Abstract
Dissolution of cellulose having different viscosity-average molecular weight (M η ) in 7 wt%NaOH/12 wt%urea aqueous solution at temperature from 60 to −12.6°C was investigated with optical microscope, viscosity measurements and wide X-ray diffraction (WXRD). The solubility (Sa) of cellulose in NaOH/urea aqueous solution strongly depended on the temperature, and molecular weight. Their Sa values increased with a decrease in temperature, and cellulose having M η below 10.0 × 104 could be dissolved completely in NaOH/urea aqueous solution pre-cooled to −12.6°C. The activation energy of dissolution (Ea,s) of the cellulose dissolution was a negative value, suggesting that the cellulose solution state had lower enthalpy than the solid cellulose. The cellulose concentration in this system increased with a decrease of M η to achieve about 8 wt% for M η of 3.1 × 104. Moreover, cellulose having 12.7 × 104 could be dissolved completely in the solvent pre-cooled to −12.6°C as its crystallinity (χ c) decreased from 0.62 to 0.53. We could improve the solubility of cellulose in NaOH/urea aqueous system by changing M η , χ c and temperature. In addition, the zero-shear viscosity (η 0 ) at 0°C for the 4 wt% cellulose solution increased rapidly with an increase of M η , as a result of the enhancement of the aggregation and entanglement for the relatively long chains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As well known, the cellulose is the most abundant material resource in the nature, especially the petrolic resource do not newborn and the crude oil prices fly to $100/per barrel. However, cellulose forms unique microcrystal structures through strong hydrogen bonding networks (Zhao et al. 2007), leading to the difficulty of its dissolution in common solvent. In traditional viscose route for production regenerated cellulose fibers, CS2 (toxic gas) leads to the serious environmental pollution and the poor health of the human body. Therefore, several new solvent systems have been developed to dissolve cellulose at high temperature. The dissolution of cellulose in the N-methyl-morpholine-N-oxide (NMMO) occurs at 85–130°C (Heinze and Liebert 2001; Michael et al. 2000). By heating or refluxing cellulose in N,N-dimethylacetamide (DMAc), or in DMAc containing LiCl at about 150°C, transparent cellulose solution can be obtained (Tosh et al. 2000; Potthast et al. 2002). Recently, various ionic liquids (ILs) have been found to dissolve cellulose (Zhu et al. 2006), such as cellulose can be dissolved in the 1-N-butyl-3-methylimidazolium chloride at about 85–100°C (Heinze et al. 2005; Kosan et al. 2008). At high temperature, ion pairs in 1-allyl-3-methylimidazolium chloride (AMIMCl) can be dissociated to individual Cl− and AMIM+ ions, and then free Cl− ions associated with the cellulose hydroxyl proton and the free cations disrupte the hydrogen bonding in cellulose, leading to its dissolution (Zhang et al. 2005). In addition, solution of cellulose in heavy metal–amine complex solutions system (such as Cuoxam, Ni-tren, Cd-tren, and so on) is usually prepared at room temperature (Saalwaechter et al. 2000). Microcrystalline cellulose (MCC) having low degree of polymerization (DP < 250) can be dissolved in 8–10%NaOH aqueous system via a subsequent freeze and thaw thermal cycling (Yamashiki et al. 1988; Kuo and Hong 2005; Isogai and Atalla 1998).
In our laboratory, new solvents, such as aqueous NaOH/urea, NaOH/thiourea and LiOH/urea aqueous solutions have been used to dissolve cellulose at low temperature (Cai and Zhang 2006; Cai et al. 2007a; Ruan et al. 2004; Cai and Zhang 2005). These solvents are attractable because cellulose can be easily and quickly dissolved them and produce stable cellulose solutions (Yan et al. 2007). It is worth noting that NaOH/urea aqueous solution is an economical and environmentally friendly solvent of cellulose. Cellulose could be rapidly dissolved in 7 wt% NaOH/12 wt%urea aqueous solution pre-cooled to −12°C (Cai and Zhang 2005). Moreover, the regenerated cellulose membranes and fibers have been prepared successfully (Cai et al. 2007b; Mao et al. 2006). However, native cellulose (cellulose I) having high viscosity-molecular weight (M η ) (M η > 14 × 104) could not be dissolved completely in 7 wt% NaOH/12 wt% urea aqueous solution pre-cooled to −12°C. Namely, the dissolution of cellulose is related to its molecular weight and conditions. A basic understanding of the effects of temperature and molecular weight of cellulose on its dissolution is essential for the successful development and application of cellulose. In this work, the cellulose samples having different M η were dissolved in 7 wt% NaOH/12 wt% urea aqueous solution at temperature from 60 to −12.6°C, and their solubility and viscosity of the cellulose in the solution were investigated and discussed.
