al. 2016a) yielded further hydro- or aerogels, respectively. One-pot method for the synthesis of sub-micron microgels, as effective stabilizers of oil-in-water emulsions, from cellulose applying a combination of alkylation with sodium monochloroacetate and subsequent Ugi reaction was proposed (Shulepov et al. 2016).
Graphene oxide/cellulose composite films were obtained by chemical crosslinking with epichlorohydrin (Liu et al. 2016b). The resulting films exhibited superior mechanical performances and excellent ultraviolet-shielding making it an excellent candidate for high performance bioplastics. A highly conductive cellulose/graphene oxide film was prepared by in situ chemical reduction of the graphene oxide in NaOH/urea/cellulose aqueous solution (Chen et al. 2018b). The composite films can be applied as multifunctional sensor materials responding to different external stimuli, such as temperature, humidity, stress/strain, and liquids by electrical resistance changes. To our knowledge, aqueous solutions of [NR4]OHs with or without addition of organic solvents were not yet applied for derivatization of cellulose. However, they were used for cellulose extraction form sugarcane bagasse (Zhong et al. 2016) or the preparation of hydrogels in the presence of β-cyclodextrin (Medronho et al. 2016, 2017). Figure 21 summarizes the applications of cellulose-based materials obtained in base/urea aqueous solutions.

Reprinted from (Luo and Zhang 2013), copyright (2013), with permission from Elsevier
Cellulosic materials prepared in NaOH/urea solutions and its applications.
Cellulose derivatization in quaternary ammonium electrolytes
Cellulose dissolution in quaternary ammonium electrolytes (QAEs)
The majority of cellulose dissolving QAEs consist of tetraalkylammonium cations with carboxylate or chloride anions. In particular, [TBA]AcO mixed with varying amounts DMSO is of great interest. The good solubility of cellulose in this solvent system is mainly attributed to the (hard) basicity of the acetate anion. Huang et al. investigated the dependence of solvent efficiency on the [TBA]AcO/DMSO weight ratio and found, the degree of dissociation of the QAE is larger at W[TBA]AcO < 0.15 than at W[TBA]AcO > 0.15 (W[TBA]AcO = mass of [TBA]AcO/[mass of [TBA]AcO + mass of DMSO]) because of the interaction of QAE ions with cellulose (Huang et al. 2016). This is illustrated schematically in Fig. 22. A rapid increase of the [TBA]AcO/DMSO/cellulose solution conductivity until W[TBA]AcO ~ 0.15 followed by subsequent slower increase corroborated this result. NMR and IR spectroscopy also revealed increased interaction of the α-methylene group of the [TBA]+ cation with the DMSO-oxygen and decreased cation/anion interaction with increasing electrolyte concentration. Weaker interactions between cation and cellobiose were found as well, which agrees with the results found for [NR4]OH solvent systems mentioned in point 3.2 above (Zhong et al. 2017). Thus, the balance between ion concentration and mobility is important to cellulose dissolution. This balance is largely controlled by hydrogen bonding and solvophobic interactions of the species present, [TBA]AcO, cellulose and DMSO. The latter has the favorable effect of reducing the viscosity and stabilizing the QAE/complex.

Reprinted with permission from Huang et al. (2016), Copyright (2016) American Chemical Society
Equilibrium of association/dissociation of [TBA]AcO as a function of the mass ratio W[TBA]AcO of the [TBA]AcO with DMSO.
Investigations of the same solvent system at 60 °C revealed a ratio acetate/AGU of 1/1 at maximum cellulose solubilization (Idström et al. 2017). This remarkable efficiency is due to the acetate anion binding to more than one hydroxyl group of the AGU. Strong anion···HO-Cel hydrogen bonding was concluded from theoretical calculations that indicated longer contact time between acetate and the AGU OH-groups compared any other pair of species present in the system (cation–cellulose, cation–DMSO, DMSO–DMSO). The simultaneous binding of halides to more than one OH-group in the same AGU (OH-2 and OH-3) was also concluded from MD simulations of cellulose solutions in [R4N]F·xH2O/DMSO (Casarano et al. 2014) and [AlMeIm]Cl/DMSO (Nawaz et al. 2015).
In a systematic study triethylalkyl- and tripropylalkylammonium acetates and propionates and their mixtures with DMSO were investigated with regard of the effect of QAE cation volume on cellulose dissolution in QAEs/DMSO (Meng et al. 2017). The cellulose dissolving QAEs are those with a fourth alkyl-substituent longer than n-hexyl. The most efficient QAE is [N2228]AcO. With 20 wt% DMSO the calculated molar ratio QAE/AGU is 2.1, which is smaller than that observed for [TBA]AcO/DMSO (Idström et al. 2017). It may be concluded, that above a certain threshold volume of the cation cellulose solubilization gets less efficient because the penetration in between the chains is sterically hindered.
Etherification of cellulose in QAEs
Water-soluble CMC with DS = 1.55 was isolated from triethylmetylammonium formate in the presence of solid NaOH (Köhler et al. 2009). A block-like distribution of substituents along the polymer backbone and partial DS-values of 0.56 (O-6), 0.55 (O-2) and 0.41 (O-3), i.e. O-6 ≥ O-2 > O-3, were found. This block-like or non-statistical distribution of substituents is in contrast to those determined in the aforementioned aqueous cellulose solvents under heterogeneous and homogeneous conditions. Hence, the homogeneous conversion of cellulose to CMC in triethylmetylammonium formate/solid NaOH follows the principle of reactive microstructures as observed in non‐aqueous solvents such as DMSO or LiCl/DMAc (Liebert and Heinze 1998). Hydroxypropylation of MCC, cotton linter, and spruce sulfite pulp in N1222Fo in the presence of magnesium acetate yielded water-soluble products (Köhler et al. 2010). Without addition of this catalyst negligible conversion was observed. Interestingly, the homogeneous preparation of HPC in [EMIM]AcO in the absence of magnesium acetate results in a less regioselective distribution of substituents with partial DS in the order O-2 ≥ O-6 > O-3, which was attributed to the higher acidity of O-2 (Saric and Schofield 1946). Benzyldimethyltetradecyl-ammonium chloride dihydrate was also used for the homogeneous etherification of cellulose to obtain HPC but no analytical data are available (Moellmann et al. 2013).
Esterification of cellulose in QAEs
The enzyme catalyzed transesterification is a promising method for cellulose esterification. However, enzyme denaturation in ionic solvents such as QAEs and ILs is a problem to overcome. Thus, bis- and tris(2-methoxyethyl)triethyl-ammonium acetates were prepared and successfully used for enzyme catalyzed transesterification of glucose and cellulose (Zhao et al. 2008). A DS of 0.89 was obtained. However, substitution exclusively occurred in position O-6. Cellulose acetate, propionate and butyrate with DS-values between 2 and 3 were obtained by acylation in [TBA]AcO/DMAc (Lin et al. 2015) or [TBA]AcO/DMSO (Yu et al. 2016, 2018). Also, acetate/propionate and acetate/butyrate mixed esters with high DS were synthesized. The conversion of cellulose with succinic anhydride under catalyst free conditions yielded products with DSSuccinate between 0.3 and 1.2 (Xin et al. 2017). The reaction was more efficient compared to other catalyst free conversions in ILs with and without DMSO as co-solvent (Liu et al. 2006, 2007). Substitution occurred mainly in position O-6. Acetylation reactions were conducted successfully in the binary mixtures of [N2228]Cl/co-solvent (Achtel and Heinze 2016; Achtel et al. 2017, 2018). Organo-soluble cellulose acetates with DSAc ranging from 0.16 to 2.79 depending on the reaction conditions were isolated. Maximum DSAc of 2.79 was obtained after 2 h at 50 °C using acetyl chloride/pyridine. Under the same reaction conditions using LiCl/DMAc and [BuMeIm]Cl as solvents the DSAc were almost identical with 2.83 and 2.81. As expected, cellulose acetate with low DS exhibit partial DS-values in the order O-6 > O-2 > O-3. On the contrary, the distribution changes to O-6 > O-3 > O-2 for samples with high DS values. This result is difficult to explain but might be a consequence of the cleavage of the O-3/O-5′ hydrogen bond as well as a slightly stronger (electrostatic or hydrophobic) interaction of the cation with C-1 of the AGU than with other regions of the AGU. Thus, the adjacent OH-2 group is sterically hindered and less accessible for the reagent. Solvatochromic investigations revealed that the acetylation is little dependent on the molecular solvent used of the binary [N2228]Cl/co-solvent mixture (Achtel et al. 2018). The medium polarity showed a “levelling-off” effect due to the relative high concentration of QAE. Moreover, the chloride ions interact with the cellulose OH-groups whereas the voluminous cation surrounds the negatively charged chains. Thus, the cellulose-QAE complex is less sensitive to the nature of the molecular solvent employed, as schematically depicted in Fig. 23.

Reprinted with permission from Achtel et al. (2018), copyright (2018) WILEY
Schematic representation of sheath formation during cellulose dissolution. The chloride anions (bright red spheres) interact with the hydroxyl units, the cations (bright blue spheres) build the sheath and “isolate” the cellulose from the molecular solvent (dark blue droplet).
Tetraalkylammonium dimethylphosphates have been reported as solvents for cellulose and reaction media for the production of cellulose esters (Buchanan et al. 2013). Neat tri-(n-butyl)methylammonium dimethylphosphate allowed the homogeneous, catalyst-free synthesis of cellulose acetate with a DSAc = 2.5. DMSO, DMF and NMP were efficiently used as co-solvents to obtain cellulose acetate and cellulose acetate/propionate mixed esters. Here, the above-mentioned change of regioselectivity at high DS-values from O-6 > O-2 > O-3 to O-6 > O-3 > O-2 was found too.
The salts of super bases were employed for acetylation of cellulose under homogeneous conditions (Jogunola et al. 2016). The derivatization was carried out with acetic anhydride in 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) mixtures with different co-solvents (acetone, acetonitrile, DMSO). Organo-soluble esters with DSAc-values in the range from 0.90 to 2.89 were obtained depending on the chosen reaction conditions with acetonitrile being the most efficient molecular solvent. Partial substituent distribution in the order O-6 > O-2 > O-3 were confirmed by NMR spectroscopy, which is in accordance with products obtained from 1-allyl-3-methylimidazolium chloride (Cao et al. 2007) and LiCl/DMAc (Marson and Seoud 1999). Other publications, however, reported partial DSAc-values in the order O-6 > O-3 > O-2 (Wu et al. 2004; Cao et al. 2010). This renders an explanation of the effects of solvent and reaction conditions uncertain. There is still the possibility that these differences are only statistical, especially for cellulose acetates with high DS. Therefore, a systematic study of the regioselectivity of the acetylation of cellulose in 3 different imidazolium-based ILs was carried out indicating a strong dependence of the reactivity of OH-2 and 3 on the IL anion and the acetylating reagent (Abe et al. 2016). This issue will be discussed in more detail below.
The acetylation of cellulose in neat [DBN]AcO using various acetylating agents was reported (Kakko et al. 2017). The reagents efficiency was equal for DSAc-values between 0.1 and 1. Isopropenyl acetate was the most efficient reagent to obtain highly or fully substituted cellulose actates. Distribution of the substituent was found to be O-6 > O-2 ≈ O-3, i.e., different from the results reported elsewhere (Abe et al. 2016). The switchable IL, produced from 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU), methanol and CO2 was used as reaction media to acylate cellulose and yielded acetate, propionate and butyrate esters with maximum DSAc = 2.94, DSPr = 2.91 and DSBu = 2.59 (Yang et al. 2014). The common reactivity for the three OH-groups was elucidated by 1H NMR spectroscopy: O-6 > O-2 > O-3.
A somewhat special solvent is the system consisting of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)/DMSO/CO2 (Xie et al. 2014; Yang et al. 2015) which belongs in principle to the group of switchable ILs (Jessop et al. 2005). The superbase DBU functions as catalyst and solvent. Commercially important, organo-soluble cellulose mixed esters (acetate-propionate, acetate butyrate) with DS between 2.33 and 3.0 were obtained after 2 h at 80 °C employing a molar ratio reagent/AGU of 5/1. The order of reactivity was O-6 > O-2 > O-3, as confirmed by 13C NMR spectroscopy (Xu et al. 2018). In the same solvent system cellulose was acylated via direct transesterification with vinyl esters of long chain fatty acids, and aromatic acids. Even at room temperature high DS-values of 2.6 was reached within 4 h. A broad range of substitution was achieved in dependence on reaction time, temperature and molar ratio reagent/AGU (Chen et al. 2018a). This reaction scheme was found to outperform comparable acylation/transesterifications reported for other solvent systems (Cao et al. 2013, 2014; Hanabusa et al. 2018; Hinner et al. 2016). The dissolved cellulose was also allowed to react with succinic anhydride without any catalyst under very mild conditions yielding cellulose succinates with DS in the range 1.51–2.59, depending on the reaction conditions and the molar ratio of succinic anhydride. The carboxylic acid moiety introduced by the succinylation was modified by Passerini three-component reactions (carboxylic acid; carbonyl compound; isocyanide) and Ugi four-component reactions ((carboxylic acid; carbonyl compound; isocyanide; amine) (Söyler et al. 2018). After reaction, DBU can be effectively recycled and reused which is desirable with respect to the development of sustainable, “greener” processes for cellulose modification.
Carboxyl groups of various polysaccharide derivatives were efficiently modified applying the Ugi-reaction in water, leading to novel polysaccharide derivatives with peptide-like substituents (Gabriel and Heinze 2018).