Experimental
Materials
Cellulose (cotton linters pulp) with an α-cellulose content of about 92% was supplied by Hubei Chemical Fiber Co. Ltd. (Xiangfan, China). The viscosity-average molecular weight (M η ) of the cellulose samples was 13.1 × 104, 11.1 × 104, 9.2 × 104, 8.3 × 104, 7.8 × 104, 6.3 × 104, 4.8 × 104 and 3.1 × 104, and coded as C13, C11, C9, C8, C7, C6, C5 and C3, respectively. The cellulose samples were shredded and dried at 60°C for 24 h in a vacuum oven, stored in a desiccator until used. Two cellulose samples (C13, C9) were beated circularly in distilled water at 20°C by Valley beating machine (ZQS2-23, Shaanxi University of Science and Technology Machine Works, Xianyang, China) for 72 h to reduce their crystallinity, and then were dried upon bronze-net. All of the chemical reagents were purchased from commercial resources in China, and were of analytical grade.
Solubility test
7 wt% NaOH/12 wt% urea aqueous solution was prepared by mixing of NaOH, urea and distilled water (7:12:81 by weight). After 192 g solvent was prepared to a desired temperature, 8 g cellulose sample was added immediately into it with stirring vigorously for 10 min. The dispersed solution was then centrifuged at 8,000 rpm at 5°C for 2 min. The remaining undissolved fractions were washed using water and acetone, respectively, and then dried at 60°C for 24 h in a vacuum oven. Thus, the solubility of cellulose (Sa) in NaOH/urea aqueous solution was calculated by
where W0 is weight of original cellulose, and Wi is weight of the undissolved fractions.
Characterization
Intrinsic viscosity ([η]) of the dilute cellulose solution in LiOH/urea system was determined using a Ubbelohde capillary viscometer at 25 ± 0.1°C, and its M η value was calculated according to [η] = 3.72 × 10−2 M 0.77w (mL g−1) (Cai et al. 2006). The zero-shear viscosity (η 0) of the cellulose solution was determined using the steady shear tests on an ARES-RFS III rheometer (TA Instrument, USA). Double-concentric cylinder geometry with a gap of 2 mm was used to measure shear viscosity as function of shear rate. Temperature control was established by connection with a julabo FS18 cooling/heating bath kept within 0.2°C over an extended time.
Optical microscope (Axiovert 200 M, ZEISS, Germany) was used to compare the morphological change and solubility of cellulose in the 7 wt% NaOH/12 wt% urea aqueous solution. The cellulose solutions were pressed between two glass slides directly after being prepared, and then sealed by paraffin to be observed and photographed. Wide-angle X-Ray diffraction (WAXD) measurement was carried out with an X-Ray diffractometry (D/MAX-1200, Rigaku Denki Co. Ltd., Japan). The X-ray radiation used was Ni-filtered Cu-Kα with a wavelength of 1.5406 Å. The voltage was set at 40 kV and the current was set at 30 mA. The samples were mounted on a solid circular holder, and the proportional counter detector was set to collect data at a rate of 2θ = 1°/min over the 2θ range from 4° to 40°. All samples were cut into particle-like size to erase the influence of the crystalline orientation. The crystallinity (χ c) of each sample was calculated according to the usual method (Rabek 1980).
Results and discussion
Influence of temperature on dissolution
The dissolution state of the cellulose in 7 wt% NaOH/12 wt% urea aqueous solution at different temperature has been investigated. Figure 1 shows the pictures of the cellulose solution dissolved at different temperature for 2 min. The results reveal that, at high temperature from 0°C to 25°C, cellulose changes hardly, and at 60°C it only swells in the solvent, showing fiber with diameter of 20–30 μm. By decreasing temperature from 0°C to −10°C, the swelling degree of the cellulose fiber increased. Transparent cellulose solution has occurred at −12.6°C, indicating that the cellulose has been dissolved completely. To clarify the change of solubility, the dependence of the Sa value on temperature for C9 in solvent is shown in Fig. 2. At temperature from 60°C to 10°C, the Sa value maintains in a constant (about 7.6–8.3%), indicating that the few fractions with the low molecular weight cellulose could be dissolved. The average molecular weight of undissolved fractions is close to that of its initial cellulose (9.2 × 104) as shown in Table 1. Clearly, the cellulose could not be dissolved at temperature above 10°C in NaOH/urea aqueous solution. However, with a decrease of the temperature from 10°C to −12°C, the Sa value of cellulose increased rapidly, and it could be dissolved in the range from −10 to −12.6°C. Ice will form in the solvent below −12.6°C (freezing point), which is a critical temperature (Tc) of this mixture solution. Although the solvent is a liquid-solid two-phase below Tc, cellulose could also be dissolved completely in the solvent system.
The relationship between Sa value and temperature can be described by the Arrhenius equation as
where Ea,s is the apparent activation energy of dissolution (kJ/mol), R is the molar gas constant (0.008314 kJ mol−1 K−1), T is the absolute temperature (K), and A is a preexponential factor (Su and Puls 1999). The Ea,s could be obtained from the slope of a plot of lnSa vs 1/T using linear least-squares analysis (Fig. 3). Interestingly, the calculated Ea value is −101 kJ/mol in the range from 5 to −10°C, as a result of the dissolution at low temperature. Usually, increasing the temperature can promote the dissolution of normal polymers, because of the solvation being enhanced by heat movement. However, the solubility of a little of aqueous soluble polymers decreases when temperature increases (Spelzini et al. 2005). Negative activation energies have been observed for dimer formation in self-complementary oligonucleotides (Craig et al. 1971) and in β-hairpin and α-helical formation in polypeptides (Muñoz et al. 1997; Lednev et al. 1999), which imply that the transition “state” along the effective reaction coordinate has a lower enthalpy than the random coil state, and that the free energy barrier arises from a significant loss of entropy (Ansari et al. 2001). The negative apparent activation energy in our system suggests that the cellulose solution state has a lower enthalpy than the solid cellulose, and the dissolution of cellulose in aqueous NaOH/urea solution pre-cooled to low temperature could be described as an entropy-driven process. When solid cellulose was immersed in NaOH/urea aqueous solution pre-cooled to −12°C, the hydrogen-bonded network structure between the cellulose macromolecules and small molecules in the solvent was created rapidly to form a inclusion complex, bringing cellulose into the aqueous solution (Cai et al. 2007a, b).
Figure 4 shows X-Ray diffractograms of the undissolved fractions at different temperature. The diffraction peaks at 2θ = 14.8°, 16.3°, and 22.6° for \( {\left( {1\ifmmode\expandafter\bar\else\expandafter\=\fi{1}0} \right)}, \) (110), and (200) planes are characteristic for cellulose I crystal, and those at 2θ = 12.1°, 19.8°, and 22.0° for \( {\left( {1\ifmmode\expandafter\bar\else\expandafter\=\fi{1}0} \right)}, \) (110), and (200) planes are signed to cellulose II crystal. The initial cellulose (C9) has typical crystalline peaks of cellulose I, whereas the dissolved fractions in cellulose solution exhibit peaks of the cellulose II. At 25°C the X-Ray diffractograms of undissolved fractions is similar to that of cellulose I, this further confirms that the solvent cannot destroy the structure of cellulose at above 25°C. However, with the decreasing of temperature, the diffraction peaks at 2θ = 14.8°, 16.3°, and 22.6° corresponding to cellulose I in undissolved fractions gradually disappear, and those at 2θ = 12.1°, 19.8°, and 22.0° corresponding to cellulose II increase. It is worth noting that the diffraction peaks corresponding to cellulose I disappear almost completely below −10°C, suggesting that they have changed to cellulose II. The results strongly indicate that the structure of native cellulose could be destroyed in the present solvent system at low temperature. On the basis of the data, the degree of mercerization of the undissolved fractions increases with a decrease of temperature, indicating that the dissolution power of NaOH/urea aqueous solution on cellulose increases. The experimental results of M η and χ c of dissolved and undissolved fractions of C9 at different temperature are summarized in Table 1. The χ c values of undissolved fractions decrease with the decreasing of temperature, which suggests that the crystalline structure of cellulose can be destroyed more easily by NaOH at low temperature. Furthermore, M η of regenerated cellulose from the dissolved fractions is close to that of initial cellulose, indicating that no obvious degradation of cellulose occurred in the dissolution processes.