Cellulose derivatization in imidazolium based ionic liquids
Cellulose dissolution in ionic liquids
In the past 15 years, ILs have been amongst the most frequently studied “new solvents” for cellulose. The term “ionic liquid” describes a highly diverse class of compounds that are entirely composed of an organic cation with an organic or inorganic anion and that are characterized by a low melting point (operationally < 100 °C) (Plechkova et al. 2009; Anastas et al. 2013). Imidazolium based ILs are most frequently employed and will be discussed in this review. However, also pyridinium-based ILs, and low-melting quaternary phosphonium and ammonium compounds have been described in the literature (Sashina and Kashirskii 2015; Kostag et al. 2018).
Most cellulose dissolving ILs contain 1-alkyl-3-methyl imidazolium cations with different n-alkyl chains (Fig. 24). Allyl, ethyl-, and butyl- are the most frequent side chains (Gericke et al. 2012a; van Osch et al. 2017). In addition, imidazole-based ILs with benzyl- and methylnapthyl substituents dissolve cellulose albeit less efficiently (Dissanayake et al. 2018). Chloride and acetate are the most common anions of ImILs that are employed as solvents for cellulose processing.
The ability of ILs to dissolve cellulose is closely related to their unique molecular structures. Due to the broad structural diversity of this class, it is difficult to propose a general dissolution mechanism. ILs are non-derivatizing cellulose solvents and it is generally accepted that the anion should be a strong hydrogen bond acceptor (i.e., with high Lewis basicity) in order to facilitate dissolution of the polysaccharide (Table 2) (Gupta and Jiang 2015; Yuan and Cheng 2015; Li et al. 2018). A major contribution that leads to cellulose dissolution is the interaction of IL anions with hydroxyl groups of the polymer backbone leading to breaking of the strong intra molecular hydrogen bond network. However, interaction of IL cations with cellulose, the anions, and the cations themselves have to be considered also. It is generally accepted that cellulose dissolution is favored by decreasing cation size (volume) because of their easier intercalation between the cellulose chains (Dissanayake et al. 2018).
ILs are renowned for their extremely low vapor pressure and their good solvent properties. They are studied intensively in the context of green chemistry in many applications such as organic and inorganic synthesis, electrolytes, separation technologies, and in general as environmentally benign alternatives to molecular solvents (Plechkova et al. 2009; Anastas et al. 2013).
ILs received extensive interest for the fractionation of lignocellulosic biomass into cellulose, hemicellulose, and lignin-based components (van Osch et al. 2017). Their unique trait as customizable “designer solvents” is of particular interest in this regard. Another research aspect is the use of ILs for the valorization of lignocellulose into biofuels and platform chemicals (Dutta et al. 2015; Zhang et al. 2017b). ILs have been studied as solvents for shaping cellulose into fibers and other cellulosic materials (Zhang et al. 2017a). However, in the field of fiber production, the development of IL-based processes is hampered by strong competition from the viscose and NMMO-processes that are both well established and commercialized for decades.
In the field of monomolecular chemistry, ILs were employed as highly efficient reaction media for a broad variety of organic synthesis (Hallett and Welton 2011). Likewise, ILs were used intensively as solvents for the homogeneous derivatization of cellulose. Mixtures of ILs and dipolar aprotic solvents are frequently used to enhance miscibility and decrease solution viscosity (Gericke et al. 2011; Gale et al. 2016; Stolarska et al. 2017). For example, cellulose is dissolved molecularly in solvent mixtures of [EtMeIm]AcO containing chloroform, dichloromethane, DMF, acetonitrile or propylene carbonate. The most efficient cellulose dissolution was achieved in solvent mixtures at maximum electrical conductivity (Rein et al. 2014). The research activities in this field was driven by the following aspects:
-
1.
Is it possible to perform a particular cellulose derivatization under homogeneous conditions using a particular IL?
-
2.
How can IL based procedures be compared with other homogeneous and heterogeneous procedures for cellulose derivatization (e.g., in terms of reaction efficiency and regioselectivity)?
-
3.
Can novel synthesis approaches be developed by exploiting the unique traits of ILs (e.g., by adopting procedures from the reactions of simple compounds)?
-
4.
Do ILs provide a particular advantage, e.g., by introducing new properties or in terms of economical and ecological considerations (sustainability of the process, green chemistry” aspects)?
Esterification of cellulose in ionic liquids
Cellulose alkyl and aryl esters
Cellulose esters, such as cellulose acetates, propionate, butyrates, and mixed esters therefrom, are of huge commercial importance, e.g., in coatings, filter materials, drug delivery, plastics, composites, and optical films (Edgar et al. 2001; Glasser 2004). Following the first reports on successful esterification of cellulose in [BuMeIm]Cl and [AlMeIm]Cl, many publications followed each with a different focus (Heinze et al. 2005; Barthel and Heinze 2006; Cao et al. 2007):
-
1.
Feasibility studies to see if esterification of cellulose in IL is possible.
-
2.
Comprehensive studies on efficiency, regioselectivity, and/or sustainability in comparison to other homogeneous and heterogeneous procedures.
-
3.
Product oriented studies aiming for the efficient synthesis of cellulose esters with specific properties and/or new types of functional derivatives.
Due to the unique solvent properties of the ILs, completely homogeneous esterification of cellulose is feasible and fully functionalized esters with DS up to 3 can be obtained in a single reaction step. The only exception are long-chain fatty acid esters that might become insoluble in the IL when exceeding a certain DS (Barthel and Heinze 2006). The reactions usually require only slight excess of acylation reagents to achieve the desired DS values. They are performed at elevated temperatures (80–100 °C), which guarantees that the viscosity of the reaction mixtures is sufficiently low.
Cellulose acetates and other commercially available cellulose esters are currently prepared by heterogeneous procedures only. To obtain uniform products, two steps are required. First, cellulose is fully converted into a cellulose ester with DS of 3. Second, controlled partial saponification yields the desired DS values and properties of the cellulose esters (Steinmeier 2004). Novel sustainable processes for production of cellulose acetate and other cellulose esters are highly desired. Homogeneous procedures have the potential to yield the products in a facile one-step-process due to the uniform reaction conditions. Acetylation of cellulose in IL such as [AlMeIm]Cl and [BuMeIm]Cl yields cellulose acetates with tunable DS and acetone soluble products are directly accessible (Cao et al. 2007). This is required for shaping into fiber filaments by solution spinning, which involves regeneration by evaporation of acetone (Law 2004). ILs also enable an innovative approach for producing cellulose ester materials by using it as reaction medium and solvent for the shaping process (Kosan et al. 2010). Directly after the homogeneous derivatization reaction is completed, the reaction mixture is subjected to a wet-spinning without prior isolation of the cellulose ester.
The regioselectivity of the acetylation of cellulose in IL, depends on type of IL and the derivatization reagent used (Table 3) (Abe et al. 2016). The primary O-6 position was preferably acetylated in most cases. The partial DS for position O-2 on the other hand was significantly affected by the anion species present in the system. A low partial DSO-2 and higher DSO-3 were observed for conversions performed with acetyl chloride in imidazolium chlorides. However, the partial DSO-2 increased at the expense of DSO-3 when acetate ions were introduced, by using IL acetates as solvents and/or acetic anhydride as reagent. The differences were attributed to a formation of carbene species by deprotonation of the imidazolium cation in the presence of acetate. Another possibility to alter regioselectivity provides the esterification of cellulose with more bulky moieties, such as pivaloate, adamantoate, and 2,4,6-trimethylbenzoate (Xu et al. 2011). A preferential esterification at position O-6 but no true regioselectivity was observed for the three solvent systems tested (LiCl/DMAc, DMSO/TBAF·3H2O, [AlMeIm]Cl).
In addition to cellulose acetates, esters with short alky chains (propionate to hexanoate) have been prepared in different IsL (Fidale et al. 2009; Possidonio et al. 2010; Luan et al. 2013; Olsson and Westman 2017). Moreover, mixed cellulose esters such as cellulose acetate/propionate and cellulose acetate/butyrate that are of considerable commercial importance have obtained by homogeneous derivatization in ILs (Huang et al. 2011a; Cao et al. 2011). In addition to conventional heating, microwave irradiation was employed in these experiments (Possidonio et al. 2010; El Seoud et al. 2011). The reaction rates for esterification of cellulose in [AlBuIm]Cl with the corresponding acid anhydrides decreased with increasing length of the alkyl chain from ethanoate to butanoate (Fig. 25). However, it increased again for pentanoate and hexanoate. This behavior was explained by two competing effects. With increasing chain length, steric hindrance increases, which decreases reactivity. At the same time, hydrophobic interaction between the anhydride and lipophilic cellulose ester chains increases which lowers reaction enthalpy (Fig. 25). An interesting option to alter the reaction kinetics of the acylation of cellulose in IL is the addition of co-solvents. They reduce viscosity and thus increase diffusion coefficients of the reactants (Nawaz et al. 2015). For the acetylation of cellulose, a reactivity order of IL/DMSO > LiCl/DMAc > IL/sulfolane was observed.

Reprinted and modified with permission from Possidonio et al. (2010), copyright (2010) WILEY
a Degrees of substitution achieved for the homogeneous synthesis of cellulose esters with different chain length in 1-allyl-3-butylimidazolium chloride under identical reaction conditions (4.5 mol acid anhydride per repeating unit, 8 h, microwave irradiation 30 W, 80 °C); b Schematic interaction of hexanoic acid anhydride with partially acylated cellulose.
ILs were employed successfully for the synthesis of cellulose aryl esters, most notably cellulose benzoates (Zhang et al. 2009; Chen et al. 2015). Thereby, a strong effect of the substituents on the phenyl ring on the derivatization was found. Long alkyl chain fatty acid cellulose esters (Huang et al. 2011b; Tarasova et al. 2013; Singh et al. 2015) and cellulose ester with dicarboxylic acids were prepared in ILs (Li et al. 2009, 2011b; Liu et al. 2010; Wang et al. 2016).
Whether or not ILs are beneficial in these processes over other homogeneous reaction media significantly depends on the objective of the particular work, e.g., improving the synthesis of an already established product, development of specific novel derivatives. Two key issues that should be considered in this context are recycling and specific side reactions. The recycling strategy employed to recover the IL strongly depends on the process in which it is used as solvent for cellulose processing. Most of these procedures involve a regeneration step in which a large excess of non-solvent (usually water or alcohol) is used to recover cellulose or the cellulose derivatives synthesized. The non-solvent is usually removed by evaporation and the crude IL can be used again to dissolve cellulose. However, thermal “aging” of the IL should be considered. Upon heating, some ILs degrade by different mechanisms that may involve common impurities (e.g., residual N-alkylimidazole), carbene species (formed in situ by deprotonation), and dealkylation products (Liebner et al. 2010; Wendler et al. 2012; Efimova et al. 2018). The thermal degradation products require specific purification strategies because they accumulate upon consecutive processing/recycling steps. Recycling becomes even more complex for ILs used as homogeneous reaction media for cellulose derivatization because it also includes removal of excesses reagents, bases, catalysts, and monomolecular side products. In addition to thermal degradation, ILs can undergo side reactions with particular derivatization reagents that may lead to unexpected products. Carbene species can be formed in ILs by deprotonation of the imidazolium cation in position 2 (Amyes et al. 2004; Rodríguez et al. 2011). This process is accelerated by the addition of bases but can also occur in pure IL with relatively basic carboxylate anions. It has been reported that imidazolium carbenes forms covalent bonds with the reducing end group of cellulose (Zweckmair et al. 2015). Moreover, the highly reactive carbenes can participate in the derivatization reactions and thus alter selectivity and/or reagent efficiency (Canal et al. 2006; Enders et al. 2007; Wang et al. 2017).
The IL anion can also influence the derivatization reaction, e.g., the acetate anion catalyzes the ring opening of oxiranes and facilitate hydroxalkylation of cellulose in ILs (Köhler et al. 2010). EMIMAc is one of the most frequently employed ILs for dissolution and processing of cellulose. However, the acetate anion can react with acid chlorides (e.g., from carboxylic acid and sulfonic acids) to form mixed anhydrides (Fig. 26) (Köhler et al. 2007). Thus, acylation of cellulose in imidazolium acetate-based IL resulted in predominant acetylation of the polysaccharide instead of yielding the desired cellulose ester.

Reprinted and modified with permission from Köhler et al. (2007), copyright (2007) WILEY
13C NMR spectra of mixtures of 1-ethyl-3-methylimidazolium acetate ([EtMeIm]AcO) with a 2-furoyl chloride and pyridine and b tosyl chloride.
As discussed above, transesterification is an interesting alternative for cellulose ester synthesis. A series of different aryl- and alkyl esters of cellulose were prepared by homogeneous conversion of cellulose, dissolved in [BuMeIm]Cl, with monomolecular methyl esters (Table 4) (Schenzel et al. 2014). The reaction was catalyzed by triazabicyclo[4.4.0]dec-5-en and yielded DS values of up to 0.7 using up to fivefold excess of the reagents. Thus, efficiency of the transesterification appears to be lower compared to the use of acid chlorides or anhydrides. However, an obvious advantage is that only methanol is liberated as a side product, which can easily be removed by evaporation. Thus IL recycling is simpler than when acid anhydrides or acyl chloride are used where the side products are carboxylic acid or chloride ion, respectively.
As discussed for LiCl/DMAc vinyl and isopropenyl esters are efficient acylating agents, the reaction is driven to completion by volatilization of the produced acetaldehyde or acetone (simpler IL recycling). Cellulose acetates with DS close to 3 have been prepared in [EtMeIm]AcO/DMSO mixtures by conversion of cellulose with an excess of vinyl acetate (Nguyen et al. 2017). As could be demonstrated for the synthesis of cellulose esters with octanoate (C8), laurate (C12), and palmitate (C16) substituents, transesterification with vinyl esters in IL is rather efficient (Hinner et al. 2016). As an example, DSlaurate of 1.6 and 2.4 are achieved using 2 and 3 mol vinyl laurate per AGU, which corresponds to reaction efficiencies of 80%. As described above, conversion of cellulose in EMIMAc with lauryl chloride or anhydride mostly results in acetylation. Transesterification with vinyl esters on the other hands mostly yields the desired products and only a minor DSacetate (Hinner et al. 2016). The result may be traced to a different mechanism, namely the reaction does not proceed via the formation of a mixed anhydride but involves the transfer of the acyl moiety to an intermediate carbene specie that is formed by deprotonation of the imidazolium cation in position 2 (Chen et al. 2017).