Influence of M η on dissolution
Figure 5 shows the dependence of the Sa value on temperature in the range from −12.6°C to 15°C. The Sa values increase with a decrease of M η of cellulose. At 15°C, the Sa value is about 8% and there is no obvious difference for the cellulose samples with different M η , indicating that cellulose cannot be dissolved at this temperature. The C13 cellulose sample (M η = 13.1 × 104) could not be dissolved completely even at −12.6°C, whereas C3 (M η = 3.1 × 104) can be dissolved completely at about −9°C. The results indicate that solubility depends strongly on molecular weight of cellulose and temperature.
From Arrhenius plots of temperature dependence of Sa, the activation energy of dissolution (Ea,s) of the cellulose samples having different M η has been calculated, and the result is shown in Fig. 6. Interestingly, the Ea,s values decrease rapidly with an increase of molecular weight of cellulose, which differ significantly from normal polymers. For normal polymer, with their long chains, the dissolution is slightly relative to the whole polymer molecular weight. This suggests that every glucose unit in cellulose contributes to the formation of their inter- and intra-molecular hydrogen bonds to remain the super-molecular structure. Thus, the high sensitivity of the Ea,s value on M η indicates that the cellulose dissolution has been controlled by their molecular weight. The Ea,s value for the C13 cellulose sample having M η = 3.1 × 104, which could not be dissolved completely even at −12.6°C, is −134 kJ/mol. This suggests that cellulose could not be dissolved completely in the solvent when Ea,s value was as low as −134 kJ/mol. Therefore, the cellulose samples having M η below 10.0 × 104 (Ea, s > −120 kJ/mol) could be dissolved completely in NaOH/urea aqueous solution pre-cooled to −12°C.
Influence of χc on dissolution
The solubility of the cellulose samples are summarized in Table 2. The Sa value at −6°C increases with the decreasing of M η , supporting above conclusion that cellulose with lower M η is more easily to be dissolved in this solvent. Interestingly, the solvent can dissolve cellulose (C13) with M η of 9.8 × 104 and value of 0.67 at −6°C, whereas it cannot dissolve cellulose (C3) with M η of 4.0 × 104 and value of 0.76 at the same condition. This can be explained that the relatively high χ c could prevent the dissolution of cellulose. Figure 7 shows X-Ray diffractograms of undissolved fractions at −6°C, which indicates that there is both cellulose I and cellulose II. Although there is slightly decreasing with the decreasing of M η , the χ c value of undissolved fractions is almost the same.
Two cellulose samples (C13 and C9) are selected to be treated by Valley beating machine to change their crystallinity. Table 3 shows that M η of the treated cellulose decrease slightly from 13.1 × 104 to 12.7 × 104 for C13 and from 9.2 × 104 to 9.0 × 104 for C9. However, the χ c value of the treated cellulose significantly decreases from 0.62 to 0.53 for C13 and from 0.66 to 0.55 for C9. Figure 8 shows the dependence of the Sa value of the treated and untreated cellulose samples on temperature. The solubility of the treated cellulose samples increase obviously. It is noted that the treated C13 having 12.7 × 104 of M η can be dissolved in the solvent pre-cooled to −12.6°C completely, as a result of the decreased crystallinity. We also obtained Ea,s value of the cellulose dissolution from the slope of a plot of lnSa vs 1/T using linear least-squares analysis (Table 3). The Ea,s value of the treated cellulose is decreased remarkably from 134 kJ/mol to 85 kJ/mol for C13 and from 101 kJ/mol to 76 kJ/mol for C9. Obviously, the solubility of the cellulose can be enhanced by the decreasing of the χ c value of cellulose. In view of the above results, we can improve the solubility of the cellulose in this solvent obviously through the physical treating of cellulose.
Solution behavior
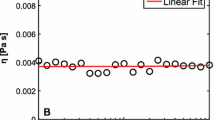
It is difficult to disperse cellulose molecules in this solvent at the molecular level. The cellulose molecules in the NaOH/urea aqueous solution could form aggregates demonstrated by combined static and dynamic laser light scattering (Chen et al. 2007). The cellulose solution in NaOH/urea aqueous system could remain in a liquid state for a long time period at about 0 to 5°C (Cai and Zhang 2006). Figure 9 shows the M η dependence of the zero-shear viscosity (η 0 ) at 0°C for the 4 wt% cellulose solution. We can obtain an equation following from the slope of a plot of logη 0 vs logM η using linear least-squares analysis:
In view of the exponent (α = 4.01), the η 0 of the cellulose solution increases more rapidly with the increasing of M η than that of normal polymers. It indicates that the aggregation and entanglement exist among the cellulose chains, and increase rapidly with an increase of M η in the cellulose solution at 0°C.
We need to control the viscosity of cellulose solution in producing regenerated cellulose products such as regenerated fibers and films. Thus, the cellulose concentration in the solution is important. Figure 10 shows dependence of the cellulose concentration (c) on M η in the solvent system pre-cooled to −12.6°C. It indicates that the polymer concentration increases with a decrease of M η to achieve about 8 wt% for M η of 3.1 × 104. Therefore, we can reduce the molecular weight of cellulose to increase its concentration in the solution.
Conclusion
The solubility of cellulose in NaOH/urea aqueous solution strongly depended on temperature of solvent, molecular weight and crystallinity. All of the cellulose samples having below M η below 10.0 × 104 had good solubility in aqueous NaOH/urea pre-cooled to −12.6°C. Interestingly, the Ea,s was negative value and decreased rapidly with an increase of molecular weight of cellulose, indicating a high sensitivity of Ea,s on M η , which significantly differed from normal polymers. Moreover, the solubility of the cellulose could be enhanced by decreasing the χ c value of cellulose. We could reduce M η , χ c to appropriate extent to obtain high concentration of the cellulose solution. The zero-shear viscosity of the cellulose solution increased more rapidly with the increasing of M η than that of the normal polymers.
References
Ansari A, Kuznetsov SV, Shen Y (2001) Configurational diffusion down a folding funnel describes the dynamics of DNA hairpins. PNAS 98:7771–7776
Cai J, Liu Y, Zhang L (2006) Dilute solution properties of cellulose in LiOH/urea aqueous system. J Polym Sci Part B: Polym Phys 44:3093–3101
Cai J, Zhang L (2005) Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol Biosci 5:539–548
Cai J, Zhang L (2006) Unique gelation behavior of cellulose in NaOH/Urea aqueous solution. Biomacromolecules 7:183–189
Cai J, Zhang L, Chang C, Cheng G, Chen X, Chu B (2007a) Hydrogen-bond-induced inclusion complex in aqueous cellulose/LiOH/Urea solution at low temperature. Chem Phys Chem 8:1572–1579
Cai J, Zhang L, Zhou J, Qi H, Chen H, Kondo T, Chen X, Chu B (2007b) Multifilament fibers based on dissolution of cellulose in NaOH/urea aqueous solution: structure and properties. Adv Mater 19:821–825
Chen X, Burger C, Wan F, Zhang J, Rong L, Hsiao BS, Chu B, Cai J, Zhang L (2007) Structure study of cellulose fibers wet-spun from environmentally friendly NaOH/urea aqueous solutions. Biomacromolecules 8:1918–1926
Craig ME, Crothers DM, Doty P (1971) Relaxation kinetics of dimer formation by self complementary oligonucleotides. J Mol Biol 62:383–401
Heinze T, Liebert T (2001) Unconventional methods in cellulose functionalization. Prog Polym Sci 26:1689–1762