Other cellulose esters
Acylation of cellulose in IL is usually performed at ≥ 80 °C. At these elevated temperatures, the viscosity of IL and cellulose/IL solutions is reasonably low, which guarantees efficient heat- and mass-transfer during the reaction. The synthesis of cellulose sulfonates and cellulose sulfates (i.e., sulfuric acid half esters) requires milder reaction conditions of ≤ 25 °C to prevent severe polymer degradation. Thus, co-solvents have to be added to diminish the adverse effect of high solution viscosity (Fig. 27) (Gericke et al. 2011; Lv et al. 2012). It should be noted in this context that sulfation and tosylation in the lower viscous imidazoliuam acetates yield acetylated cellulose instead of the desired products (Köhler et al. 2007; Ebner et al. 2008). The derivatization reagents form mixed anhydrides with acetate counterions that act as acetylation agents.

Reprinted and modified with permission from Lv et al. (2012), copyright (2012), with permission from Elsevier
Viscosity (η) of a ionic liquid (AmimCl: 1-allyl-3-methylimidazolium chloride; BmimCl: 1-butyl-3-methylimidazolium chloride)/DMS) mixtures a without and b with dissolved cellulose at 25 °C and different molar fractions (χs) of DMSO.
Cellulose sulfates with DS up to 1.7 were prepared in mixtures of [BuMeIm]Cl and DMF (Gericke et al. 2009). As a result of the completely homogeneous reaction course, uniform distribution of sulfate groups along the polymer back-bone was achieved, which resulted in good water solubility of the products even at rather low DS around 0.2–0.3. Comparable products cannot be prepared in DAS/LiCl based cellulose solvents due to coagulation of the reaction mixture during the sulfation.
Synthesis of p-toluensulfonic acid ester (tosylates) of cellulose in IL is performed at room temperature in order to prevent the excessive formation of 6-chloro-6-deoxy cellulose (Gericke et al. 2012a, b). Thus, co-solvents such as pyridine or DAS need to be employed. In the latter case, an additional base such as 1-butylimidazole is required. Ethylenediamine that is usually employed in the tosylation of cellulose in LiCl/DMAc cannot be used because it is immiscible with IL (Rahn et al. 1996). Under identical reaction conditions, tosylation in LiCl/DMAc at 25 °C proceeds with a higher reaction efficiency whereas the tendency to produce 6-chloro-6-deoxy moieties is less pronounced for the synthesis in IL.
In addition to cellulose sulfonates and sulfates, aryl phosphates have been prepared in [AlMeIm]Cl by conversion of the polysaccharide with diphenyl chlorophosphate (Xiao et al. 2014). DS values up to 1.23 were obtained under mild conditions (50 °C, 5–10 h) using 3 mol-equivalents of the reagent. It was also possible to obtain mixed cellulose acetate phosphate esters. The advantage of IL is that the synthesis can be performed in a homogeneous, one-pot reaction because all reagents and products are soluble in the reaction medium.
The synthesis of cellulose aryl carbonates is a good example for reactions in which ILs are superior over DAS based cellulose solvents. The conversion of cellulose with phenyl chloroformate yields cellulose phenyl carbonates. If the reaction is carried out in LiCl/DMAc, DS values of up to 2.0 are obtained but only with 10 equivalents of reagent/AGU, which corresponds to a reaction efficiency of only 20% (Elschner et al. 2013). This is due to Vilsmeier-type side reactions of chloroformate with DAS such as DMSO and DMAc that consume the reagent (Thota et al. 2009; Kwak and Gong 2013). Using a [BuMeIm]Cl/pyridine mixture as reaction medium, undesired side reactions is suppressed. Reaction efficiency increases significantly up to 90% (Elschner et al. 2014). Even fully functionalized cellulose phenyl carbonates with a DS of 3 were obtained that provided NMR-spectra with a high resolution due to the very uniform molecular structure (Fig. 28). In addition to aryl carbonates, cellulose alkyl carbonates were prepared in two different IL, [EtMeIm]AcO and methyltrioctylphosphonium acetate.

Reprinted and modified with permission from Elschner et al. (2014), copyright (2014) WILEY
a1H NMR and, b13C NMR spectra of cellulose phenyl carbonate with a degree of substitution of 3.
ImIL proved to be efficient solvents for the homogeneous esterification of cellulose. They should always be considered as potential reaction media when developing new synthesis approaches. It is important to consider critically the individual advantages (e.g., high dissolution power for cellulose, reagents and products) and disadvantages (e.g., high viscosity, need for recycling strategies). IL are often described as “designer solvents” due to the vast number of combinations of potential anions and cations. Thus, new developments can be expected in the future.
Etherification of cellulose in ionic liquids
Despite the commercial importance of cellulose ethers such as CMC and cellulose hydroxyalkyl ethers, reports on etherification of cellulose in IL are scarce. This is mostly a result of the limited miscibility of the derivatization reagents used (e.g., solid alkali, gaseous/volatile and/or hydrophobic alkylation reagents). The synthesis of CMC, one of the most important commercial cellulose derivatives, was not successful in IL and yielded small DS-values < 0.5 (Heinze et al. 2005). Solid NaOH was used as a base, which resulted in gelation of the reaction mixture despite the use of DMSO as co-solvent. Hydroxyalkylation of cellulose was achieved in [EtMeIm]AcO, [BuMeIm]Cl, and several ammonium-based ILs (Köhler et al. 2010). In this process, co-solvents such as DMSO and DMF were added to improve the dissolution of the gaseous reagents, namely ethylene oxide and propylene oxide. The ring opening reaction is catalyzed by acetate either coming from the IL counterion (for [EtMeIm]AcO) or magenesium-/potassium acetate. Additionally, the etherification of cellulose with glycidol in IL has been reported (Kakibe et al. 2017).
Trityl ethers of cellulose are of interest for the preparation of cellulose derivatives with regioselective substitution pattern (Kondo and Gray 1991). The bulky trityl moieties are preferably introduced at the primary OH-group (O-6) and can be removed selectively under acidic conditions. Thus, it can be employed as protecting group for obtaining regioselective derivatization at position O-2 and O-3. Trityl celluloses with DS values of up to 1.5 were obtained in ILs by conversion of the polysaccharide with trityl chloride in the presence of excess pyridine (Erdmenger et al. 2007; Xia et al. 2014; Lv et al. 2015). The maximum DS was increased to 1.8 using the more reactive p-methoxytrityl chloride. Mild reaction conditions and DS values close to 1.0 (i.e., complete protection of O-6) are desired for TC in the context of protecting group applications. IL appear to be favorable in this regard because said DS values are achieved within 1–10 h whereas tritylation in LiCl/DMAc requires ≥ 24 h.
Cellulose silyl ethers are renowned for their self-assembly into thin films (e.g., by spin-coating, Langmuir–Blodgett-/Langmuir–Schaefer deposition) that can be converted into well-defined cellulosic films by acid hydrolysis (Kontturi et al. 2003; Tammelin et al. 2006). Different ILs, including [EtMeIm]AcO, [BuMeIm]Cl, and [BuMeIm]propionate were studied as reaction media for the synthesis of trimethylsilyl cellulose (Köhler et al. 2008; Mormann and Wezstein 2009; Kostag et al. 2010; Liebert et al. 2011). The reaction proceeds heterogeneously because 1,1,1,3,3,3-hexamethylendisilazane, the silylation reagent employed, is immiscible with IL. Moreover, trimethylsilyl cellulose with a DS > 2.0 is insoluble in IL. Completely homogeneous synthesis of TMSC with a DS up to 2.9 was achieved in mixtures of IL with chloroform as co-solvent (Köhler et al. 2008).
Conclusions and perspectives
The last 25 years witnessed the introduction of diverse cellulose solvents that allowed cellulose dissolution and derivatization under homogeneous conditions, with much better yields and control of regioselectivity. These solvents include strong electrolytes/DAS; aqueous and nonaqueous solutions of QAEs, and a myriad of pure ILs and their solutions in DAS. Although we focused our attention on esters and ethers of cellulose, the above-mentioned advantages apply to other classes of cellulose derivatives. Thanks to the introduction of these novel solvents, additional themes are being actively investigated, including potential applications of cellulose solutions for its regeneration as fibers (the Ioncell-F is an example), systematic investigation of solvent structure/performance relationship, increasing the atom-efficiency of the derivatization reactions, and environmentally acceptable and efficient solvent component recovery. An important purpose of using these solvents is production of specialty cellulose derivatives where structure control and reproducible physical forms (nanospheres, nanofibers, etc.) is crucial to their (biomedical and pharmacological) applications. As the demand for such products is expected to grow, there is an urgent need to assess the green chemistry aspects of the homogeneous reaction scheme, in particular, economics of the process and its environmental impact (Table 5).
Abbreviations
- AcO:
-
Acetate
- AGU:
-
Anhydroglucose unit
- [AlMeIm]Cl:
-
1-Allyl-3-methylimidazolium chloride
- BC:
-
Bacterial cellulose
- [BuMeIm]Cl:
-
1-(n-Butyl)-3-methylimidazolium chloride
- CDI:
-
Carbonyldiimidazole
- CT:
-
Cellulose tosylate
- CHPTMA Cl:
-
(3-Chloro-2-hydroxypropyl)trimethyl-ammonium chloride
- DAS:
-
Dipolar aprotic solvent
- DBN:
-
1,5-Diazabicyclo[4.3.0]non-5-ene
- DBU:
-
1,8-Diazabicyclo[5.4.0]undec-7-ene
- DCC:
-
N,N′-Dicyclohexylcarbodiimide
- DLS:
-
Dynamic light scattering
- DMAc:
-
N,N-Dimethylacetamide
- DMF:
-
N,N-Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- DP:
-
Average degree of polymerization
- DS:
-
Average degree of substitution
- EPTMA Cl:
-
(2,3-Epoxypropyl)trimethylammonium chloride
- E T(30):
-
Solvent empirical polarity parameter (in kcal mol−1) as determined by the solvatochromic probe 2,6-diphenyl-4-(2,4,6-triphenylpyridin-1-ium-1-yl)phenolate
- [EtMeIm]AcO:
-
1-Ethyl-3-methylimidazolium acetate
- HEC:
-
Hydroxyethyl cellulose
- HPC:
-
Hydroxypropyl cellulose
- Ic :
-
Index of crystallinity
- IL:
-
Ionic liquid
- ImIL:
-
Imidazolium based IL
- log P:
-
Partition coefficient of a substance between (mutually saturated) n-octanol and water
- MALS:
-
Multiangle light scattering
- MC:
-
Methyl cellulose
- MD:
-
Molecular dynamic simulations
- MM:
-
Average molar mass5
- MS:
-
Average degree of molar substitution
- [N2228]Cl:
-
Triethyl(n-octyl)ammonium chloride
- NMMO:
-
N-Methylmorpholine-N-oxide
- QAE:
-
Quaternary ammonium electrolyte
- SA:
-
Solvent Lewis acidity
- SB:
-
Solvent Lewis basicity
- [TBA]F·3H2O:
-
Tetra(n-butyl)ammonium fluoride trihydrate
- TC:
-
Trityl cellulose
- TsCl:
-
Tosyl chloride
References
Abe M, Sugimura K, Nishio Y (2016) Regioselectivity in acetylation of cellulose in ionic liquids. ChemistrySelect 1:2474–2478. https://doi.org/10.1002/slct.201600520
Abe M, Sugimura K, Nishiyama Y, Nishio Y (2017) Rapid benzylation of cellulose in tetra-n-butylphosphonium hydroxide aqueous solution at room temperature. ACS Sustain Chem Eng 5:4505–4510. https://doi.org/10.1021/acssuschemeng.7b00492
Achtel C, Heinze T (2016) Homogeneous acetylation of cellulose in the new solvent triethyloctylammonium chloride in combination with organic liquids. Macromol Chem Phys 217:2041–2048. https://doi.org/10.1002/macp.201600217
Achtel C, Jedvert K, Kosan B et al (2017) Dissolution capacity of novel cellulose solvents based on triethyloctylammonium chloride. Macromol Chem Phys 218:1700208. https://doi.org/10.1002/macp.201700208
Achtel C, Jedvert K, Kostag M et al (2018) Surprising insensitivity of homogeneous acetylation of cellulose dissolved in triethyl(n-octyl)ammonium chloride/molecular solvent on the solvent polarity. Macromol Mater Eng 305:1800032. https://doi.org/10.1002/mame.201800032
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications—a review. J Adv Res 6:105–121. https://doi.org/10.1016/j.jare.2013.07.006
Alves L, Medronho B, Antunes FE et al (2016a) Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. ellulose 23:247–258. https://doi.org/10.1007/s10570-015-0809-6
Alves L, Medronho B, Antunes FE et al (2016b) Dissolution state of cellulose in aqueous systems. 2. Acidic solvents. Carbohydr Polym 151:707–715. https://doi.org/10.1016/j.carbpol.2016.06.015
Amigues E, Hardacre C, Keane G et al (2006) Ionic liquids—media for unique phosphorus chemistry. Chem Commun 1:72–74. https://doi.org/10.1039/b509248e
Amyes TL, Diver ST, Richard JP et al (2004) Formation and stability of N-heterocyclic carbenes in water: the carbon acid pKa of imidazolium cations in aqueous solution. J Am Chem Soc 126:4366–4374. https://doi.org/10.1021/ja039890j
Anastas PT, Wasserscheid P, Stark A (2013) Green solvents: ionic liquids, 1st edn. Wiley-VCH, Weinheim
Aono H, Tatsumi D, Matsumoto T (2006) Characterization of aggregate structure in mercerized cellulose/LiCl·DMAc solution using light scattering and rheological measurements. Biomacromolecules 7:1311–1317. https://doi.org/10.1021/bm050889h
Ass BAP, Frollini E, Heinze T (2004) Studies on the homogeneous acetylation of cellulose in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride trihydrate. Macromol Biosci 4:1008–1013. https://doi.org/10.1002/mabi.200400088
ASTM D871 - 96 (2004) Standard test methods of testing cellulose acetate. https://doi.org/10.1520/d0871-96r04
Austin PR (1977) Chitin solution, US05728257
Baba K, Ono H, Itoh E et al (2006) Kinetic study of thermal Z to E isomerization reactions of azobenzene and 4-dimethylamino-4′-nitroazobenzene in ionic liquids [1-R-3-methylimidazolium bis(trifluoromethylsulfonyl)imide with R = butyl, pentyl, and hexyl]. Chem A Eur J 12:5328–5333. https://doi.org/10.1002/chem.200600081
Bao Y, Qian H, Lu Z, Cui S (2015) Revealing the hydrophobicity of natural cellulose by single-molecule experiments. Macromolecules 48:3685–3690. https://doi.org/10.1021/acs.macromol.5b00260
Barthel S, Heinze T (2006) Acylation and carbanilation of cellulose in ionic liquids. Green Chem 8:301–306. https://doi.org/10.1039/B513157J
Bergenstråhle M, Wohlert J, Himmel ME, Brady JW (2010) Simulation studies of the insolubility of cellulose. Carbohyd Res 345:2060–2066. https://doi.org/10.1016/j.carres.2010.06.017
Berger S, Braun S (2004) 200 and more NMR experiments. Wiley-VCH, London
Bialik E, Stenqvist B, Fang Y et al (2016) Ionization of cellobiose in aqueous alkali and the mechanism of cellulose dissolution. J Phys Chem Lett 7:5044–5048. https://doi.org/10.1021/acs.jpclett.6b02346
Bioni TA, Malek NI, Seoud OAE (2018) Kinetics of cellulose acylation with carboxylic anhydrides and N-acylimidazoles in ionic liquid/molecular solvent mixtures: relevance to the synthesis of mixed cellulose esters. Lenzinger Berichte 94:57–66
Buchanan CM, Buchanan NL, Guzman-Morales E (2013) Regioselectively substituted cellulose esters produced in a tetraalkylammonium alkylphosphate ionic liquid process and products produced therefrom EP2419453A1, 22 Feb 2012
Budtova T, Navard P (2015) Cellulose in NaOH–water based solvents: a review. Cellulose 23:5–55. https://doi.org/10.1007/s10570-015-0779-8
Burchard W (2003) Solubility and solution structure of cellulose derivatives. Cellulose 10:213–225. https://doi.org/10.1023/A:1025160620576
Burchard W, Habermann N, Klüfers P et al (1994) Cellulose in Schweizer’s reagent: a stable, polymeric metal complex with high chain stiffness. Angew Chem Int Ed Engl 33:884–887. https://doi.org/10.1002/anie.199408841
Cai J, Zhang L (2005) Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol Biosci 5:539–548. https://doi.org/10.1002/mabi.200400222
Cai J, Zhang L (2006) Unique gelation behavior of cellulose in NaOH/urea aqueous solution. Biomacromol 7:183–189. https://doi.org/10.1021/bm0505585
Cai J, Liu Y, Zhang L (2006) Dilute solution properties of cellulose in LiOH/urea aqueous system. J Polym Sci Part B Polym Phys 44:3093–3101. https://doi.org/10.1002/polb.20938
Cai J, Zhang L, Chang C et al (2007) Hydrogen-bond-induced inclusion complex in aqueous cellulose/LiOH/urea solution at low temperature. ChemPhysChem 8:1572–1579. https://doi.org/10.1002/cphc.200700229
Cai J, Zhang L, Liu S et al (2008) Dynamic self-assembly induced rapid dissolution of cellulose at low temperatures. Macromolecules 41:9345–9351. https://doi.org/10.1021/ma801110g
Canal JP, Ramnial T, Dickie DA, Clyburne JAC (2006) From the reactivity of N-heterocyclic carbenes to new chemistry in ionic liquids. Chem Commun 17:1809–1818. https://doi.org/10.1039/b512462j
Cao Y, Wu J, Meng T et al (2007) Acetone-soluble cellulose acetates prepared by one-step homogeneous acetylation of cornhusk cellulose in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Carbohyd Polym 69:665–672. https://doi.org/10.1016/j.carbpol.2007.02.001
Cao Y, Zhang J, He J et al (2010) Homogeneous acetylation of cellulose at relatively high concentrations in an ionic liquid. Chin J Chem Eng 18:515–522. https://doi.org/10.1016/S1004-9541(10)60252-2
Cao Y, Li H, Zhang J (2011) Homogeneous synthesis and characterization of cellulose acetate butyrate (CAB) in 1-allyl-3-methylimidazolium chloride (AmimCl) ionic liquid. Ind Eng Chem Res 50:7808–7814. https://doi.org/10.1021/ie2004362
Cao X, Sun S, Peng X et al (2013) Rapid synthesis of cellulose esters by transesterification of cellulose with vinyl esters under the catalysis of NaOH or KOH in DMSO. J Agric Food Chem 61:2489–2495. https://doi.org/10.1021/jf3055104
Cao X, Peng X, Zhong L et al (2014) A novel transesterification system to rapidly synthesize cellulose aliphatic esters. Cellulose 21:581–594. https://doi.org/10.1007/s10570-013-0102-5
Casarano R, Fidale LC, Lucheti CM et al (2011) Expedient, accurate methods for the determination of the degree of substitution of cellulose carboxylic esters: application of UV–Vis spectroscopy (dye solvatochromism) and FTIR. Carbohyd Polym 83:1285–1292. https://doi.org/10.1016/j.carbpol.2010.09.035
Casarano R, Pires PAR, El Seoud OA (2014) Acylation of cellulose in a novel solvent system: solution of dibenzyldimethylammonium fluoride in DMSO. Carbohyd Polym 101:444–450. https://doi.org/10.1016/j.carbpol.2013.09.043
Chang C, Duan B, Zhang L (2009a) Fabrication and characterization of novel macroporous cellulose–alginate hydrogels. Polymer 50:5467–5473. https://doi.org/10.1016/j.polymer.2009.06.001
Chang C, Peng J, Zhang L, Pang D-W (2009b) Strongly fluorescent hydrogels with quantum dots embedded in cellulose matrices. J Mater Chem 19:7771–7776. https://doi.org/10.1039/B908835K
Chang C, Duan B, Cai J, Zhang L (2010a) Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur Polym J 46:92–100. https://doi.org/10.1016/j.eurpolymj.2009.04.033
Chang C, Zhang L, Zhou J et al (2010b) Structure and properties of hydrogels prepared from cellulose in NaOH/urea aqueous solutions. Carbohyd Polym 82:122–127. https://doi.org/10.1016/j.carbpol.2010.04.033
Chang C, He M, Zhou J, Zhang L (2011) Swelling behaviors of pH- and salt-responsive cellulose-based hydrogels. Macromolecules 44:1642–1648. https://doi.org/10.1021/ma102801f
Chang C, Teramoto Y, Nishio Y (2013) Synthesis of O-(2,3-dihydroxypropyl) cellulose in NaOH/urea aqueous solution: as a precursor for introducing “necklace-like” structure. J Polym Sci Part A Polym Chem 51:3590–3597. https://doi.org/10.1002/pola.26773
Chen J, Su M, Zhang X et al (2014) The role of cations in homogeneous succinoylation of mulberry wood cellulose in salt-containing solvents under mild conditions. Cellulose 21:4081–4091. https://doi.org/10.1007/s10570-014-0429-6
Chen W, Feng Y, Zhang M et al (2015) Homogeneous benzoylation of cellulose in 1-allyl-3-methylimidazolium chloride: hammett correlation, mechanism and regioselectivity. RSC Adv 5:58536–58542. https://doi.org/10.1039/C5RA08911E
Chen M-J, Li R-M, Zhang X-Q et al (2017) Homogeneous transesterification of sugar cane bagasse toward sustainable plastics. ACS Sustain Chem Eng 5:360–366. https://doi.org/10.1021/acssuschemeng.6b01735
Chen H, Yang F, Du J et al (2018a) Efficient transesterification reaction of cellulose with vinyl esters in DBU/DMSO/CO2 solvent system at low temperature. Cellulose 25:6935–6945. https://doi.org/10.1007/s10570-018-2078-7
Chen Y, Pötschke P, Pionteck J et al (2018b) Smart cellulose/graphene composites fabricated by in situ chemical reduction of graphene oxide for multiple sensing applications. J Mater Chem A 6:7777–7785. https://doi.org/10.1039/C8TA00618K
Ciacco GT, Liebert TF, Frollini E, Heinze TJ (2003) Application of the solvent dimethyl sulfoxide/tetrabutyl-ammonium fluoride trihydrate as reaction medium for the homogeneous acylation of Sisal cellulose. Cellulose 10:125–132. https://doi.org/10.1023/A:1024064018664
Ciacco GT, Morgado DL, Frollini E et al (2010) Some aspects of acetylation of untreated and mercerized sisal cellulose. J Braz Chem Soc 21:71–77. https://doi.org/10.1590/S0103-50532010000100012
Ciolacu D, Rudaz C, Vasilescu M, Budtova T (2016) Physically and chemically cross-linked cellulose cryogels: structure, properties and application for controlled release. Carbohyd Polym 151:392–400. https://doi.org/10.1016/j.carbpol.2016.05.084
Das D, Das B, Hazra DK (2002) Conductance of some 1:1 electrolytes in N,N-dimethylacetamide at 25°C. J Solut Chem 31:425–431. https://doi.org/10.1023/A:1015815500299
Dissanayake N, Thalangamaarachchige VD, Troxell S et al (2018) Substituent effects on cellulose dissolution in imidazolium-based ionic liquids. Cellulose 25:6887–6900. https://doi.org/10.1007/s10570-018-2055-1
Dupont A-L (2003) Cellulose in lithium chloride/N,N-dimethylacetamide, optimisation of a dissolution method using paper substrates and stability of the solutions. Polymer 44:4117–4126. https://doi.org/10.1016/S0032-3861(03)00398-7
Dutta T, Shi J, Sun J et al (2015) Ionic liquid pretreatment of lignocellulosic biomass for biofuels and chemicals. In: Bogel-Lukasik R (ed) Ionic liquids in the biorefinery concept. The Royal Society of Chemistry, Cambridge, pp 65–94
Ebner G, Schiehser S, Potthast A, Rosenau T (2008) Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett 49:7322–7324. https://doi.org/10.1016/j.tetlet.2008.10.052
Edgar KJ, Buchanan CM, Debenham JS et al (2001) Advances in cellulose ester performance and application. Prog Polym Sci 26:1605–1688. https://doi.org/10.1016/S0079-6700(01)00027-2
Efimova A, Varga J, Matuschek G et al (2018) Thermal resilience of imidazolium-based ionic liquids—studies on short- and long-term thermal stability and decomposition mechanism of 1-alkyl-3-methylimidazolium halides by thermal analysis and single-photon ionization time-of-flight mass spectrometry. J Phys Chem B 122:8738–8749. https://doi.org/10.1021/acs.jpcb.8b06416
Egal M, Budtova T, Navard P (2008) The dissolution of microcrystalline cellulose in sodium hydroxide-urea aqueous solutions. Cellulose 15:361–370. https://doi.org/10.1007/s10570-007-9185-1
El Seoud OA, Marson GA, Ciacco GT, Frollini E (2000) An efficient, one-pot acylation of cellulose under homogeneous reaction conditions. Macromol Chem Phys 201:882–889. https://doi.org/10.1002/(SICI)1521-3935(20000501)201:8%3c882:AID-MACP882%3e3.0.CO;2-I
El Seoud OA, Fidale LC, Ruiz N et al (2008) Cellulose swelling by protic solvents: which properties of the biopolymer and the solvent matter? Cellulose 15:371–392. https://doi.org/10.1007/s10570-007-9189-x
El Seoud OA, da Silva VC, Possidonio S et al (2011) Microwave-assisted derivatization of cellulose, 2—the surprising effect of the structure of ionic liquids on the dissolution and acylation of the biopolymer. Macromol Chem Phys 212:2541–2550. https://doi.org/10.1002/macp.201100348
El Seoud OA, Nawaz H, Arêas E et al (2013) Chemistry and applications of polysaccharide solutions in strong electrolytes/dipolar aprotic solvents: an overview. Molecules 18:1270–1313. https://doi.org/10.3390/molecules18011270