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium in cellulose functionalization. Macromol Biosci 5:520–525
Isogai A, Atalla RH (1998) Dissolution of cellulose in aqueous NaOH solutions. Cellulose 5:309–319
Lednev IK, Karnoup AS, Sparrow MC, Asher SA (1999) Nanosecond UV resonance raman examination of initial steps in α-helix secondary structure evolution. J Am Chem Soc 121:4076–4077
Mao Y, Zhou J, Cai J, Zhang L (2006) Effects of coagulants on porous structure of membranes prepared from cellulose in NaOH/urea aqueous solution. J Membr Sci 279:246–255
Michael M, Ibbett RN, Howarth OW (2000) Interaction of cellulose with amine oxide solvents. Cellulose 7:21–33
Muñoz V, Thompson PA, Hofrichter J, Eaton WA (1997) Folding dynamics and mechanism of β-hairpin formation. Nature 390:196–199
Kosan B, Michels C, Meister F (2008) Dissolution and forming of cellulose with ionic liquids. Cellulose 15:59–66
Kuo Y, Hong J (2005) Investigation of solubility of microcrystalline cellulose in aqueous NaOH. Polym Adv Technol 16:425–428
Potthast A, Rosenau T, Sixta H, Kosma P (2002) Degradation of cellulosic materials by heating in DMAc/LiCl. Tetrahedron Lett 43:7757–7759
Rabek JF (1980) Experimental methods in polymer chemistry: application of wide-angle X-ray diffraction (WAXS) to the study of the structure of polymers. Wiley, Chichester, 505 pp
Ruan D, Zhang L, Mao Y, Zeng M, Li X (2004) Microporous membranes prepared from cellulose in NaOH/thiourea aqueous solution. J Membr Sci 241:265–274
Saalwaechter K, Burchard W, Kluefers P, Kettenbach G, Mayer P, Klemm D, Dugarmaa S (2000) Cellulose solutions in water containing metal complexes. Macromolecules 33:4094–4107
Spelzini D, Rigatusso R, Farruggia B, Picó G (2005) Thermal aggregation of methyl cellulose in aqueous solution: a thermodynamic study and protein partitioning behaviour. Cellulose 12:293–304
Su C, Puls RW (1999) Kinetics of trichloroethene reduction by zerovalent iron and tin: pretreatment effect, apparent activation energy, and intermediate products. Environ Sci Technol 33:163–168
Tosh B, Saikia CN, Dass NN (2000) Homogeneous esterification of cellulose in the lithium chloride-N, N-dimethylacetamide solvent system: effect of temperature and catalyst. Carbohydr Res 327:345–352
Yamashiki T, Kamide K, Okajima K, Kowsaka K, Matsui T, Fukase H (1988) Some characteristic features of dilute aqueous alkali solutions of specific alkali concentration (2.5 mol.l-1) which possess maximum solubility power against cellulose. Polym J 20:447–457
Yan L, Chen J, Bangal PR (2007) Dissolving cellulose in a NaOH/thiourea aqueous solution: a topochemical investigation. Macromol Biosci 7:1139–1148
Zhang H, Wu J, Zhang J, He J (2005) 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38:8272–8277
Zhao H, Kwak J, Wang Y, Franz JA, White JM, Holladay JE (2007) Interactions between cellulose and N-methylmorpholine-N-oxide. Carbohydr Polym 67:97–103
Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S, Ding Y, Wu G (2006) Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem 8:325–327
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (863 Program, 2003AA333040), National Supporting Project for Science and Technology (2006BAF02A09), and the National Natural Science Foundation of China (20474048). And Institute of Chemical Industry of Forest Product (Nanjing, China) is acknowledged for providing Valley beating machine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qi, H., Chang, C. & Zhang, L. Effects of temperature and molecular weight on dissolution of cellulose in NaOH/urea aqueous solution. Cellulose 15, 779–787 (2008). https://doi.org/10.1007/s10570-008-9230-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-008-9230-8