Eliza MY, Shahruddin M, Noormaziah J, Rosli WDW (2015) Carboxymethyl cellulose (CMC) from oil palm empty fruit bunch (OPEFB) in the new solvent dimethyl sulfoxide (DMSO)/tetrabutylammonium fluoride (TBAF). J Phys Conf Ser 622:012026. https://doi.org/10.1088/1742-6596/622/1/012026
Elschner T, Ganske K, Heinze T (2013) Synthesis and aminolysis of polysaccharide carbonates. Cellulose 20:339–353. https://doi.org/10.1007/s10570-012-9819-9
Elschner T, Kötteritzsch M, Heinze T (2014) Synthesis of cellulose tricarbonates in 1-butyl-3-methylimidazolium chloride/pyridine. Macromol Biosci 14:161–165. https://doi.org/10.1002/mabi.201300345
Enders D, Niemeier O, Henseler A (2007) Organocatalysis by N-heterocyclic carbenes. Chem Rev 107:5606–5655. https://doi.org/10.1021/cr068372z
Erdmenger T, Haensch C, Hoogenboom R, Schubert US (2007) Homogeneous tritylation of cellulose in 1-butyl-3-methylimidazolium chloride. Macromol Biosci 7:440–445. https://doi.org/10.1002/mabi.200600253
Ferreira DC, Bastos GS, Pfeifer A et al (2016) Cellulose carboxylate/tosylate mixed esters: synthesis, properties and shaping into microspheres. Carbohyd Polym 152:79–86. https://doi.org/10.1016/j.carbpol.2016.06.075
Fidale LC, Köhler S, Prechtl MHG et al (2006) Simple, expedient methods for the determination of water and electrolyte contents of cellulose solvent systems. Cellulose 13:581–592. https://doi.org/10.1007/s10570-005-9036-x
Fidale LC, Possidonio S, Seoud OAE (2009) Application of 1-allyl-3-(1-butyl)imidazolium chloride in the synthesis of cellulose esters: properties of the ionic liquid, and comparison with other solvents. Macromol Biosci 9:813–821. https://doi.org/10.1002/mabi.200900034
Fidale LC, Heinze T, El Seoud OA (2013) Perichromism: a powerful tool for probing the properties of cellulose and its derivatives. Carbohyd Polym 93:129–134. https://doi.org/10.1016/j.carbpol.2012.06.061
Fox SC, Edgar KJ (2012) Staudinger reduction chemistry of cellulose: synthesis of selectively O-acylated 6-amino-6-deoxy-cellulose. Biomacromolecules 13:992–1001. https://doi.org/10.1021/bm2017004
Fox SC, Li B, Xu D, Edgar KJ (2011) Regioselective esterification and etherification of cellulose: a review. Biomacromolecules 12:1956–1972. https://doi.org/10.1021/bm200260d
Freire CSR, Silvestre AJD, Pascoal Neto C, Rocha RMA (2005) An efficient method for determination of the degree of substitution of cellulose esters of long chain aliphatic acids. Cellulose 12:449–458. https://doi.org/10.1007/s10570-005-2203-2
Furuhata K, Koganei K, Chang H-S et al (1992) Dissolution of cellulose in lithium bromide-organic solvent systems and homogeneous bromination of cellulose with N-bromosuccinimide-triphenylphosphine in lithium bromide-N,N-dimethylacetamide. Carbohyd Res 230:165–177. https://doi.org/10.1016/S0008-6215(00)90519-6
Gabriel L, Heinze T (2018) Diversity of polysaccharide structures designed by aqueous Ugi-multi-compound reaction. Cellulose 25:2849–2859. https://doi.org/10.1007/s10570-018-1754-y
Gagnaire D, Saint-Germain J, Vincendon M (1983) NMR evidence of hydrogen bonds in cellulose solutions. J Appl Polym Sci Appl Polym Symp 37:261–275
Gale E, Wirawan RH, Silveira RL et al (2016) Directed discovery of greener cosolvents: new csolvents for use in ionic liquid based organic electrolyte solutions for cellulose dissolution. ACS Sustain Chem Eng 4:6200–6207. https://doi.org/10.1021/acssuschemeng.6b02020
Geng H (2018) A one-step approach to make cellulose-based hydrogels of various transparency and swelling degrees. Carbohyd Polym 186:208–216. https://doi.org/10.1016/j.carbpol.2018.01.031
Gentile L, Olsson U (2016) Cellulose–solvent interactions from self-diffusion NMR. Cellulose 23:2753–2758. https://doi.org/10.1007/s10570-016-0984-0
Gericke M, Liebert T, Heinze T (2009) Interaction of ionic liquids with polysaccharides, 8—synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol Biosci 9:343–353. https://doi.org/10.1002/mabi.200800329
Gericke M, Liebert T, Seoud OAE, Heinze T (2011) Tailored media for homogeneous cellulose chemistry: ionic liquid/co-solvent mixtures. Macromol Mater Eng 296:483–493. https://doi.org/10.1002/mame.201000330
Gericke M, Fardim P, Heinze T (2012a) Ionic liquids—promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 17:7458–7502. https://doi.org/10.3390/molecules17067458
Gericke M, Schaller J, Liebert T et al (2012b) Studies on the tosylation of cellulose in mixtures of ionic liquids and a co-solvent. Carbohyd Polym 89:526–536. https://doi.org/10.1016/j.carbpol.2012.03.040
Glasser WG (2004) 6. Prospects for future applications of cellulose acetate. Macromol Symp 208:371–394. https://doi.org/10.1002/masy.200450416
Gomez JAC, Erler UW, Klemm DO (1996) 4-methoxy substituted trityl groups in 6-O protection of cellulose: homogeneous synthesis, characterization, detritylation. Macromol Chem Phys 197:953–964. https://doi.org/10.1002/macp.1996.021970316
Gubitosi M, Duarte H, Gentile L et al (2016) On cellulose dissolution and aggregation in aqueous tetrabutylammonium hydroxide. Biomacromolecules 17:2873–2881. https://doi.org/10.1021/acs.biomac.6b00696
Gupta KM, Jiang J (2015) Cellulose dissolution and regeneration in ionic liquids: a computational perspective. Chem Eng Sci 121:180–189. https://doi.org/10.1016/j.ces.2014.07.025
Hallett JP, Welton T (2011) Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev 111:3508–3576. https://doi.org/10.1021/cr1003248
Hanabusa H, Izgorodina EI, Suzuki S et al (2018) Cellulose-dissolving protic ionic liquids as low cost catalysts for direct transesterification reactions of cellulose. Green Chem 20:1412–1422. https://doi.org/10.1039/C7GC03603E
Heinze T (1998) New ionic polymers by cellulose functionalization. Macromol Chem Phys 199:2341–2364. https://doi.org/10.1002/(SICI)1521-3935(19981101)199:11%3c2341:AID-MACP2341%3e3.0.CO;2-J
Heinze T, Liebert T (2012) Celluloses and polyoses/hemicelluloses. In: Matyjaszewski K, Möller M (eds) Polymer science: a comprehensive reference. Elsevier, Amsterdam, pp 83–152
Heinze T, Rahn K (1997) Cellulose-p-toluenesulfonates: a valuable intermediate in cellulose chemistry. Macromol Symp 120:103–113. https://doi.org/10.1002/masy.19971200112
Heinze T, Erler U, Nehls I, Klemm D (1994a) Determination of the substituent pattern of heterogeneously and homogeneously synthesized carboxymethyl cellulose by using high-performance liquid chromatography. Die Angew Makromol Chem 215:93–106. https://doi.org/10.1002/apmc.1994.052150108
Heinze T, Röttig K, Nehls I (1994b) Synthesis of 2,3-O-carboxymethylcellulose. Macromol Rapid Commun 15:311–317. https://doi.org/10.1002/marc.1994.030150403
Heinze T, Rahn K, Jaspers M, Berghmans H (1996) p-Toluenesulfonyl esters in cellulose modifications: acylation of remaining hydroxyl groups. Macromol Chem Phys 197:4207–4224. https://doi.org/10.1002/macp.1996.021971218
Heinze T, Liebert T, Klüfers P, Meister F (1999) Carboxymethylation of cellulose in unconventional media. Cellulose 6:153–165. https://doi.org/10.1023/A:1009271427760
Heinze T, Dicke R, Koschella A et al (2000) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol Chem Phys 201:627–631. https://doi.org/10.1002/(SICI)1521-3935(20000301)201:6%3c627:AID-MACP627%3e3.0.CO;2-Y
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium in cellulose functionalization. Macromol Biosci 5:520–525. https://doi.org/10.1002/mabi.200500039
Heinze T, Liebert T, Koschella A (2006) Esterification of polysaccharides. Springer, Berlin
Heinze T, Lincke T, Fenn D, Koschella A (2008) Efficient allylation of cellulose in dimethyl sulfoxide/tetrabutylammonium fluoride trihydrate. Polym Bull 61:1–9. https://doi.org/10.1007/s00289-008-0919-5
Heinze T, Seoud OAE, Koschella A (2018a) Principles of cellulose derivatization. In: Cellulose derivatives. Springer, Cham, pp 259–292
Heinze T, Seoud OAE, Koschella A (2018b) Cellulose esters. In: Cellulose derivatives. Springer, Cham, pp 293–427
Heinze T, Seoud OAE, Koschella A (2018c) Cellulose activation and dissolution. In: Cellulose derivatives. Springer, Cham, pp 173–257
Heinze T, Seoud OAE, Koschella A (2018d) Etherification of cellulose. In: Cellulose derivatives. Springer, Cham, pp 429–477
Hinner LP, Wissner JL, Beurer A et al (2016) Homogeneous vinyl ester-based synthesis of different cellulose derivatives in 1-ethyl-3-methyl-imidazolium acetate. Green Chem 18:6099–6107. https://doi.org/10.1039/C6GC02005D
Hirrien M, Desbrières J, Rinaudo M (1996) Physical properties of methylcelluloses in relation with the conditions for cellulose modification. Carbohyd Polym 31:243–252. https://doi.org/10.1016/S0144-8617(96)00118-X
Hu H, You J, Gan W et al (2015) Synthesis of allyl cellulose in NaOH/urea aqueous solutions and its thiol–ene click reactions. Polym Chem 6:3543–3548. https://doi.org/10.1039/C5PY00301F
Huang F-Y (2012) Thermal properties and thermal degradation of cellulose tri-stearate (CTs). Polymers 4:1012–1024. https://doi.org/10.3390/polym4021012
Huang K, Wang B, Cao Y et al (2011a) Homogeneous preparation of cellulose acetate propionate (CAP) and cellulose acetate butyrate (CAB) from sugarcane bagasse cellulose in ionic liquid. J Agric Food Chem 59:5376–5381. https://doi.org/10.1021/jf104881f
Huang K, Xia J, Li M et al (2011b) Homogeneous synthesis of cellulose stearates with different degrees of substitution in ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohyd Polym 83:1631–1635. https://doi.org/10.1016/j.carbpol.2010.10.020
Huang Y-B, Xin P-P, Li J-X et al (2016) Room-temperature dissolution and mechanistic investigation of cellulose in a tetra-butylammonium acetate/dimethyl sulfoxide system. ACS Sustain Chem Eng 4:2286–2294. https://doi.org/10.1021/acssuschemeng.5b01749
Hussain MA, Liebert T, Heinze T (2004) Acylation of cellulose with N,N-carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol Rapid Commun 25:916–920. https://doi.org/10.1002/marc.200300308
Idström A, Gentile L, Gubitosi M et al (2017) On the dissolution of cellulose in tetrabutylammonium acetate/dimethyl sulfoxide: a frustrated solvent. Cellulose 24:3645–3657. https://doi.org/10.1007/s10570-017-1370-2
Ikeda I, Washino K, Maeda Y (2003) Graft polymerization of cyclic compounds on cellulose dissolved in tetrabutylammonium fluoride/dimethyl sulfoxide. Sen’i Gakkaishi 59:110–114. https://doi.org/10.2115/fiber.59.110
Ishii D, Tatsumi D, Matsumoto T (2008) Effect of solvent exchange on the supramolecular structure, the molecular mobility and the dissolution behavior of cellulose in LiCl/DMAc. Carbohyd Res 343:919–928. https://doi.org/10.1016/j.carres.2008.01.035
Isogai A (1997) NMR analysis of cellulose dissolved in aqueous NaOH solutions. Cellulose 4:99–107. https://doi.org/10.1023/A:1018471419692
Isogai A, Ishizu A, Nakano J (1984a) Distribution of substituents in cellulose ethers prepared in aqueous and non-aqueous systems. Sen’i Gakkaishi 40:T504–T511. https://doi.org/10.2115/fiber.40.12_T504
Isogai A, Ishizu A, Nakano J (1984b) Preparation of tri-O-benzylcellulose by the use of nonaqueous cellulose solvents. J Appl Polym Sci 29:2097–2109. https://doi.org/10.1002/app.1984.070290617
Iwata T, Azuma J-I, Okamura K et al (1992) Preparation and n.m.r. assignments of cellulose mixed esters regioselectively substituted by acetyl and propanoyl groups. Carbohyd Res 224:277–283. https://doi.org/10.1016/0008-6215(92)84113-7
Jessop PG, Heldebrant DJ, Li X et al (2005) Green chemistry: reversible nonpolar-to-polar solvent. Nature 436:1102. https://doi.org/10.1038/4361102a
Jogunola O, Eta V, Hedenström M et al (2016) Ionic liquid mediated technology for synthesis of cellulose acetates using different co-solvents. Carbohyd Polym 135:341–348. https://doi.org/10.1016/j.carbpol.2015.08.092
Joly N, Granet R, Branland P et al (2005) New methods for acylation of pure and sawdust-extracted cellulose by fatty acid derivatives—thermal and mechanical analyses of cellulose-based plastic films. J Appl Polym Sci 97:1266–1278. https://doi.org/10.1002/app.21783
Kakibe T, Nakamura S, Mizuta W, Kishi H (2017) Etherification of cellulose in binary ionic liquid as solvent and catalyst. Chem Lett 46:737–739. https://doi.org/10.1246/cl.170089
Kakko T, King AWT, Kilpeläinen I (2017) Homogenous esterification of cellulose pulp in [DBNH][OAc]. Cellulose 24:5341–5354. https://doi.org/10.1007/s10570-017-1521-5
Kamida K, Okajima K, Matsui T, Kowsaka K (1984) Study on the solubility of cellulose in aqueous alkali solution by deuteration IR and 13C NMR. Polym J 16:857–866. https://doi.org/10.1295/polymj.16.857
Kamide K, Okajima K (1981) Determination of distribution of O-acetyl group in trihydric alcohol units of cellulose acetate by carbon-13 nuclear magnetic resonance analysis. Polym J 13:127–133. https://doi.org/10.1295/polymj.13.127
Kamide K, Okajima K, Saito M (1981) Nuclear magnetic resonance study of thermodynamic interaction between cellulose acetate and solvent. Polym J 13:115–125. https://doi.org/10.1295/polymj.13.115
Ke H, Zhou J, Zhang L (2006) Structure and physical properties of methylcellulose synthesized in NaOH/urea solution. Polym Bull 56:349–357. https://doi.org/10.1007/s00289-006-0507-5
Khupse ND, Kumar A (2011) The cosolvent-directed Diels–Alder reaction in ionic liquids. J Phys Chem A 115:10211–10217. https://doi.org/10.1021/jp205181e
Klemm D, Stein A (1995) Silylated cellulose materials in design of supramolecular structures of ultrathin cellulose films. J Macromol Sci Part A 32:899–904. https://doi.org/10.1080/10601329508010304
Klüfers P, Schuhmacher J (1994) Linear coordination polymers of copper(II) and fourfold deprotonated sugar alcohols. Angew Chem Int Ed Engl 33:1742–1744. https://doi.org/10.1002/anie.199417421
Köhler S, Heinze T (2007) New solvents for cellulose: dimethyl sulfoxide/ammonium fluorides. Macromol Biosci 7:307–314. https://doi.org/10.1002/mabi.200600197
Köhler S, Liebert T, Schöbitz M et al (2007) Interactions of ionic liquids with polysaccharides 1. Unexpected acetylation of cellulose with 1-ethyl-3-methylimidazolium acetate. Macromol Rapid Commun 28:2311–2317. https://doi.org/10.1002/marc.200700529
Köhler S, Liebert T, Heinze T (2008) Interactions of ionic liquids with polysaccharides. VI. Pure cellulose nanoparticles from trimethylsilyl cellulose synthesized in ionic liquids. J Polym Sci Part A Polym Chem 46:4070–4080. https://doi.org/10.1002/pola.22749
Köhler S, Liebert T, Heinze T (2009) Ammonium-based cellulose solvents suitable for homogeneous etherification. Macromol Biosci 9:836–841. https://doi.org/10.1002/mabi.200900156
Köhler S, Liebert T, Heinze T et al (2010) Interactions of ionic liquids with polysaccharides 9. Hydroxyalkylation of cellulose without additional inorganic bases. Cellulose 17:437–448. https://doi.org/10.1007/s10570-009-9379-9
Kondo T, Gray DG (1991) The preparation of O-methyl- and O-ethyl-celluloses having controlled distribution of substituents. Carbohyd Res 220:173–183. https://doi.org/10.1016/0008-6215(91)80015-F
Kontturi EJ, Thuene PC, Niemantsverdriet JW (2003) Cellulose model surfaces—simplified preparation by spin coating and characterization by X-ray photoelectron spectroscopy, infrared spectroscopy, and atomic force microscopy. Langmuir 19:5735–5741. https://doi.org/10.1021/la0340394
Kosan B, Dorn S, Meister F, Heinze T (2010) Preparation and subsequent shaping of cellulose acetates using ionic liquids. Macromol Mater Eng 295:676–681. https://doi.org/10.1002/mame.201000022
Koschella A, Klemm D (1997) Silylation of cellulose regiocontrolled by bulky reagents and dispersity in the reaction media. Macromol Symp 120:115–125. https://doi.org/10.1002/masy.19971200113
Koschella A, Heinze T, Klemm D (2001) First synthesis of 3-O-functionalized cellulose ethers via 2,6-di-O-protected silyl cellulose. Macromol Biosci 1:49–54. https://doi.org/10.1002/1616-5195(200101)1:1%3c49:AID-MABI49%3e3.0.CO;2-C
Kostag M, Köhler S, Liebert T, Heinze T (2010) Pure cellulose nanoparticles from trimethylsilyl cellulose. Macromol Symp 294:96–106. https://doi.org/10.1002/masy.200900095
Kostag M, Liebert T, El Seoud OA, Heinze T (2013) Efficient cellulose solvent: quaternary ammonium chlorides. Macromol Rapid Commun 34:1580–1584. https://doi.org/10.1002/marc.201300497
Kostag M, Jedvert K, Achtel C et al (2018) Recent advances in solvents for the dissolution, shaping and derivatization of cellulose: quaternary ammonium electrolytes and their solutions in water and molecular solvents. Molecules 23:511. https://doi.org/10.3390/molecules23030511
Kuzmina O, Sashina ES, Troshenkowa S, Wawro D (2010) Dissolved state of cellulose in ionic liquids—the impact of water. Fibers Text Eastern Eur 18:32–37
Kwak SH, Gong Y-D (2013) Unexpected route for the synthesis of N,N-dialkyl formamidines using phenyl chloroformate and N,N-dialkyl formamides. Tetrahedron 69:7107–7111. https://doi.org/10.1016/j.tet.2013.06.026
Kwolek SL, Morgan PW, Schaefgen JR, Gulrich LW (1977) Synthesis, anisotropic solutions, and fibers of poly(1,4-benzamide). Macromolecules 10:1390–1396. https://doi.org/10.1021/ma60060a041
Law RC (2004) 5. Applications of cellulose acetate 5.1 Cellulose acetate in textile application. Macromol Symp 208:255–266. https://doi.org/10.1002/masy.200450410
Letters K (1932) Viskosimetrische Untersuchungen über die Reaktion von Zellulose mit konzentrierten Chlorzinklösungen. Kolloid-Zeitschrift 58:229–239. https://doi.org/10.1007/BF01460731
Lewis WG, Green LG, Grynszpan F et al (2002) Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew Chem Int Ed 41:1053–1057. https://doi.org/10.1002/1521-3773(20020315)41:6%3c1053:AID-ANIE1053%3e3.0.CO;2-4
Li WY, Jin AX, Liu CF et al (2009) Homogeneous modification of cellulose with succinic anhydride in ionic liquid using 4-dimethylaminopyridine as a catalyst. Carbohyd Polym 78:389–395. https://doi.org/10.1016/j.carbpol.2009.04.028
Li M-F, Sun S-N, Xu F, Sun R-C (2011a) Cold NaOH/urea aqueous dissolved cellulose for benzylation: synthesis and characterization. Eur Polym J 47:1817–1826. https://doi.org/10.1016/j.eurpolymj.2011.06.013
Li W, Wu L, Chen D et al (2011b) DMAP-catalyzed phthalylation of cellulose with phthalic anhydride in [BMIM]Cl. BioResources 6:2375–2385. https://doi.org/10.15376/biores.6.3.2375-2385
Li Y, Wang J, Liu X, Zhang S (2018) Towards a molecular understanding of cellulose dissolution in ionic liquids: anion/cation effect, synergistic mechanism and physicochemical aspects. Chem Sci 9:4027–4043. https://doi.org/10.1039/C7SC05392D
Liebert T, Heinze T (1998) Induced phase separation: a new synthesis concept in cellulose chemistry. In: Heinze TJ, Glasser WG (eds) Cellulose derivatives. American Chemical Society, pp 61–72. https://doi.org/10.1021/bk-1998-0688.ch004
Liebert TF, Heinze T (2005) Tailored cellulose esters: synthesis and structure determination. Biomacromolecules 6:333–340. https://doi.org/10.1021/bm049532o
Liebert T, Kostag M, Wotschadlo J, Heinze T (2011) Stable cellulose nanospheres for cellular uptake. Macromol Biosci 11:1387–1392. https://doi.org/10.1002/mabi.201100113
Liebner F, Patel I, Ebner G et al (2010) Thermal aging of 1-alkyl-3-methylimidazolium ionic liquids and its effect on dissolved cellulose. Holzforschung 64:161–166. https://doi.org/10.1515/hf.2010.033
Lilienfeld L (1924) Manufacture of cellulose solutions, US1460A German Patent Application No. 443095 C, 16 April 1927
Lin L, Yamaguchi H, Tsuchii K (2015) Solvent used for dissolving polysaccharide and method for manufacturing molded article and polysaccharide derivative using this solvent, EP 2 690 132 A1
Lindman B, Medronho B, Alves L et al (2017) The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys Chem Chem Phys 19:23704–23718. https://doi.org/10.1039/C7CP02409F
Liu C, Baumann H (2002) Exclusive and complete introduction of amino groups and their N-sulfo and N-carboxymethyl groups into the 6-position of cellulose without the use of protecting groups. Carbohyd Res 337:1297–1307. https://doi.org/10.1016/S0008-6215(02)00132-5
Liu S, Zhang L (2009) Effects of polymer concentration and coagulation temperature on the properties of regenerated cellulose films prepared from LiOH/urea solution. Cellulose 16:189–198. https://doi.org/10.1007/s10570-008-9268-7
Liu CF, Sun RC, Zhang AP et al (2006) Structural and thermal characterization of sugarcane bagasse cellulose succinates prepared in ionic liquid. Polym Degrad Stab 91:3040–3047. https://doi.org/10.1016/j.polymdegradstab.2006.08.004
Liu CF, Sun RC, Zhang AP et al (2007) Homogeneous modification of sugarcane bagasse cellulose with succinic anhydride using a ionic liquid as reaction medium. Carbohyd Res 342:919–926. https://doi.org/10.1016/j.carres.2007.02.006
Liu CF, Zhang AP, Li WY et al (2010) Succinoylation of cellulose catalyzed with iodine in ionic liquid. Ind Crops Prod 31:363–369. https://doi.org/10.1016/j.indcrop.2009.12.002
Liu H, Wang A, Xu X et al (2016a) Porous aerogels prepared by crosslinking of cellulose with 1,4-butanediol diglycidyl ether in NaOH/urea solution. RSC Adv 6:42854–42862. https://doi.org/10.1039/C6RA07464B
Liu X, Zhang T, Pang K et al (2016b) Graphene oxide/cellulose composite films with enhanced UV-shielding and mechanical properties prepared in NaOH/urea aqueous solution. RSC Adv 6:73358–73364. https://doi.org/10.1039/C6RA16535D
Lu F, Ralph J (2003) Non-degradative dissolution and acetylation of ball-milled plant cell walls: high-resolution solution-state NMR. Plant J 35:535–544. https://doi.org/10.1046/j.1365-313X.2003.01817.x
Luan Y, Zhang J, Zhan M et al (2013) Highly efficient propionylation and butyralation of cellulose in an ionic liquid catalyzed by 4-dimethylminopyridine. Carbohyd Polym 92:307–311. https://doi.org/10.1016/j.carbpol.2012.08.111
Luo X, Zhang L (2013) New solvents and functional materials prepared from cellulose solutions in alkali/urea aqueous system. Food Res Int 52:387–400. https://doi.org/10.1016/j.foodres.2010.05.016
Lv Y, Wu J, Zhang J et al (2012) Rheological properties of cellulose/ionic liquid/dimethylsulfoxide (DMSO) solutions. Polymer 53:2524–2531. https://doi.org/10.1016/j.polymer.2012.03.037
Lv Y, Chen Y, Shao Z et al (2015) Homogeneous tritylation of cellulose in 1-allyl-3-methylimidazolium chloride and subsequent acetylation: the influence of base. Carbohyd Polym 117:818–824. https://doi.org/10.1016/j.carbpol.2014.10.041
Marson GA, Seoud OAE (1999) A novel, efficient procedure for acylation of cellulose under homogeneous solution conditions. J Appl Polym Sci 74:1355–1360. https://doi.org/10.1002/(SICI)1097-4628(19991107)74:6%3c1355:AID-APP5%3e3.0.CO;2-M
McCormick CL, Callais PA (1987) Derivatization of cellulose in lithium chloride and N,N-dimethylacetamide solutions. Polymer 28:2317–2323. https://doi.org/10.1016/0032-3861(87)90393-4
McCormick CL, Callais PA, Hutchinson BH (1985) Solution studies of cellulose in lithium chloride and N,N-dimethylacetamide. Macromolecules 18:2394–2401. https://doi.org/10.1021/ma00154a010
McCormick CL, Dawsey TR, Newman JK (1990) Competitive formation of cellulose p-toluenesulfonate and chlorodeoxycellulose during homogeneous reaction of p-toluenesulfonyl chloride with cellulose in N,N-dimethylacetamide-lithium chloride. Carbohyd Res 208:183–191. https://doi.org/10.1016/0008-6215(90)80098-N
Medronho B, Duarte H, Alves L et al (2016) The role of cyclodextrin-tetrabutylammonium complexation on the cellulose dissolution. Carbohyd Polym 140:136–143. https://doi.org/10.1016/j.carbpol.2015.12.026
Medronho B, Duarte H, Magalhães S et al (2017) From a new cellulose solvent to the cyclodextrin induced formation of hydrogels. Colloids Surf A 532:548–555. https://doi.org/10.1016/j.colsurfa.2017.03.047
Meng X, Devemy J, Verney V et al (2017) Improving cellulose dissolution in ionic liquids by tuning the size of the Ions: impact of the length of the alkyl chains in tetraalkylammonium carboxylate. Chemsuschem 10:1749–1760. https://doi.org/10.1002/cssc.201601830
Miyamoto H, Umemura M, Aoyagi T et al (2009) Structural reorganization of molecular sheets derived from cellulose II by molecular dynamics simulations. Carbohyd Res 344:1085–1094. https://doi.org/10.1016/j.carres.2009.03.014
Moellmann E, Heinze T, Liebert T, Koehler S (2013) Homogeneous synthesis of cellulose ethers in ionic liquids US 2009/0221813 A1 1813A1
Morgan PW (1977) Synthesis and properties of aromatic and extended chain polyamides. Macromolecules 10:1381–1390. https://doi.org/10.1021/ma60060a040
Morgenstern B, Berger W (1993) Investigations about dissolution of cellulose in the LiCl/N,N-dimethylformamide system. Acta Polym 44:100–102. https://doi.org/10.1002/actp.1993.010440208
Morgenstern B, Kammer H-W (1999) On the particulate structure of cellulose solutions. Polymer 40:1299–1304. https://doi.org/10.1016/S0032-3861(98)00267-5
Morgenstern B, Kammer HW, Berger W, Skrabal P (1992) 7Li-NMR study on cellulose/LiCl/N,N-dimethylacetamide solutions. Acta Polym 43:356–357. https://doi.org/10.1002/actp.1992.010430612
Mormann W, Wezstein M (2009) Trimethylsilylation of cellulose in ionic liquids. Macromol Biosci 9:369–375. https://doi.org/10.1002/mabi.200800192
Nawaz H, Pires PAR, El Seoud OA (2013) Kinetics and mechanism of imidazole-catalyzed acylation of cellulose in LiCl/N,N-dimethylacetamide. Carbohyd Polym 92:997–1005. https://doi.org/10.1016/j.carbpol.2012.10.009
Nawaz H, Pires PAR, Arêas EPG et al (2015) Probing cellulose acetylation in binary mixtures of an ionic liquid with dimethylsulfoxide and sulfolane by chemical kinetics, viscometry, spectroscopy, and molecular dynamics simulations. Macromol Chem Phys 216:2368–2376. https://doi.org/10.1002/macp.201500315
Nguyen QV, Nomura S, Hoshino R et al (2017) Recyclable and scalable organocatalytic transesterification of polysaccharides in a mixed solvent of 1-ethyl-3-methylimidazolium acetate and dimethyl sulfoxide. Polym J 49:783–787. https://doi.org/10.1038/pj.2017.49
Olsson C, Westman G (2017) Co-solvent facilitated in situ esterification of cellulose in 1-ethyl-3-methylimidazolium acetate. BioResources 12:1395–1402. https://doi.org/10.15376/biores.12.1.1395-1402
Östlund Å, Lundberg D, Nordstierna L et al (2009) Dissolution and gelation of cellulose in TBAF/DMSO solutions: the roles of fluoride ions and water. Biomacromolecules 10:2401–2407. https://doi.org/10.1021/bm900667q
Otera J (1993) Transesterification. Chem Rev 93:1449–1470. https://doi.org/10.1021/cr00020a004
Papanyan Z, Roth C, Wittler K et al (2013) The dissolution of polyols in salt solutions and ionic liquids at molecular level: ions, counter ions, and hofmeister effects. ChemPhysChem 14:3667–3671. https://doi.org/10.1002/cphc.201300465
Parthasarathi R, Bellesia G, Chundawat SPS et al (2011) Insights into hydrogen bonding and stacking interactions in cellulose. J Phys Chem A 115:14191–14202. https://doi.org/10.1021/jp203620x
Parviainen A, King AWT, Mutikainen I et al (2013) Predicting cellulose solvating capabilities of acid–base conjugate ionic liquids. Chemsuschem 6:2161–2169. https://doi.org/10.1002/cssc.201300143
Peng N, Wang Y, Ye Q et al (2016) Biocompatible cellulose-based superabsorbent hydrogels with antimicrobial activity. Carbohyd Polym 137:59–64. https://doi.org/10.1016/j.carbpol.2015.10.057
Petruš L, Gray DG, BeMiller JN (1995) Homogeneous alkylation of cellulose in lithium chloride/dimethyl sulfoxide solvent with dimsyl sodium activation. A proposal for the mechanism of cellulose dissolution in LiCl/Me2SO. Carbohyd Res 268:319–323. https://doi.org/10.1016/0008-6215(94)00330-I
Peydecastaing J, Vaca-Garcia C, Borredon E (2008a) Accurate determination of the degree of substitution of long chain cellulose esters. Cellulose 16:289. https://doi.org/10.1007/s10570-008-9267-8
Peydecastaing J, Vaca-Garcia C, Borredon E (2008b) Quantitative analysis of mixtures of various linear anhydrides and carboxylic acids. Chroma 68:685–688. https://doi.org/10.1365/s10337-008-0765-5
Peydecastaing J, Vaca-Garcia C, Borredon E (2009) Consecutive reactions in an oleic acid and acetic anhydride reaction medium. Eur J Lipid Sci Technol 111:723–729. https://doi.org/10.1002/ejlt.200800189
Pinkert A, Marsh KN, Pang S (2010) Reflections on the solubility of cellulose. Ind Eng Chem Res 49:11121–11130. https://doi.org/10.1021/ie1006596
Plechkova NV, Rogers RD, Seddon KR (2009) Ionic liquids: from knowledge to application. In: Ionic liquids: from knowledge to application. American Chemical Society, Washington
Possidonio S, Fidale LC, Seoud OAE (2010) Microwave-assisted derivatization of cellulose in an ionic liquid: an efficient, expedient synthesis of simple and mixed carboxylic esters. J Polym Sci Part A Polym Chem 48:134–143. https://doi.org/10.1002/pola.23770
Potthast A, Rosenau T, Buchner R et al (2002) The cellulose solvent system N,N-dimethylacetamide/lithium chloride revisited: the effect of water on physicochemical properties and chemical stability. Cellulose 9:41–53. https://doi.org/10.1023/A:1015811712657
Potthast A, Rosenau T, Sartori J et al (2003) Hydrolytic processes and condensation reactions in the cellulose solvent system N,N-dimethylacetamide/lithium chloride. Part 2: degradation of cellulose. Polymer 44:7–17. https://doi.org/10.1016/S0032-3861(02)00751-6
Potthast A, Radosta S, Saake B et al (2015) Comparison testing of methods for gel permeation chromatography of cellulose: coming closer to a standard protocol. Cellulose 22:1591–1613. https://doi.org/10.1007/s10570-015-0586-2
Qi H, Chang C, Zhang L (2008) Effects of temperature and molecular weight on dissolution of cellulose in NaOH/urea aqueous solution. Cellulose 15:779–787. https://doi.org/10.1007/s10570-008-9230-8
Qi H, Liebert T, Meister F, Heinze T (2009) Homogeneous carboxymethylation of cellulose in the NaOH/urea aqueous solution. React Funct Polym 69:779–784. https://doi.org/10.1016/j.reactfunctpolym.2009.06.007
Qi H, Liebert T, Meister F et al (2010) Homogeneous carboxymethylation of cellulose in the new alkaline solvent LiOH/urea aqueous solution. Macromol Symp 294:125–132. https://doi.org/10.1002/masy.200900166
Rahn K, Diamantoglou M, Klemm D et al (1996) Homogeneous synthesis of cellulose p-toluenesulfonates in N,N-dimethylacetamide/LiCl solvent system. Die Angew Makromol Chem 238:143–163. https://doi.org/10.1002/apmc.1996.052380113
Ramos LA, Frollini E, Heinze T (2005a) Carboxymethylation of cellulose in the new solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Carbohyd Polym 60:259–267. https://doi.org/10.1016/j.carbpol.2005.01.010
Ramos LA, Frollini E, Koschella A, Heinze T (2005b) Benzylation of cellulose in the solvent dimethylsulfoxide/tetrabutylammonium fluoride trihydrate. Cellulose 12:607–619. https://doi.org/10.1007/s10570-005-9007-2
Ramos LA, Morgado DL, El Seoud OA et al (2011) Acetylation of cellulose in LiCl-N,N-dimethylacetamide: first report on the correlation between the reaction efficiency and the aggregation number of dissolved cellulose. Cellulose 18:385–392. https://doi.org/10.1007/s10570-011-9496-0
Ratanakamnuan U, Atong D, Aht-Ong D (2012) Cellulose esters from waste cotton fabric via conventional and microwave heating. Carbohyd Polym 87:84–94. https://doi.org/10.1016/j.carbpol.2011.07.016
Rebière J, Rouilly A, Durrieu V et al (2017) Characterization of non-derivatized cellulose samples by size exclusion chromatography in tetrabutylammonium fluoride/dimethylsulfoxide (TBAF/DMSO). Molecules 22:1985. https://doi.org/10.3390/molecules22111985
Regiani AM, Frollini E, Marson GA et al (1999) Some aspects of acylation of cellulose under homogeneous solution conditions. J Polym Sci Part A Polym Chem 37:1357–1363. https://doi.org/10.1002/(SICI)1099-0518(19990501)37:9%3c1357:AID-POLA16%3e3.0.CO;2-Y
Reichardt C, Welton T (2010) Empirical parameters of solvent polarity. In: Solvents and solvent effects in organic chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 425–508
Rein DM, Khalfin R, Szekely N, Cohen Y (2014) True molecular solutions of natural cellulose in the binary ionic liquid-containing solvent mixtures. Carbohyd Polym 112:125–133. https://doi.org/10.1016/j.carbpol.2014.05.059
Rinaudo M (1993) Polysaccharide characterization in relation with some original properties. J Appl Polym Sci Appl Polym Symp 52:11–17
Röder T, Morgenstern B, Schelosky N, Glatter O (2001) Solutions of cellulose in N,N-dimethylacetamide/lithium chloride studied by light scattering methods. Polymer 42:6765–6773. https://doi.org/10.1016/S0032-3861(01)00170-7
Rodríguez H, Gurau G, Holbrey JD, Rogers RD (2011) Reaction of elemental chalcogens with imidazolium acetates to yield imidazole-2-chalcogenones: direct evidence for ionic liquids as proto-carbenes. Chem Commun 47:3222–3224. https://doi.org/10.1039/C0CC05223J
Rosenau T, Potthast A, Hofinger A et al (2001) Hydrolytic processes and condensation Reactions in the cellulose solvent system N,N-dimethylacetamide/lithium chloride. Part 1. Holzforschung 55:661–666. https://doi.org/10.1515/HF.2001.107
Rosenau T, Potthast A, Kosma P (2006) Trapping of reactive intermediates to study reaction mechanisms in cellulose chemistry. In: Klemm D (ed) Polysaccharides II. Springer, Berlin Heidelberg, pp 153–197
Ruan D, Lue A, Zhang L (2008) Gelation behaviors of cellulose solution dissolved in aqueous NaOH/thiourea at low temperature. Polymer 49:1027–1036. https://doi.org/10.1016/j.polymer.2007.12.044
Saalwächter K, Burchard W, Klüfers P et al (2000) Cellulose solutions in water containing metal complexes. Macromolecules 33:4094–4107. https://doi.org/10.1021/ma991893m
Saric SP, Schofield RK (1946) The dissociation constants of the carboxyl and hydroxyl groups in some insoluble and sol-forming polysaccharides. Proc R Soc Lond A 185:431–447. https://doi.org/10.1098/rspa.1946.0029
Sashina ES, Kashirskii DA (2015) Pyridinium-based ionic liquids—application for cellulose processing. Ionic Liquids—Current State of the Art 389–417. https://doi.org/10.5772/59286
Schenzel A, Hufendiek A, Barner-Kowollik C, Meier MAR (2014) Catalytic transesterification of cellulose in ionic liquids: sustainable access to cellulose esters. Green Chem 16:3266–3271. https://doi.org/10.1039/C4GC00312H
Schneider S, Linse P (2002) Swelling of cross-linked polyelectrolyte gels. Eur Phys J E 8:457–460. https://doi.org/10.1140/epje/i2002-10043-y
Schneider S, Linse P (2003) Monte Carlo simulation of defect-free cross-linked polyelectrolyte gels. J Phys Chem B 107:8030–8040. https://doi.org/10.1021/jp022336w
Schult T, Hjerde T, Inge Optun O et al (2002) Characterization of cellulose by SEC-MALLS. Cellulose 9:149–158. https://doi.org/10.1023/A:1020139409903
Sealey JE, Samaranayake G, Todd JG, Glasser WG (1996) Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose. J Polym Sci Part B Polym Phys 34:1613–1620. https://doi.org/10.1002/(SICI)1099-0488(19960715)34:9%3c1613:AID-POLB10%3e3.0.CO;2-A
Sharma RK, Fry JL (1983) Instability of anhydrous tetra-n-alkylammonium fluorides. J Org Chem 48:2112–2114. https://doi.org/10.1021/jo00160a041
Shulepov ID, Kozhikhova KV, Panfilova YS et al (2016) One-pot synthesis of cross-linked sub-micron microgels from pure cellulose via the Ugi reaction and their application as emulsifiers. Cellulose 23:2549–2559. https://doi.org/10.1007/s10570-016-0957-3
Singh RK, Gupta P, Sharma OP, Ray SS (2015) Homogeneous synthesis of cellulose fatty esters in ionic liquid (1-butyl-3-methylimidazolium chloride) and study of their comparative antifriction property. J Ind Eng Chem 24:14–19. https://doi.org/10.1016/j.jiec.2014.09.031
Sjöholm E, Gustafsson K, Pettersson B, Colmsjö A (1997) Characterization of the cellulosic residues from lithium chloride/N,N-dimethylacetamide dissolution of softwood kraft pulp. Carbohyd Polym 32:57–63. https://doi.org/10.1016/S0144-8617(96)00129-4
Song Y, Sun Y, Zhang X et al (2008a) Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromolecules 9:2259–2264. https://doi.org/10.1021/bm800429a
Song Y, Zhou J, Zhang L, Wu X (2008b) Homogeneous modification of cellulose with acrylamide in NaOH/urea aqueous solutions. Carbohyd Polym 73:18–25. https://doi.org/10.1016/j.carbpol.2007.10.018
Song J, He A, Jin Y, Cheng Q (2013) Synthesis of amphoteric cellulose in aqueous NaOH–urea solution in one pot and its application in paper strength enhancement. RSC Advances 3:24586–24592. https://doi.org/10.1039/C3RA44628J
Söyler Z, Onwukamike KN, Grelier S et al (2018) Sustainable succinylation of cellulose in a CO2-based switchable solvent and subsequent Passerini 3-CR and Ugi 4-CR modification. Green Chem 20:214–224. https://doi.org/10.1039/C7GC02577G
Spange S, Reuter A, Vilsmeier E et al (1998) Determination of empirical polarity parameters of the cellulose solvent N,N-dimethylacetamide/LiCl by means of the solvatochromic technique. J Polym Sci Part A Polym Chem 36:1945–1955. https://doi.org/10.1002/(SICI)1099-0518(199808)36:11%3c1945:AID-POLA30%3e3.0.CO;2-C
Steinmeier H (2004) 3. Acetate manufacturing, process and technology 3.1 Chemistry of cellulose acetylation. Macromolecular Symposia 208:49–60. https://doi.org/10.1002/masy.200450405
Stolarska O, Pawlowska-Zygarowicz A, Soto A et al (2017) Mixtures of ionic liquids as more efficient media for cellulose dissolution. Carbohyd Polym 178:277–285. https://doi.org/10.1016/j.carbpol.2017.09.025
Striegel AM (2003) Advances in the understanding of the dissolution mechanism of cellulose in DMAc/LiCl. J Chil Chem Soc 48:73–77. https://doi.org/10.4067/S0717-97072003000100013
Striegel AM, Timpa JD (1996) Size exclusion chromatography of polysaccharides in dimethylacetamide-lithium chloride. In: Potschka M, Dubin PL (eds) Strategies in size exclusion chromatography. American Chemical Society, pp 366–378
Strlič M, Kolar J (2003) Size exclusion chromatography of cellulose in LiCl/N,N-dimethylacetamide. J Biochem Biophys Methods 56:265–279. https://doi.org/10.1016/S0165-022X(03)00064-2
Sun H, DiMagno SG (2005) Anhydrous tetrabutylammonium fluoride. J Am Chem Soc 127:2050–2051. https://doi.org/10.1021/ja0440497
Takahashi S-I, Fujimoto T, Barua BM et al (1986) 13C-NMR spectral studies on the distribution of substituents in some cellulose derivatives. J Polym Sci Part A Polym Chem 24:2981–2993. https://doi.org/10.1002/pola.1986.080241125
Tammelin T, Saarinen T, Österberg M, Laine J (2006) Preparation of Langmuir/Blodgett-cellulose surfaces by using horizontal dipping procedure. Application for polyelectrolyte adsorption studies performed with QCM-D. Cellulose 13:519. https://doi.org/10.1007/s10570-005-9002-7
Tarasova E, Šumigin D, Kudrjašova M, Krumme A (2013) Preparation of cellulose stearate and cellulose acetate stearate in 1-butyl-3-methylimidazolium chloride. Key Eng Mater 559:105–110. https://doi.org/10.4028/www.scientific.net/KEM.559.105
Thomas R (1970) New process for the partial esterification of cellulose with carboxylic acids under practice conditions. Textilveredlung 5:361–368
Thota N, Mukherjee D, Reddy MV et al (2009) Reaction of carbohydrates with Vilsmeier reagent: a tandem selective chloro O-formylation of sugars. Org Biomol Chem 7:1280–1283. https://doi.org/10.1039/B900026G
Tiller J, Berlin P, Klemm D (2000) Novel matrices for biosensor applications by structural design of redox-chromogenic aminocellulose esters. J Appl Polym Sci 75:904–915. https://doi.org/10.1002/(SICI)1097-4628(20000214)75:7%3c904:AID-APP7%3e3.0.CO;2-8
Tiwari S, Kumar A (2012) Viscosity dependence of intra- and intermolecular Diels–Alder reactions. J Phys Chem A 116:1191–1198. https://doi.org/10.1021/jp208989z
Trulove PC, Reichert WM, Long HCD et al (2009) The structure and dynamics of silk and cellulose dissolved in ionic liquids. ECS Trans 16:111–117. https://doi.org/10.1149/1.3159315
Vaca-Garcia C, Borredon ME (1999) Solvent-free fatty acylation of cellulose and lignocellulosic wastes. Part 2: reactions with fatty acids. Biores Technol 70:135–142. https://doi.org/10.1016/S0960-8524(99)00034-6
Vaca-Garcia C, Thiebaud S, Borredon ME, Gozzelino G (1998) Cellulose esterification with fatty acids and acetic anhydride in lithium chloride/N,N-dimethylacetamide medium. J Am Oil Chem Soc 75:315–319. https://doi.org/10.1007/s11746-998-0047-2
van Osch DJGP, Kollau LJBM, van den Bruinhorst A et al (2017) Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys Chem Chem Phys 19:2636–2665. https://doi.org/10.1039/c6cp07499e
Vasudevan V, Mushrif SH (2015) Insights into the solvation of glucose in water, dimethyl sulfoxide (DMSO), tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) and its possible implications on the conversion of glucose to platform chemicals. RSC Adv 5:20756–20763. https://doi.org/10.1039/C4RA15123B
Wang Z, Yokoyama T, Chang H, Matsumoto Y (2009) Dissolution of beech and spruce milled woods in LiCl/DMSO. J Agric Food Chem 57:6167–6170. https://doi.org/10.1021/jf900441q
Wang H-H, Zhang X-Q, Long P et al (2016) Reaction behavior of cellulose in the homogeneous esterification of bagasse modified with phthalic anhydride in ionic liquid 1-allyl-3-methylimidazium chloride. Int J Polym Sci. https://doi.org/10.1155/2016/2361284
Wang B, Qin L, Mu T et al (2017) Are ionic liquids chemically stable? Chem Rev 117:7113–7131. https://doi.org/10.1021/acs.chemrev.6b00594
Wang Y, Liu L, Chen P et al (2018) Cationic hydrophobicity promotes dissolution of cellulose in aqueous basic solution by freezing–thawing. Phys Chem Chem Phys 20:14223–14233. https://doi.org/10.1039/C8CP01268G
Wei Y, Cheng F (2007) Effect of solvent exchange on the structure and rheological properties of cellulose in LiCl/DMAc. J Appl Polym Sci 106:3624–3630. https://doi.org/10.1002/app.26886
Wendler F, Todi L-N, Meister F (2012) Thermostability of imidazolium ionic liquids as direct solvents for cellulose. Thermochim Acta 528:76–84. https://doi.org/10.1016/j.tca.2011.11.015
Wu J, Zhang J, Zhang H et al (2004) Homogeneous acetylation of cellulose in a newionic liquid. Biomacromolecules 5:266–268. https://doi.org/10.1021/bm034398d
Würfel H, Kayser M, Heinze T (2018) Efficient and catalyst-free synthesis of cellulose acetoacetates. Cellulose 25:4919–4928. https://doi.org/10.1007/s10570-018-1908-y
Xia K, Chen J, Yang R et al (2014) Green synthesis and crystal structure of regioselectively substituting 6-O-tritylcellulose derivatives. J Biobased Mater Bioenergy 8:587–593. https://doi.org/10.1166/jbmb.2014.1472
Xiao P, Zhang J, Feng Y et al (2014) Synthesis, characterization and properties of novel cellulose derivatives containing phosphorus: cellulose diphenyl phosphate and its mixed esters. Cellulose 21:2369–2378. https://doi.org/10.1007/s10570-014-0256-9
Xie H, Yu X, Yang Y, Zhao ZK (2014) Capturing CO2 for cellulose dissolution. Green Chem 16:2422–2427. https://doi.org/10.1039/C3GC42395F
Xin P-P, Huang Y-B, Hse C-Y et al (2017) Modification of cellulose with succinic anhydride in TBAA/DMSO mixed solvent under catalyst-free conditions. Materials 10:526. https://doi.org/10.3390/ma10050526
Xu Q, Chen L-F (1999) Ultraviolet spectra and structure of zinc–cellulose complexes in zinc chloride solution. J Appl Polym Sci 71:1441–1446. https://doi.org/10.1002/(SICI)1097-4628(19990228)71:9%3c1441:AID-APP8%3e3.0.CO;2-G
Xu D, Edgar KJ (2012) TBAF and cellulose esters: unexpected deacylation with unexpected regioselectivity. Biomacromolecules 13:299–303. https://doi.org/10.1021/bm201724s
Xu A, Wang J, Wang H (2010) Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems. Green Chem 12:268–275. https://doi.org/10.1039/B916882F
Xu D, Li B, Tate C, Edgar KJ (2011) Studies on regioselective acylation of cellulose with bulky acid chlorides. Cellulose 18:405–419. https://doi.org/10.1007/s10570-010-9476-9
Xu Q, Song L, Zhang L et al (2018) Synthesis of cellulose acetate propionate and cellulose acetate butyrate in a CO2/DBU/DMSO system. Cellulose 25:205–216. https://doi.org/10.1007/s10570-017-1539-8
Yang Y, Xie H, Liu E (2014) Acylation of cellulose in reversible ionic liquids. Green Chem 16:3018–3023. https://doi.org/10.1039/C4GC00199K
Yang Y, Song L, Peng C et al (2015) Activating cellulose via its reversible reaction with CO2 in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene for the efficient synthesis of cellulose acetate. Green Chem 17:2758–2763. https://doi.org/10.1039/C5GC00115C
Yu Y, Miao J, Jiang Z et al (2016) Cellulose esters synthesized using a tetrabutylammonium acetate and dimethylsulfoxide solvent system. Appl Phys A 122:656. https://doi.org/10.1007/s00339-016-0205-6
Yu Y, Jiang Z, Miao J et al (2018) Application of the solvent dimethyl sulfoxide/tetrabutyl-ammonium acetate as reaction medium for mix-acylation of pulp. Adv Polym Technol 37:955–961. https://doi.org/10.1002/adv.21742
Yuan X, Cheng G (2015) From cellulose fibrils to single chains: understanding cellulose dissolution in ionic liquids. Phys Chem Chem Phys 17:31592–31607. https://doi.org/10.1039/C5CP05744B
Yusup EM, Mahzan S, Jafferi N, Been CW (2015) The effectiveness of TBAF/DMSO in dissolving oil palm empty fruit bunch-cellulose phosphate. J Med Bioeng 4:165–169. https://doi.org/10.12720/jomb.4.2.165-169
Zhang J, Wu J, Cao Y et al (2009) Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 16:299–308. https://doi.org/10.1007/s10570-008-9260-2
Zhang C, Liu R, Xiang J et al (2014) Dissolution mechanism of cellulose in N,N-dimethylacetamide/lithium chloride: revisiting through molecular interactions. J Phys Chem B 118:9507–9514. https://doi.org/10.1021/jp506013c
Zhang H, Guo H, Wang B et al (2016) Synthesis and characterization of quaternized bacterial cellulose prepared in homogeneous aqueous solution. Carbohyd Polym 136:171–176. https://doi.org/10.1016/j.carbpol.2015.09.029
Zhang J, Wu J, Yu J et al (2017a) Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends. Mater Chem Front 1:1273–1290. https://doi.org/10.1039/C6QM00348F
Zhang Z, Song J, Han B (2017b) Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem Rev 117:6834–6880. https://doi.org/10.1021/acs.chemrev.6b00457
Zhao H, Baker GA, Song Z et al (2008) Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem 10:696–705. https://doi.org/10.1039/B801489B
Zheng X, Gandour RD, Edgar KJ (2013a) Probing the mechanism of TBAF-catalyzed deacylation of cellulose esters. Biomacromolecules 14:1388–1394. https://doi.org/10.1021/bm400041w
Zheng X, Gandour RD, Edgar KJ (2013b) TBAF-catalyzed deacylation of cellulose esters: reaction scope and influence of reaction parameters. Carbohyd Polym 98:692–698. https://doi.org/10.1016/j.carbpol.2013.06.010
Zhong C, Wang C, Wang F et al (2016) Application of tetra-n-methylammonium hydroxide on cellulose dissolution and isolation from sugarcane bagasse. Carbohyd Polym 136:979–987. https://doi.org/10.1016/j.carbpol.2015.10.001
Zhong C, Cheng F, Zhu Y et al (2017) Dissolution mechanism of cellulose in quaternary ammonium hydroxide: revisiting through molecular interactions. Carbohyd Polym 174:400–408. https://doi.org/10.1016/j.carbpol.2017.06.078
Zhou J, Zhang L (2000) Solubility of cellulose in NaOH/urea aqueous solution. Polym J 32:866–870. https://doi.org/10.1295/polymj.32.866
Zhou J, Zhang L, Deng Q, Wu X (2004) Synthesis and characterization of cellulose derivatives prepared in NaOH/urea aqueous solutions. J Polym Sci Part A Polym Chem 42:5911–5920. https://doi.org/10.1002/pola.20431
Zhou J, Qin Y, Liu S, Zhang L (2006) Homogeneous synthesis of hydroxyethylcellulose in NaOH/urea aqueous solution. Macromol Biosci 6:84–89. https://doi.org/10.1002/mabi.200500148
Zhou J, Xu Y, Wang X et al (2008) Microstructure and aggregation behavior of methylcelluloses prepared in NaOH/urea aqueous solutions. Carbohyd Polym 74:901–906. https://doi.org/10.1016/j.carbpol.2008.05.016
Zweckmair T, Hettegger H, Abushammala H et al (2015) On the mechanism of the unwanted acetylation of polysaccharides by 1,3-dialkylimidazolium acetate ionic liquids: part 1—analysis, acetylating agent, influence of water, and mechanistic considerations. Cellulose 22:3583–3596. https://doi.org/10.1007/s10570-015-0756-2
Acknowledgments
O. A. El Seoud and M. Kostag thank the FAPESP research foundation for financial support and postdoctoral fellowship (Grants 2014/22136-4 and 2016/22869-7, respectively). O. A. El Seoud thanks CNPq for research productivity fellowship (Grant 307022/2014-5). The financial support of the DFG-funded Collaborative Research Centre PolyTarget (SFB 1278, Project A02) is gratefully acknowledged by T. Heinze. We thank Gabriel O. El Seoud for the art work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kostag, M., Gericke, M., Heinze, T. et al. Twenty-five years of cellulose chemistry: innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 26, 139–184 (2019). https://doi.org/10.1007/s10570-018-2198-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2198-0